Abstract
Objective:
To determine how severity of prior history of SARS-CoV-2 infection influences postoperative outcomes following major elective inpatient surgery.
Summary Background Data:
Surgical guidelines instituted early in the COVID-19 pandemic recommended delay in surgery up to 8 weeks following an acute SARS-CoV-2 infection. This was based on the observation of elevated surgical risk following recovery from COVID-19 early in the pandemic. As the pandemic shifts to an endemic phase, it is unclear if this association remains, especially for those recovering from asymptomatic or mildly symptomatic COVID-19.
Methods:
Utilizing the National Covid Cohort Collaborative (N3C), we assessed postoperative outcomes for adults with and without a history of COVID-19 who underwent major elective inpatient surgery between January 2020 and February 2023. COVID-19 severity and time from infection to surgery were each used as independent variables in multivariable logistic regression models.
Results:
This study included 387,030 patients, of whom 37,354 (9.7%) were diagnosed with preoperative COVID-19. History of COVID-19 was found to be an independent risk factor for adverse postoperative outcomes even after a 12-week delay for patients with moderate and severe SARS-CoV-2 infection. Patients with mild COVID-19 did not have an increased risk of adverse postoperative outcomes at any time point. Vaccination decreased the odds of respiratory failure.
Conclusions:
Impact of COVID-19 on postoperative outcomes is dependent on severity of illness, with only moderate and severe disease leading to higher risk of adverse outcomes. Existing perioperative policies should be updated to include consideration of COVID-19 disease severity and vaccination status.
Keywords: COVID-19, SARS-CoV-2, postoperative outcomes, elective inpatient surgery
MINI-ABSTRACT
Postoperative outcomes were assessed for adults with and without a history of COVID-19 who underwent elective inpatient surgery. The impact of COVID-19 on postoperative outcomes was found to be associated with severity, timing, and vaccination status.
INTRODUCTION
As of February 2023, over 100 million cases of COVID-19 have been reported in the US1. Among those with prior infection, 15–20% will require elective surgery2. A prior history of COVID-19 infection is associated with adverse postoperative outcomes and complications3–7, and this risk can persist for up to eight weeks after infection5. Accordingly, early in the pandemic, several groups recommended a delay in surgery for 7–8 weeks following COVID-19 infection5,8.
While these guidelines have attempted to mitigate the risk of COVID-19 by delaying surgery, as the pandemic transitions to an endemic phase, there is a paucity of data to guide the de-implementation of these policies. This is especially important when delayed care can worsen both short- and long-term health outcomes9,10. In addition, delayed surgical care can lead to prolonged poor quality of life, including chronic pain, impaired mobility11–13, and an inability to work14.
The severity of SARS-CoV-2 infection can vary widely, from mild flu-like symptoms or no symptoms to systemic disease requiring hospitalization and even critical care. Most current guidelines for managing COVID-19 in the perioperative setting are not stratified by disease severity; however, there appears to be a relationship between severity of infection and postoperative outcomes15. Prior studies also show that compared to those with asymptomatic or mild disease, postoperative intensive care unit stay is longer among those with severe COVID-19 who require hospitalization16. This suggests that including the severity of SARS-CoV-2 infection in surgical wait time guidelines may facilitate more personalized patient recommendations and prevent unnecessary delays to surgery for those with less severe infections. In addition, since disease severity can be lowered through vaccination2,17, and as of February 2023, over 269 million people in the US received at least one dose of the COVID-19 vaccine1, the impact of preoperative vaccination among patients who present with COVID-19 prior to requiring surgery must be taken into consideration.
The primary objective of this study was to measure the association between the severity of SARS-CoV-2 infection prior to major elective surgery and postoperative adverse outcomes. Secondary objectives included investigating how severity should be considered in surgical wait time guidelines and the influence of vaccination on postoperative outcomes.
METHODS
Electronic health record data from the National COVID Cohort Collaborative (N3C), the largest multi-site centralized data source of COVID-19 patients in the United States, were retrospectively analyzed using the N3C Data Enclave. PCORnet, TriNetX, OMOP, and ACT are the primary data sources that contribute to N3C. A detailed description of N3C’s rationale, design, infrastructure, and deployment has been previously reported. Patients aged 18 years and older who underwent major inpatient surgery between January 2020 and February 2023 were included, and the surgical procedures were selected based on prior literature assessing the role of COVID-19 in surgical outcomes5. The following procedures were included: hip arthroplasty, knee arthroplasty, laminectomy, spinal arthrodesis, craniectomy, aortic aneurysm repair, lung excision, coronary artery bypass graft, esophagectomy, mastectomy, prostatectomy, colectomy, gastrectomy, hepatectomy, and pancreatectomy (Supplemental Table 1).
Patient characteristics (sex, age, race, ethnicity, and Charlson Comorbidity Index)11 were identified using the N3C’s Shared Knowledge Store patient fact table. To account for the changes in surgical care throughout the pandemic, we included an additional variable, ‘pandemic era’, which was independent of a patient’s COVID-19 status. This variable divided the pandemic into three specific eras, each characterized by the predominant SARS-CoV-2 variant: ‘Alpha’ from 01/01/2020 to 06/30/2021, ‘Delta’ from 07/01/2021 to 12/31/2021, and ‘Omicron’ from 01/01/2022 to 02/01/202318.
Standard SNOMED codes from the Observational Health Data Science and Informatics’ (OHDSI) ATLAS tool were used to identify elective inpatient procedures and patient comorbidities (Supplemental Table 1,2). The exclusion criteria included undergoing natural orifice, percutaneous, endoscopic, diagnostic (unless open surgery), or transplant procedures. In patients who underwent multiple surgeries, the first surgery after SARS-CoV-2 infection was used.
COVID-19 positivity was defined as having a positive laboratory measurement (PCR or antigen) or a positive COVID-19 diagnosis (ICD10-CM code U07.1) at any time before surgery. Patients with COVID-19 within 24 hours of surgery were excluded. The severity of a patient’s COVID-19 was determined using the World Health Organization’s (WHO) Clinical Progression Scale (CPS)19. Patient-specific severity was obtained from the N3C Knowledge Store and derived from critical visits associated with COVID positivity. Mild severity was defined as having an outpatient visit only (WHO Severity 1–3). Moderate severity was defined as requiring hospitalization without pulmonary support (WHO Severity 4–6). Severe disease was defined as requiring hospitalization with extracorporeal membrane oxygenation (ECMO or invasive ventilation, WHO Severity 7–9)20. The N3C Knowledge Store was used to determine the vaccination status of patients. Fully vaccinated patients were defined as having received either a single dose of the viral vector vaccine or two doses of mRNA vaccine at least 14 days before their first SARS-CoV-2 infection. Patients who received only one dose of the mRNA vaccine or were vaccinated after COVID-19 diagnosis were not fully vaccinated.
The primary outcome of the study was 30-day composite major morbidity and mortality and included the following: mortality, acute myocardial infarction, deep vein thrombosis, pneumonia, pulmonary embolism, renal failure, respiratory failure, and sepsis. Each 30-day complication included in the composite outcome was evaluated separately. Outcomes were constructed using SNOMED codes, and concepts associated with chronic conditions or those directly caused by COVID-19 were excluded (Supplemental Table 3).
Unadjusted baseline characteristics and comparisons between patients with and without prior COVID-19 were analyzed using standard descriptive statistics. Adjusted analyses were performed using multivariable logistic regression models. Separate models were fit for each outcome with covariates that included age, sex, race, ethnicity, Charlson Comorbidity Index (CCI), relative surgical risk, and pandemic era. To account for heterogeneity in the types of surgery included, we classified each procedure into one of three categories: low, medium, or high risk. This “Relative Surgical Risk” was a feature based on the expected median length of stay for each procedure included in this study (Supplemental Table 1). Additional logistic regression models were fitted to measure the association between 30-day postoperative adverse outcomes and (1) time between SARS-CoV-2 infection and surgery, (2) disease severity, and (3) vaccination status. Within each disease severity group, the risk of 30-day postoperative adverse outcomes as a factor of the time between infection and surgery was analyzed. All analyses were conducted using the N3C Data Enclave using the tidyverse, ggplot2, gtsummary, splines, and broom R packages.
RESULTS
A total of 387,030 patients were included in the study, and 37,354 (9.7%) were preoperatively diagnosed with COVID-19. Table 1 details the baseline demographics and characteristics of patients with and without a history of COVID-19.
Table 1.
Baseline patient characteristics and 30-day postoperative adverse outcomes in patients with and without preoperative COVID-19.
| Characteristics | No Hx of COVID-19 (N = 349,676), No. (%) |
Hx of COVID-19 (N = 37,354), No. (%) |
P value |
|---|---|---|---|
| Age, years | 63 (52, 72) | 61 (49, 70) | <0.001 |
| 18–29 | 15166 (4.3%) | 1625 (4.4%) | <0.001 |
| 30–49 | 60828 (17%) | 7734 (21%) | |
| 50–64 | 111160 (32%) | 12838 (34%) | |
| 65+ | 162522 (46%) | 15157 (41%) | |
| Sex | <0.001 | ||
| Female | 190052 (54%) | ‡20952 (≈56%) | |
| Race and Ethnicity | <0.001 | ||
| Non-Hispanic White | 257334 (74%) | 25833 (69%) | |
| Smoking Status | <0.001 | ||
| Nonsmoker | 304141 (86%) | 33151 (89%) | |
| Charlson Comorbidity Index | <0.001 | ||
| 0–2 | 178477 (51%) | 17527 (47%) | |
| 3+ | 171199 (49%) | 19827 (53%) | |
| Comorbidities | |||
| CKD | 22374 (6.4%) | 3862 (10%) | <0.001 |
| CHF | 20816 (6.0%) | 3454 (9.2%) | <0.001 |
| COPD | 21089 (6.0%) | 3134 (8.4%) | <0.001 |
| Coronary Artery Disease | 39221 (11%) | 5852 (16%) | <0.001 |
| Depression | 35956 (10%) | 5875 (16%) | <0.001 |
| Diabetes | 41584 (12%) | 7144 (19%) | <0.001 |
| GERD | 58830 (17%) | 9432 (25%) | <0.001 |
| Hypertension | 100981 (29%) | 14669 (39%) | <0.001 |
| Liver Disease | 5730 (1.6%) | 977 (2.6%) | <0.001 |
| Obesity | 51446 (15%) | 8920 (24%) | <0.001 |
| Relative Risk of Surgery | 0.058 | ||
| Low Risk | 134385 (38%) | 14364 (38%) | |
| Medium Risk | 166702 (48%) | 17642 (47%) | |
| High Risk | 48589 (14%) | 5348 (14%) | |
| Vaccine Status | <0.001 | ||
| Fully Vaccinated | 48441 (14%) | 5381 (14%) | |
| Non-Fully Vaccinated | 301235 (86%) | 31973 (86%) | |
| Pandemic Era | <0.001 | ||
| Alpha | 204624 (59%) | 9661 (26%) | |
| Delta | 61838 (18%) | 5942 (16%) | |
| Omicron | 83214 (24%) | 21751 (58%) |
To comply with N3C policy, counts below 20 are displayed as <20 and additional values were skewed by up to 5 in order to render it impossible to back-calculate precise counts in the ‘Missing or Unknown’ category. Hx: history; CKD: chronic kidney disease; CHF: congestive heart failure; COPD: chronic obstructive pulmonary disease; GERD: gastroesophageal reflux disease.
History of COVID-19 and 30-day postoperative adverse outcomes
In the unadjusted analysis, the rate of 30-day composite major morbidity or mortality was higher in patients with a history of COVID-19 than in those without (12% vs. 11%, P<0.001, Supplemental Table 4). Additionally, the rates of 30-day mortality (1.8% vs. 1.4%, P<0.001), postoperative pulmonary embolism (1.4% vs. 1.2%; P=0.008), renal failure (6.9% vs. 6.4%; P<0.001), and sepsis (3.2% vs. 2.9%; P=0.007) were all significantly greater in patients with a history of COVID-19 (Supplemental Table 4). After adjusting for age, sex, race, ethnicity, smoking status, comorbid disease, relative risk of surgery, and pandemic era, patients with a history of COVID-19 had greater odds of 30-day major morbidity or mortality (aOR 1.08 [1.05–1.12]; Figure 1).
Figure 1.
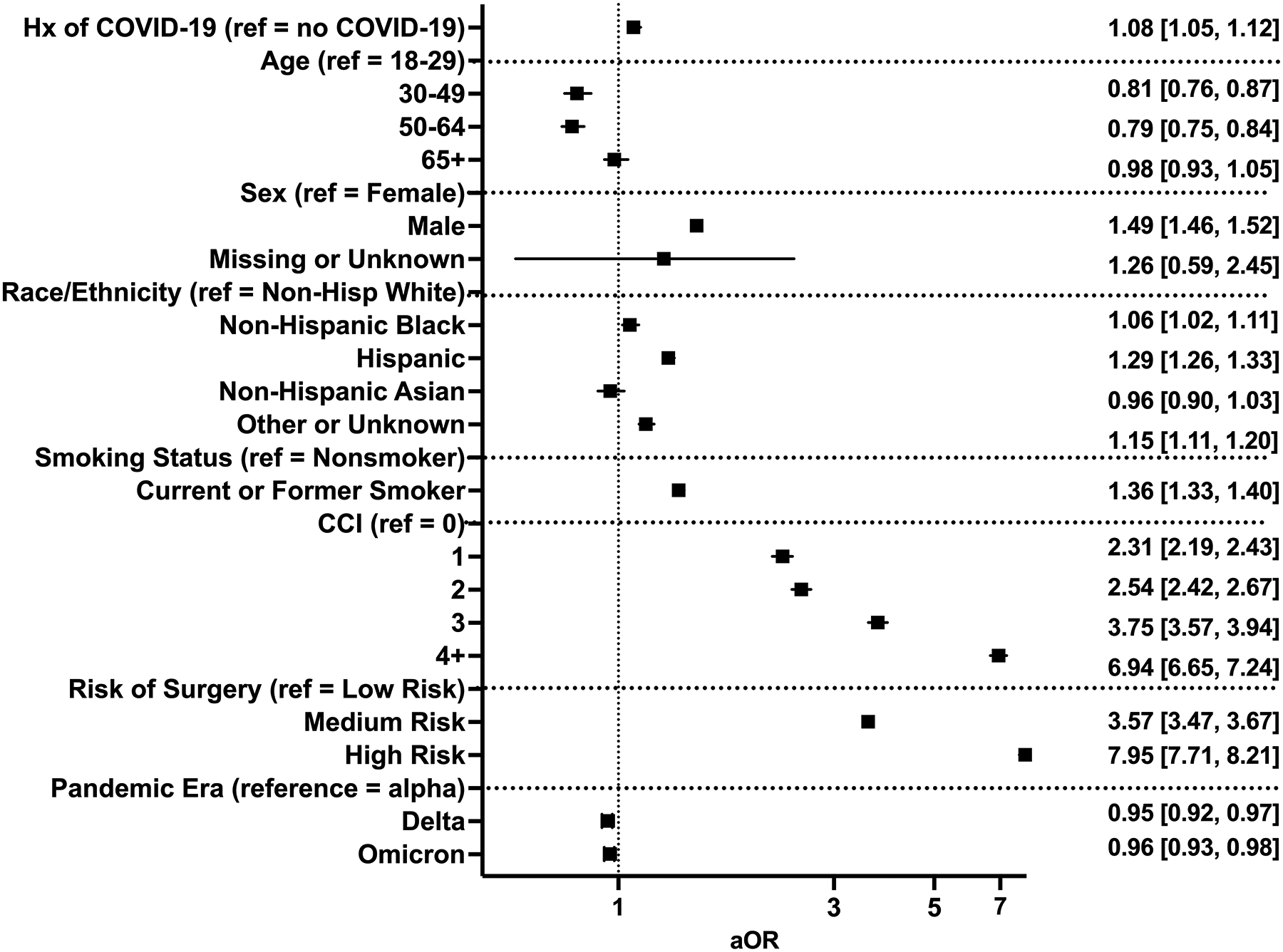
Multivariable regression assessing the association between prior history of COVID-19 and composite 30-day major morbidity or mortality. Hx: history; CCI: Charlson Comorbidity Index; aOR: adjusted odds ratio (with 95% confidence interval).
Association of time between SARS-CoV-2 infection and surgery and 30-day postoperative adverse outcomes
Patients were stratified based on the timing of surgery relative to SARS-CoV-2 infection. Groups included 0–4 weeks (N=7,425, 19.9%), 4–8 weeks (N=3,936, 10.5%), 8–12 weeks (N=2,604, 7.0%) and 12+ weeks (N=23,389, 62.6%). Patients who underwent surgery 0–4 weeks after COVID-19 had overall higher rates of postoperative adverse events, including composite major morbidity or mortality (21% vs. 15%; P<0.001), when compared to patients without COVID-19 (Supplemental Table 5). In the adjusted analysis (Figure 2, Supplemental Table 6), patients who underwent surgery within 4 weeks of SARS-CoV-2 infection had increased odds of 30-day composite major morbidity or mortality (aOR 1.44 [1.35–1.53]), mortality (aOR 2.37 [2.07–2.70]), deep vein thrombosis (aOR 1.36 [1.14–1.61]), pneumonia (aOR 1.65 [1.47–1.84]), pulmonary embolism (aOR 1.45 [1.21–1.72]), renal failure (aOR 1.29 [1.18–1.40]), respiratory failure (aOR 1.55 [1.42–1.69]), and sepsis (aOR 1.54 [1.37–1.72]). The odds of composite major morbidity or mortality returned to baseline at 12 weeks following infection (aOR 0.95 [0.91–0.99] at 12+ weeks).
Figure 2.
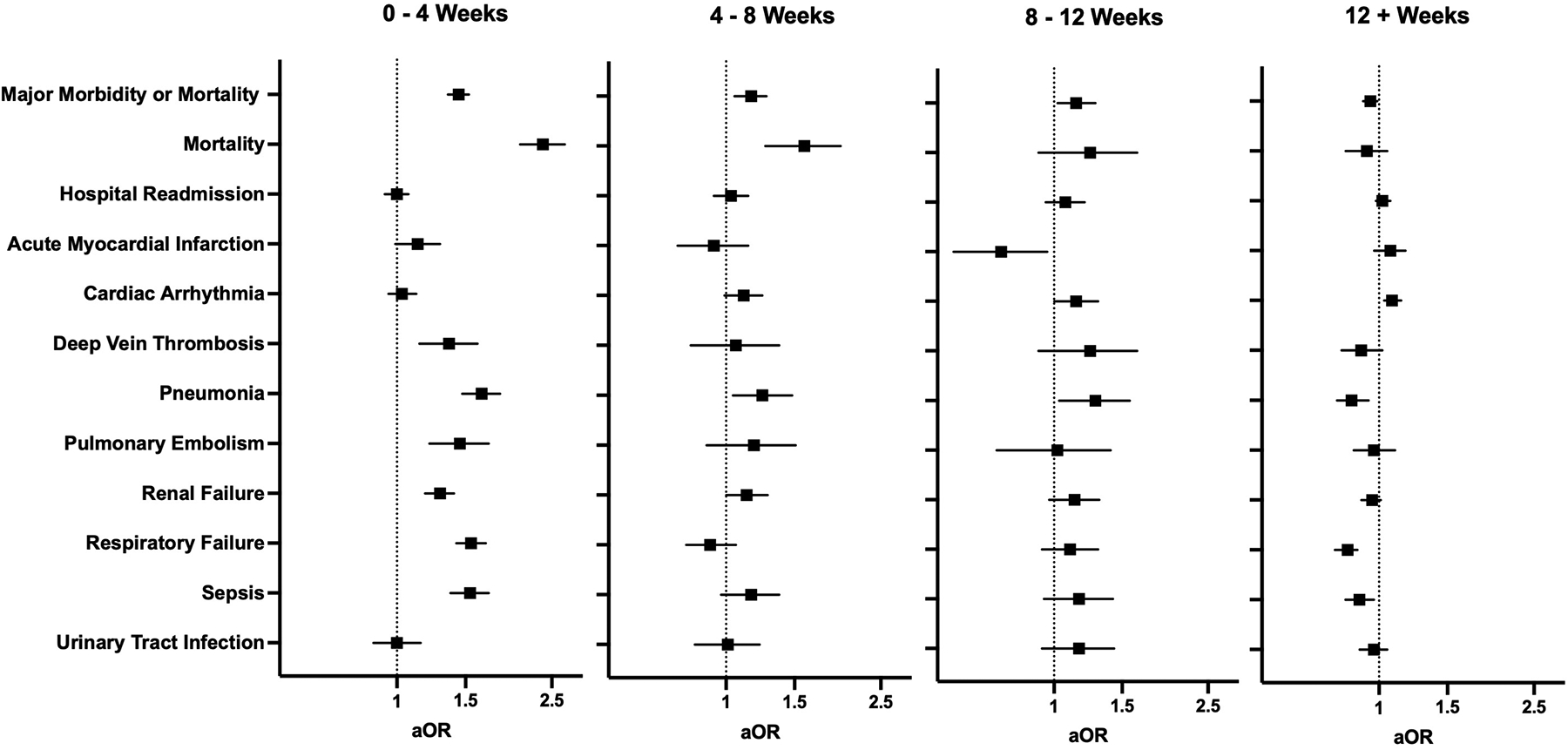
Multivariable regression assessing the association between timing of infection relative to surgery and 30-day postoperative adverse events. All models adjusted for age, sex, race and ethnicity, smoking status, comorbid disease, relative risk of surgery, and pandemic era. aOR: adjusted odds ratio (with 95% confidence interval).
Association of severity of SARS-CoV-2 infection and 30-day postoperative adverse outcomes
Patients were next stratified based on the severity of their SARS-CoV-2 infection before surgery, and 31,677 (84.8%), 5,009 (13.4%), and 655 (1.8%) patients had mild, moderate, and severe disease, respectively. The incidence of adverse 30-day postoperative outcomes increased with severity across all measured outcomes (Supplemental Table 7), and patients with severe disease had the highest rate of composite major morbidity or mortality (59% vs. 15%), mortality (14% vs. 1.4%), and hospital readmission (18% vs. 11%) compared to patients without COVID-19 history (P<0.001). After adjusting for patient demographics, risk of surgery, pandemic era, and comorbidities, patients with moderate and severe disease had higher odds for all assessed adverse events, except for pulmonary embolism for moderate COVID-19 (aOR 1.20 [0.97–1.46]) and hospital readmission for severe COVID-19 (aOR 1.20 [0.98–1.47]) (Figure 3, Supplemental Table 8).
Figure 3.
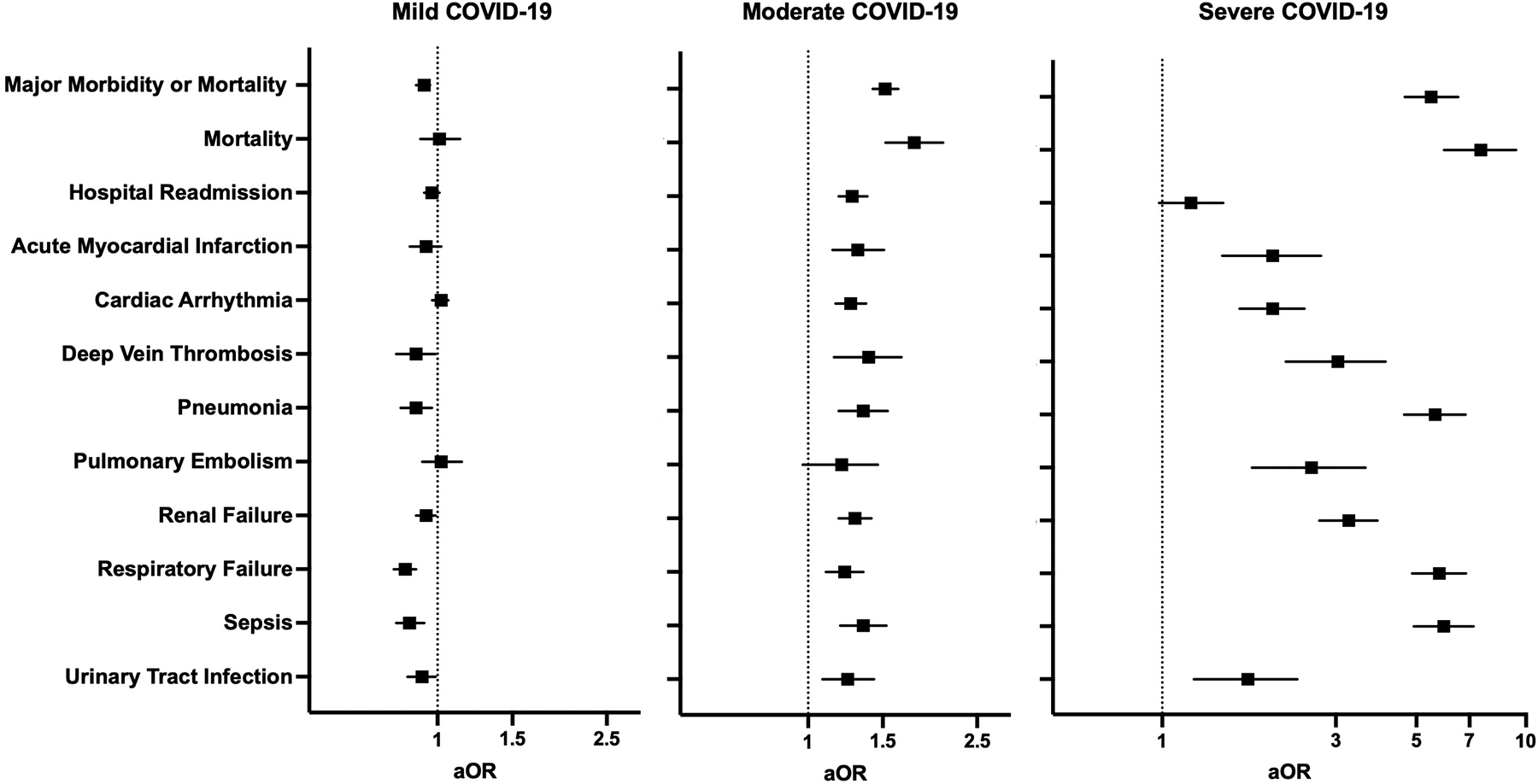
Multivariable regression assessing the association between COVID-19 disease severity and 30-day postoperative adverse outcomes. All models adjusted for age, sex, race and ethnicity, smoking status, comorbid disease, relative risk of surgery, and pandemic era. aOR: adjusted odds ratio (with 95% confidence interval).
The interplay between timing of surgery and COVID-19 disease severity in influencing risk for 30-day composite postoperative complications
For each COVID-19 severity group (mild, moderate, severe), the adjusted odds of having a composite adverse postoperative event were measured (Figure 4). Patients with mild COVID-19 did not demonstrate an increased risk, regardless of the time between infection and surgery. Patients with moderate disease were observed to have increased risk for composite adverse events at 0–4 weeks (aOR 2.16 [1.88–2.48]) that persisted beyond 12 weeks between COVID-19 and surgery (aOR 1.26 [1.15–1.37]) when compared to patients without prior history of COVID-19. The magnitude of the risk was greater for patients with severe disease, with the highest odds for an adverse event occurring if surgery was performed within 4 weeks of SARS-CoV-2 infection (aOR 10.3 [7.92–13.4]). This risk decreased with time, although it remained elevated when compared to patients who did not have prior COVID-19 (aOR 2.24 [1.67–3.00] at 12+ weeks).
Figure 4.
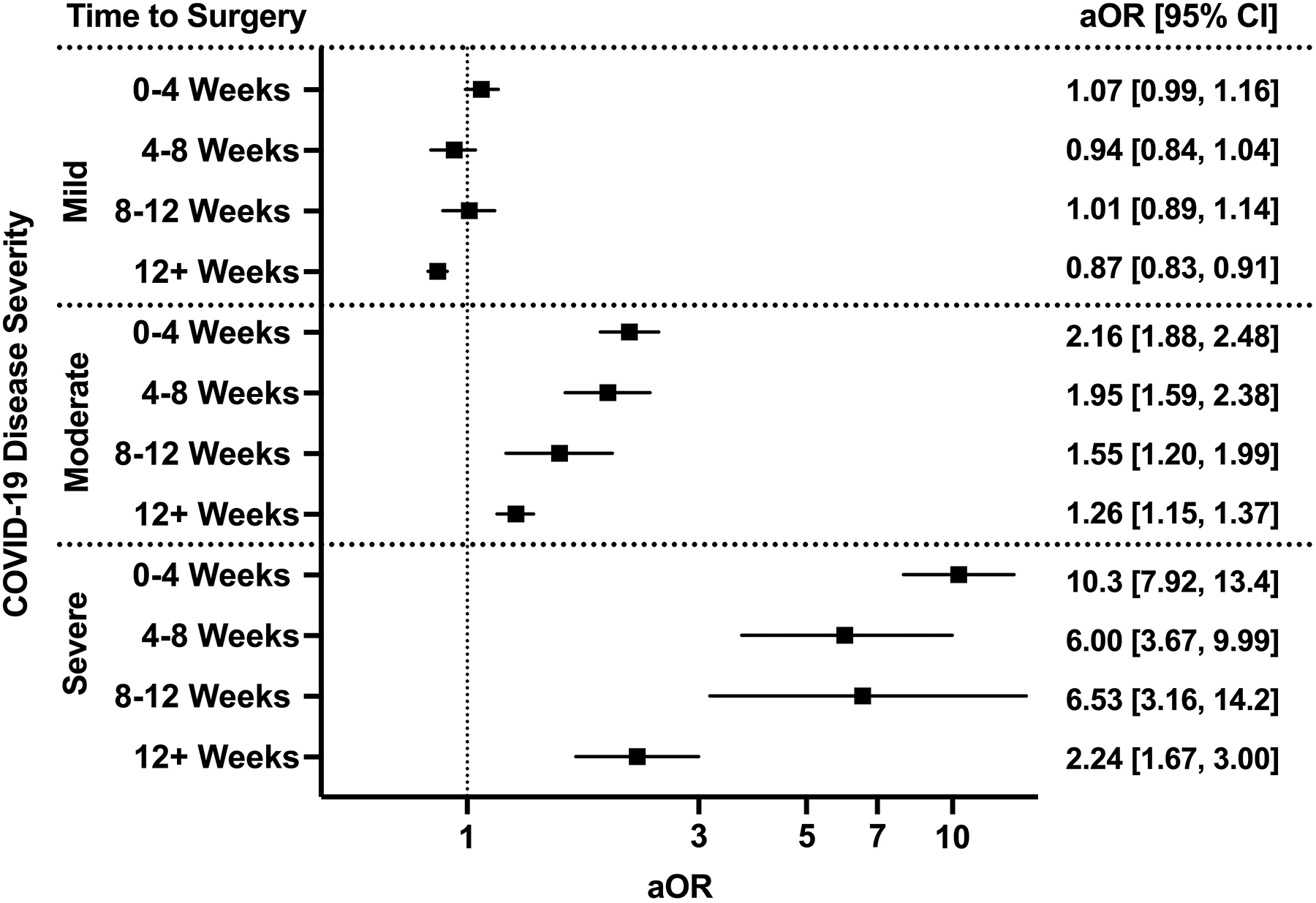
The interplay between timing of surgery and COVID-19 disease severity in influencing odds of composite 30-day major morbidity or mortality. All models adjusted for age, sex, race and ethnicity, smoking status, Charlson Comorbidity Index, relative risk of surgery, and pandemic era. aOR: adjusted odds ratio (with 95% confidence interval).
Preoperative vaccination and 30-day postoperative adverse outcomes
In patients with COVID-19 before surgery, vaccination significantly decreased the rates of composite major morbidity or mortality (14% vs. 16%; P=0.005), pneumonia (2.3% vs. 3.0%; P=0.003), respiratory failure (4.2% vs. 5.6%; P<0.001), and sepsis (2.7% vs. 3.3%; P<0.05) (Supplemental Table 9). After adjusting for patient demographics, comorbidities, relative risk of surgery, and pandemic era, vaccination was found to only be associated with decreased odds of respiratory failure (aOR 0.77 [0.66–0.90]) (Figure 5).
Figure 5.
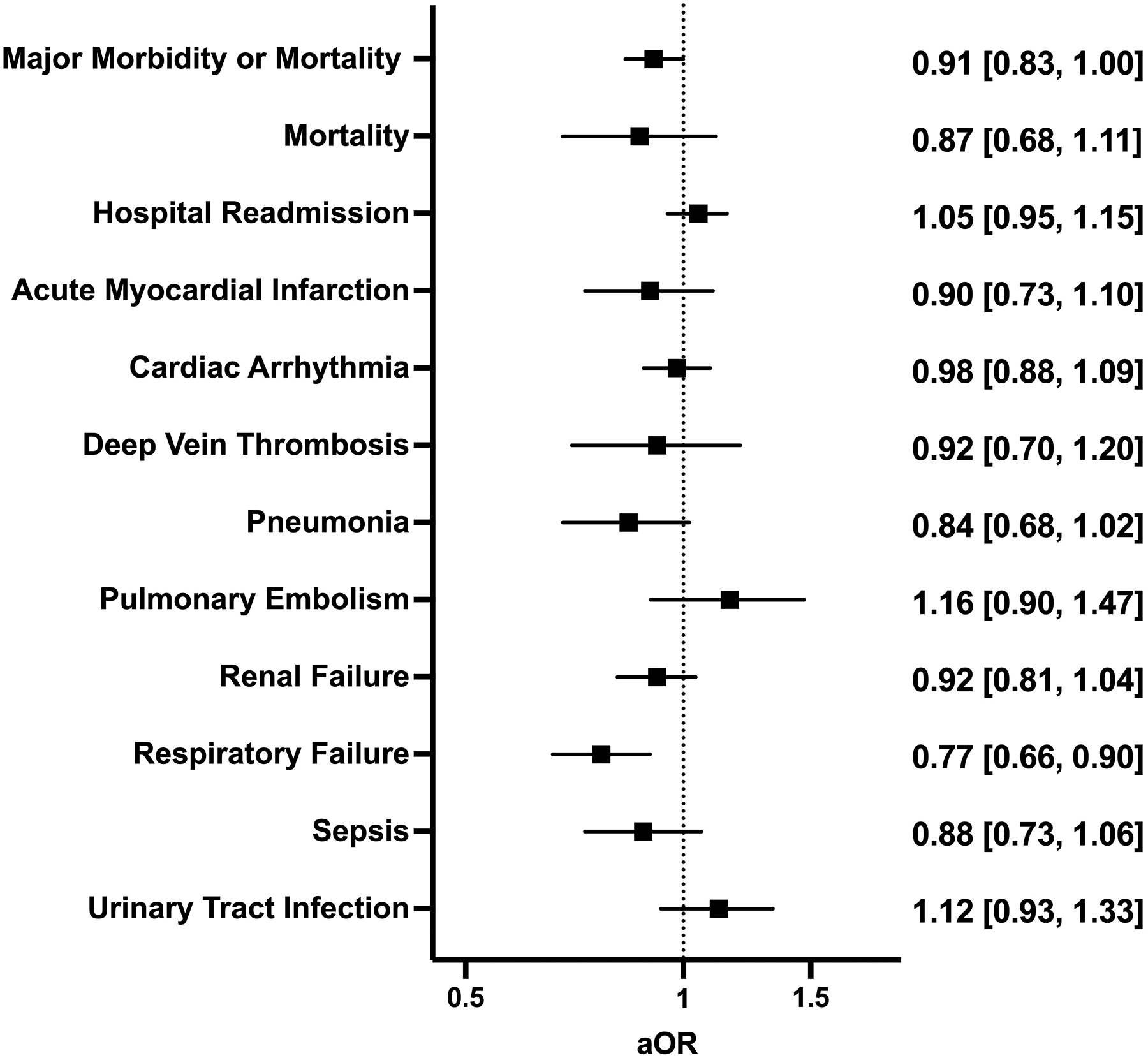
Multivariable regression assessing the association between full vaccination in patients with a history of COVID-19 and 30-day postoperative adverse events. All models adjusted for age, sex, race and ethnicity, smoking status, Charlson Comorbidity Index, relative risk of surgery, and pandemic era. aOR: adjusted odds ratio (with 95% confidence interval).
DISCUSSION
In this study, we aimed to understand how the severity of a SARS-CoV-2 infection prior to major elective inpatient surgery influences postoperative outcomes using the N3C Data Enclave, a harmonized electronic health data platform comprising over 70 institutions and more than 18,000,000 patients21. Our findings demonstrate that a prior history of COVID-19 is an independent risk factor for adverse surgical outcomes, and the risk remains elevated for 12 weeks after SARS-CoV-2 infection; however, this is dependent on the severity of COVID-19. Patients with mild disease (not requiring hospitalization) did not have an elevated risk of adverse outcomes regardless of the timing of surgery. Those with moderate and severe disease had persistently elevated surgical risk that lasted 12+ weeks after an acute infection.
The results of this study are consistent with those of previous studies examining surgical outcomes in patients with a history of SARS-CoV-2 infection conducted early during the pandemic. The COVIDSurg Collaborative, a multinational collaborative that aimed to explore the impact of COVID-19 in surgical patients and services, published the first population-level study assessing surgical outcomes following a SARS-CoV-2 infection. They found an increased risk of surgical mortality that lasted for 7 weeks following an acute infection7. Another large-scale study by Deng et al. confirmed these findings in a United States population and advocated for a wait time of 8 weeks from the time of SARS-CoV-2 infection before proceeding with elective surgery5. In response to these data (and other similar findings from a single institution series)22, expert guidelines suggested delaying elective surgery by 7 weeks while weighing the time sensitivity of the intervention23. These studies and guidelines were crucial given the novel situation that perioperative providers faced early in the COVID-19 pandemic, as an increasing number of patients with prior SARS-CoV-2 infection required elective surgery.
Nevertheless, several gaps remain in the evidence base for guiding the management of this patient population, which are now addressed by this study. We re-demonstrate that the risk for adverse 30-day composite postoperative outcomes remains elevated for 8 weeks after SARS-CoV-2 infection; however, when stratified by severity, this risk is only present in those recovered from moderate and severe infections (anyone who required hospitalization). Those with mild disease were not at an increased risk of adverse postoperative outcomes even when undergoing surgery within 4 weeks of an acute infection, which is consistent with a previous work15. We also provide a more detailed measurement of the association across a spectrum of specific postoperative complications. For example, patients with mild disease had no increased odds for all assessed adverse events, moderate disease showed an elevated risk for all assessed complications, while those with severe disease had increased odds of all assessed complications with a magnitude of effect multiple fold greater. This study also provides evidence that vaccination can reduce the risk of adverse postoperative events in patients with a history of COVID-19. This is in line with numerous other studies supporting the prioritization of preoperative vaccination24–27.
The data presented in this study included patients who underwent surgery prior to March 2023, and thus reflect the environment as the pandemic shifts to an endemic phase. For comparison, the data collected for the COVIDSurg study was in October of 2020, and the Deng et al. study of patients in the United States included surgeries performed until June of 2021. Equipped with the updated data presented in this study, we propose the following guidance for managing patients prior to elective inpatient surgery. First, all patients who are planned to undergo surgery should be assessed for their history of COVID-19, including detailed information regarding the severity of their infection. Those with a prior history should be classified as having either asymptomatic/mild disease or moderate/severe disease. After this determination is made, patients with asymptomatic/mild disease should have their surgery delayed at least 5 days from the time of the positive test to ensure they do not develop progressive symptoms that increase the severity of illness. If they remain asymptomatic/mild after this wait time, our data support the safety of proceeding with elective surgery. However, if the patient has moderate/severe disease or develops moderate/severe disease, consideration should be given to delaying surgery, while also weighing the risk/benefit ratio of potential harms related to a prolonged delay in circumstances of time-sensitive surgical indications. Since we did not observe a return to baseline risk even beyond 12 weeks in patients with moderate/severe disease, no standard time for delay can be recommended. Instead, we advocate for early engagement with perioperative medicine teams and/or dedicated long COVID clinics to identify strategies for optimal risk reduction and provide objective assessment of physiological recovery prior to proceeding with surgery. At our institution, all patients with a history of moderate/severe disease are recommended to be referred to a preoperative clinic for assessment prior to the procedure to assess recovery and help collaboratively determine the optimal timing for surgery. Developing and applying machine learning/artificial intelligence tools using the N3C Data Enclave is an active area of work in our group to help provide more specific guidance for patients with a history of moderate or severe SARS-CoV-2 infection.
Our study has several limitations that must be carefully considered when interpreting the results. The use of electronic health record data within the N3C Data Enclave may introduce biases that are well described in prior studies28. Methods specific to N3C can help manage several of these through rigorous data standardization, a highly engaged user community, and a centralized platform for handling all data-related tasks including extraction, processing, and analyses. Another source of bias is the selection of patients who underwent surgery after recovery from a prior COVID-19 infection. Detailed information on the preoperative evaluation, indications, and rationale for proceeding with surgery prior to 7 weeks in patients with prior COVID-19 is not available. Additionally, we were unable to directly measure the association between SARS-CoV-2 variants and outcomes; however, this is likely to be indirectly accounted for in analyses that included severity. Despite these potential shortcomings, leveraging the largest COVID-19-specific national dataset provides a unique opportunity to help guide the management of patients with previous SARS-CoV-2 infections undergoing elective surgery.
In conclusion, prior COVID-19 infection is a risk factor for adverse postoperative outcomes following elective major inpatient surgery. The magnitude and duration of this risk are dependent on the severity of the COVID-19 infection and are reduced in vaccinated patients. As the pandemic transitions to an endemic phase, the results of this study emphasize that a one-size-fits-all approach to risk stratification in this patient population can lead to unnecessary surgical delays. Consensus guidelines should be updated to include consideration of COVID-19 disease severity and vaccination status.
Supplementary Material
ACKNOWLEDGEMENTS
The analyses described in this publication were conducted under the DUR RP-A39709 and IRB #PRO00042159 with data or tools accessed through the NCATS N3C Data Enclave covid.cd2h.org/enclave and supported by CD2H - The National COVID Cohort Collaborative (N3C) IDeA CTR Collaboration 3U24TR002306-04S2 NCATS U24 TR002306. The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, Award Number UL1 TR001436 and 3UL1TR001436-08S3. Additional funding support provided by Advancing a Healthier Wisconsin Endowment. This research was possible because of the patients whose information is included within the data from participating organizations (covid.cd2h.org/dtas) and the organizations and scientists (covid.cd2h.org/duas) who have contributed to the on-going development of this community resource (https://doi.org/10.1093/jamia/ocaa196).
We gratefully acknowledge the following core contributors to N3C:
Adam B. Wilcox, Adam M. Lee, Alexis Graves, Alfred (Jerrod) Anzalone, Amin Manna, Amit Saha, Amy Olex, Andrea Zhou, Andrew E. Williams, Andrew Southerland, Andrew T. Girvin, Anita Walden, Anjali A. Sharathkumar, Benjamin Amor, Benjamin Bates, Brian Hendricks, Brijesh Patel, Caleb Alexander, Carolyn Bramante, Cavin Ward-Caviness, Charisse Madlock-Brown, Christine Suver, Christopher Chute, Christopher Dillon, Chunlei Wu, Clare Schmitt, Cliff Takemoto, Dan Housman, Davera Gabriel, David A. Eichmann, Diego Mazzotti, Don Brown, Eilis Boudreau, Elaine Hill, Elizabeth Zampino, Emily Carlson Marti, Emily R. Pfaff, Evan French, Farrukh M Koraishy, Federico Mariona, Fred Prior, George Sokos, Greg Martin, Harold Lehmann, Heidi Spratt, Hemalkumar Mehta, Hongfang Liu, Hythem Sidky, J.W. Awori Hayanga, Jami Pincavitch, Jaylyn Clark, Jeremy Richard Harper, Jessica Islam, Jin Ge, Joel Gagnier, Joel H. Saltz, Joel Saltz, Johanna Loomba, John Buse, Jomol Mathew, Joni L. Rutter, Julie A. McMurry, Justin Guinney, Justin Starren, Karen Crowley, Katie Rebecca Bradwell, Kellie M. Walters, Ken Wilkins, Kenneth R. Gersing, Kenrick Dwain Cato, Kimberly Murray, Kristin Kostka, Lavance Northington, Lee Allan Pyles, Leonie Misquitta, Lesley Cottrell, Lili Portilla, Mariam Deacy, Mark M. Bissell, Marshall Clark, Mary Emmett, Mary Morrison Saltz, Matvey B. Palchuk, Melissa A. Haendel, Meredith Adams, Meredith Temple-O’Connor, Michael G. Kurilla, Michele Morris, Nabeel Qureshi, Nasia Safdar, Nicole Garbarini, Noha Sharafeldin, Ofer Sadan, Patricia A. Francis, Penny Wung Burgoon, Peter Robinson, Philip R.O. Payne, Rafael Fuentes, Randeep Jawa, Rebecca Erwin-Cohen, Rena Patel, Richard A. Moffitt, Richard L. Zhu, Rishi Kamaleswaran, Robert Hurley, Robert T. Miller, Saiju Pyarajan, Sam G. Michael, Samuel Bozzette, Sandeep Mallipattu, Satyanarayana Vedula, Scott Chapman, Shawn T. O’Neil, Soko Setoguchi, Stephanie S. Hong, Steve Johnson, Tellen D. Bennett, Tiffany Callahan, Umit Topaloglu, Usman Sheikh, Valery Gordon, Vignesh Subbian, Warren A. Kibbe, Wenndy Hernandez, Will Beasley, Will Cooper, William Hillegass, Xiaohan Tanner Zhang. Details of contributions available at covid.cd2h.org/core-contributors
The following institutions whose data is released or pending:
Available: Advocate Health Care Network — UL1TR002389: The Institute for Translational Medicine (ITM) • Boston University Medical Campus — UL1TR001430: Boston University Clinical and Translational Science Institute • Brown University — U54GM115677: Advance Clinical Translational Research (Advance-CTR) • Carilion Clinic — UL1TR003015: iTHRIV Integrated Translational health Research Institute of Virginia • Charleston Area Medical Center — U54GM104942: West Virginia Clinical and Translational Science Institute (WVCTSI) • Children’s Hospital Colorado — UL1TR002535: Colorado Clinical and Translational Sciences Institute • Columbia University Irving Medical Center — UL1TR001873: Irving Institute for Clinical and Translational Research • Duke University — UL1TR002553: Duke Clinical and Translational Science Institute • George Washington Children’s Research Institute — UL1TR001876: Clinical and Translational Science Institute at Children’s National (CTSA-CN) • George Washington University — UL1TR001876: Clinical and Translational Science Institute at Children’s National (CTSA-CN) • Indiana University School of Medicine — UL1TR002529: Indiana Clinical and Translational Science Institute • Johns Hopkins University — UL1TR003098: Johns Hopkins Institute for Clinical and Translational Research • Loyola Medicine — Loyola University Medical Center • Loyola University Medical Center — UL1TR002389: The Institute for Translational Medicine (ITM) • Maine Medical Center — U54GM115516: Northern New England Clinical & Translational Research (NNE-CTR) Network • Massachusetts General Brigham — UL1TR002541: Harvard Catalyst • Mayo Clinic Rochester — UL1TR002377: Mayo Clinic Center for Clinical and Translational Science (CCaTS) • Medical University of South Carolina — UL1TR001450: South Carolina Clinical & Translational Research Institute (SCTR) • Montefiore Medical Center — UL1TR002556: Institute for Clinical and Translational Research at Einstein and Montefiore • Nemours — U54GM104941: Delaware CTR ACCEL Program • NorthShore University HealthSystem — UL1TR002389: The Institute for Translational Medicine (ITM) • Northwestern University at Chicago — UL1TR001422: Northwestern University Clinical and Translational Science Institute (NUCATS) • OCHIN — INV-018455: Bill and Melinda Gates Foundation grant to Sage Bionetworks • Oregon Health & Science University — UL1TR002369: Oregon Clinical and Translational Research Institute • Penn State Health Milton S. Hershey Medical Center — UL1TR002014: Penn State Clinical and Translational Science Institute • Rush University Medical Center — UL1TR002389: The Institute for Translational Medicine (ITM) • Rutgers, The State University of New Jersey — UL1TR003017: New Jersey Alliance for Clinical and Translational Science • Stony Brook University — U24TR002306 • The Ohio State University — UL1TR002733: Center for Clinical and Translational Science • The State University of New York at Buffalo — UL1TR001412: Clinical and Translational Science Institute • The University of Chicago — UL1TR002389: The Institute for Translational Medicine (ITM) • The University of Iowa — UL1TR002537: Institute for Clinical and Translational Science • The University of Miami Leonard M. Miller School of Medicine — UL1TR002736: University of Miami Clinical and Translational Science Institute • The University of Michigan at Ann Arbor — UL1TR002240: Michigan Institute for Clinical and Health Research • The University of Texas Health Science Center at Houston — UL1TR003167: Center for Clinical and Translational Sciences (CCTS) • The University of Texas Medical Branch at Galveston — UL1TR001439: The Institute for Translational Sciences • The University of Utah — UL1TR002538: Uhealth Center for Clinical and Translational Science • Tufts Medical Center — UL1TR002544: Tufts Clinical and Translational Science Institute • Tulane University — UL1TR003096: Center for Clinical and Translational Science • University Medical Center New Orleans — U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center • University of Alabama at Birmingham — UL1TR003096: Center for Clinical and Translational Science • University of Arkansas for Medical Sciences — UL1TR003107: UAMS Translational Research Institute • University of Cincinnati — UL1TR001425: Center for Clinical and Translational Science and Training • University of Colorado Denver, Anschutz Medical Campus — UL1TR002535: Colorado Clinical and Translational Sciences Institute • University of Illinois at Chicago — UL1TR002003: UIC Center for Clinical and Translational Science • University of Kansas Medical Center — UL1TR002366: Frontiers: University of Kansas Clinical and Translational Science Institute • University of Kentucky — UL1TR001998: UK Center for Clinical and Translational Science • University of Massachusetts Medical School Worcester — UL1TR001453: The UMass Center for Clinical and Translational Science (UMCCTS) • University of Minnesota — UL1TR002494: Clinical and Translational Science Institute • University of Mississippi Medical Center — U54GM115428: Mississippi Center for Clinical and Translational Research (CCTR) • University of Nebraska Medical Center — U54GM115458: Great Plains IDeA-Clinical & Translational Research • University of North Carolina at Chapel Hill — UL1TR002489: North Carolina Translational and Clinical Science Institute • University of Oklahoma Health Sciences Center — U54GM104938: Oklahoma Clinical and Translational Science Institute (OCTSI) • University of Rochester — UL1TR002001: UR Clinical & Translational Science Institute • University of Southern California — UL1TR001855: The Southern California Clinical and Translational Science Institute (SC CTSI) • University of Vermont — U54GM115516: Northern New England Clinical & Translational Research (NNE-CTR) Network • University of Virginia — UL1TR003015: iTHRIV Integrated Translational health Research Institute of Virginia • University of Washington — UL1TR002319: Institute of Translational Health Sciences • University of Wisconsin-Madison — UL1TR002373: UW Institute for Clinical and Translational Research • Vanderbilt University Medical Center — UL1TR002243: Vanderbilt Institute for Clinical and Translational Research • Virginia Commonwealth University — UL1TR002649: C. Kenneth and Dianne Wright Center for Clinical and Translational Research • Wake Forest University Health Sciences — UL1TR001420: Wake Forest Clinical and Translational Science Institute • Washington University in St. Louis — UL1TR002345: Institute of Clinical and Translational Sciences • Weill Medical College of Cornell University — UL1TR002384: Weill Cornell Medicine Clinical and Translational Science Center • West Virginia University — U54GM104942: West Virginia Clinical and Translational Science Institute (WVCTSI)
Submitted: Icahn School of Medicine at Mount Sinai — UL1TR001433: ConduITS Institute for Translational Sciences • The University of Texas Health Science Center at Tyler — UL1TR003167: Center for Clinical and Translational Sciences (CCTS) • University of California, Davis — UL1TR001860: UCDavis Health Clinical and Translational Science Center • University of California, Irvine — UL1TR001414: The UC Irvine Institute for Clinical and Translational Science (ICTS) • University of California, Los Angeles — UL1TR001881: UCLA Clinical Translational Science Institute • University of California, San Diego — UL1TR001442: Altman Clinical and Translational Research Institute • University of California, San Francisco — UL1TR001872: UCSF Clinical and Translational Science Institute
Pending: Arkansas Children’s Hospital — UL1TR003107: UAMS Translational Research Institute • Baylor College of Medicine — None (Voluntary) • Children’s Hospital of Philadelphia — UL1TR001878: Institute for Translational Medicine and Therapeutics • Cincinnati Children’s Hospital Medical Center — UL1TR001425: Center for Clinical and Translational Science and Training • Emory University — UL1TR002378: Georgia Clinical and Translational Science Alliance • HonorHealth — None (Voluntary) • Loyola University Chicago — UL1TR002389: The Institute for Translational Medicine (ITM) • Medical College of Wisconsin — UL1TR001436: Clinical and Translational Science Institute of Southeast Wisconsin • MedStar Health Research Institute — UL1TR001409: The Georgetown-Howard Universities Center for Clinical and Translational Science (GHUCCTS) • MetroHealth — None (Voluntary) • Montana State University — U54GM115371: American Indian/Alaska Native CTR • NYU Langone Medical Center — UL1TR001445: Langone Health’s Clinical and Translational Science Institute • Ochsner Medical Center — U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center • Regenstrief Institute — UL1TR002529: Indiana Clinical and Translational Science Institute • Sanford Research — None (Voluntary) • Stanford University — UL1TR003142: Spectrum: The Stanford Center for Clinical and Translational Research and Education • The Rockefeller University — UL1TR001866: Center for Clinical and Translational Science • The Scripps Research Institute — UL1TR002550: Scripps Research Translational Institute • University of Florida — UL1TR001427: UF Clinical and Translational Science Institute • University of New Mexico Health Sciences Center — UL1TR001449: University of New Mexico Clinical and Translational Science Center • University of Texas Health Science Center at San Antonio — UL1TR002645: Institute for Integration of Medicine and Science • Yale New Haven Hospital — UL1TR001863: Yale Center for Clinical Investigation
Funding:
National Center for Advancing Translational Sciences, National Institutes of Health, Award Number UL1 TR001436 and 3UL1TR001436-08S3. Additional funding support provided by Advancing a Healthier Wisconsin Endowment.
Footnotes
Conflicts of Interest: The authors declare that they have no affiliations with or involvement in any organization or entity with any financial interest in the subject matter or materials discussed in this manuscript.
REFERENCES
- 1.Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/us-map
- 2.El-Boghdadly K, Cook TM, Goodacre T, et al. SARS-CoV-2 infection, COVID-19 and timing of elective surgery: A multidisciplinary consensus statement on behalf of the Association of Anaesthetists, the Centre for Peri-operative Care, the Federation of Surgical Specialty Associations, the Royal College of Anaesthetists and the Royal College of Surgeons of England. Anaesthesia. Jul 2021;76(7):940–946. doi: 10.1111/anae.15464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collaborative CO. Outcomes and Their State-level Variation in Patients Undergoing Surgery With Perioperative SARS-CoV-2 Infection in the USA: A Prospective Multicenter Study. Ann Surg. Feb 1 2022;275(2):247–251. doi: 10.1097/SLA.0000000000005310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collaborative CO. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. Jul 4 2020;396(10243):27–38. doi: 10.1016/S0140-6736(20)31182-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng JZ, Chan JS, Potter AL, et al. The Risk of Postoperative Complications After Major Elective Surgery in Active or Resolved COVID-19 in the United States. Ann Surg. Feb 1 2022;275(2):242–246. doi: 10.1097/SLA.0000000000005308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verhagen NB, Koerber NK, Szabo A, et al. Vaccination Against SARS-CoV-2 Decreases Risk of Adverse Events in Patients who Develop COVID-19 Following Cancer Surgery. Ann Surg Oncol. Dec 7 2022:1–4. doi: 10.1245/s10434-022-12916-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Argandykov D, Dorken-Gallastegi A, El Moheb M, et al. Is perioperative COVID-19 really associated with worse surgical outcomes? A nationwide COVIDSurg propensity-matched analysis. J Trauma Acute Care Surg. Apr 1 2023;94(4):513–524. doi: 10.1097/TA.0000000000003859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collaborative CO, GlobalSurg C. Timing of surgery following SARS-CoV-2 infection: an international prospective cohort study. Anaesthesia. Jun 2021;76(6):748–758. doi: 10.1111/anae.15458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. Nov 4 2020;371:m4087. doi: 10.1136/bmj.m4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumont F, Kepenekian V, De Franco V, et al. Delaying Surgery After Neoadjuvant Chemotherapy Affects Survival in Patients with Colorectal Peritoneal Metastases: A BIG-RENAPE Network Multicentric Study. Ann Surg Oncol. Mar 13 2023:1–11. doi: 10.1245/s10434-023-13224-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reijman M, Eggerding V, van Es E, et al. Early surgical reconstruction versus rehabilitation with elective delayed reconstruction for patients with anterior cruciate ligament rupture: COMPARE randomised controlled trial. BMJ. Mar 9 2021;372:n375. doi: 10.1136/bmj.n375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunn KL, Lam KC, Valovich McLeod TC. Early Operative Versus Delayed or Nonoperative Treatment of Anterior Cruciate Ligament Injuries in Pediatric Patients. J Athl Train. May 2016;51(5):425–7. doi: 10.4085/1062-6050.51.5.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.investigators O, Chikuda H, Koyama Y, et al. Effect of Early vs Delayed Surgical Treatment on Motor Recovery in Incomplete Cervical Spinal Cord Injury With Preexisting Cervical Stenosis: A Randomized Clinical Trial. JAMA Netw Open. Nov 1 2021;4(11):e2133604. doi: 10.1001/jamanetworkopen.2021.33604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hah JM, Lee E, Shrestha R, et al. Return to work and productivity loss after surgery: A health economic evaluation. Int J Surg. Nov 2021;95:106100. doi: 10.1016/j.ijsu.2021.106100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SenthilKumar G, Verhagen NB, Sheriff SA, et al. Preoperative SARS-CoV-2 Infection Increases Risk of Early Postoperative Cardiovascular Complications Following Non-Cardiac Surgery. American Journal of Physiology - Heart and Circulatory Physiology. 2023;Accepted. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al Ani A, Tahtamoni R, Mohammad Y, Al-Ayoubi F, Haider N, Al-Mashhadi A. Impacts of severity of Covid-19 infection on the morbidity and mortality of surgical patients. Ann Med Surg (Lond). Jul 2022;79:103910. doi: 10.1016/j.amsu.2022.103910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tenforde MW, Self WH, Adams K, et al. Association Between mRNA Vaccination and COVID-19 Hospitalization and Disease Severity. JAMA. Nov 23 2021;326(20):2043–2054. doi: 10.1001/jama.2021.19499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambrou AS, Shirk P, Steele MK, et al. Genomic Surveillance for SARS-CoV-2 Variants: Predominance of the Delta (B.1.617.2) and Omicron (B.1.1.529) Variants - United States, June 2021-January 2022. MMWR Morb Mortal Wkly Rep. Feb 11 2022;71(6):206–211. doi: 10.15585/mmwr.mm7106a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Characterisation WHOWGotC, Management of C-i. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. Aug 2020;20(8):e192–e197. doi: 10.1016/S1473-3099(20)30483-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett TD, Moffitt RA, Hajagos JG, et al. Clinical Characterization and Prediction of Clinical Severity of SARS-CoV-2 Infection Among US Adults Using Data From the US National COVID Cohort Collaborative. JAMA Netw Open. Jul 1 2021;4(7):e2116901. doi: 10.1001/jamanetworkopen.2021.16901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haendel MA, Chute CG, Bennett TD, et al. The National COVID Cohort Collaborative (N3C): Rationale, design, infrastructure, and deployment. J Am Med Inform Assoc. Mar 1 2021;28(3):427–443. doi: 10.1093/jamia/ocaa196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butyrskii AG, Mikhaylichenko VY, Fomochkin II, et al. [How long must be an elective surgery delayed after SARS-COV-2 diagnosis? (Multiple-center regional research)]. Khirurgiia (Mosk). 2021;(8):5–10. Na skol’ko sleduet otlozhit’ operatsiyu posle vyyavleniya SARS-COV-2? (Opyt mnogotsentrovogo regional’nogo issledovaniya). doi: 10.17116/hirurgia20210815 [DOI] [PubMed] [Google Scholar]
- 23.ASA and APSF Joint Statement on Elective Surgery/Procedures and Anesthesia for Patients after COVID-19 Infection. American Society of Anesthesiologists; 2022. https://www.asahq.org/about-asa/newsroom/news-releases/2022/02/asa-and-apsf-joint-statement-on-elective-surgery-procedures-and-anesthesia-for-patients-after-covid-19-infection
- 24.Taghioff SM, Slavin BR, Narasimman M, et al. The influence of SARS-CoV-2 vaccination on post-operative outcomes in microsurgery patients. Microsurgery. Oct 2022;42(7):685–695. doi: 10.1002/micr.30940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Covidsurg Collaborative GC. SARS-CoV-2 vaccination modelling for safe surgery to save lives: data from an international prospective cohort study. Br J Surg. Sep 27 2021;108(9):1056–1063. doi: 10.1093/bjs/znab101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prasad NK, Lake R, Englum BR, et al. COVID-19 Vaccination Associated With Reduced Postoperative SARS-CoV-2 Infection and Morbidity. Ann Surg. Jan 1 2022;275(1):31–36. doi: 10.1097/SLA.0000000000005176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.SenthilKumar G, Verhagen NB, Sheriff SA, et al. Preoperative SARS-CoV-2 Infection Increases Risk of Early Postoperative Cardiovascular Complications Following Non-Cardiac Surgery. Am J Physiol Heart Circ Physiol. Mar 17 2023;doi: 10.1152/ajpheart.00097.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verheij RA, Curcin V, Delaney BC, McGilchrist MM. Possible Sources of Bias in Primary Care Electronic Health Record Data Use and Reuse. J Med Internet Res. May 29 2018;20(5):e185. doi: 10.2196/jmir.9134 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


