Abstract
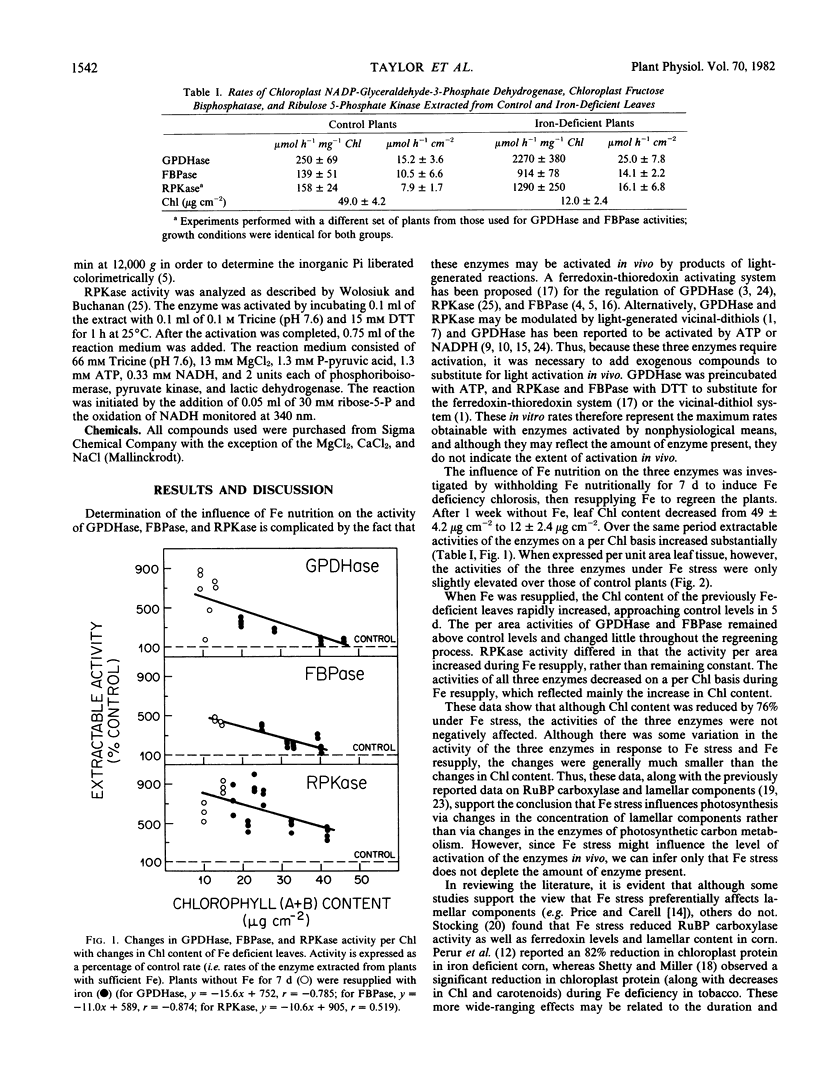
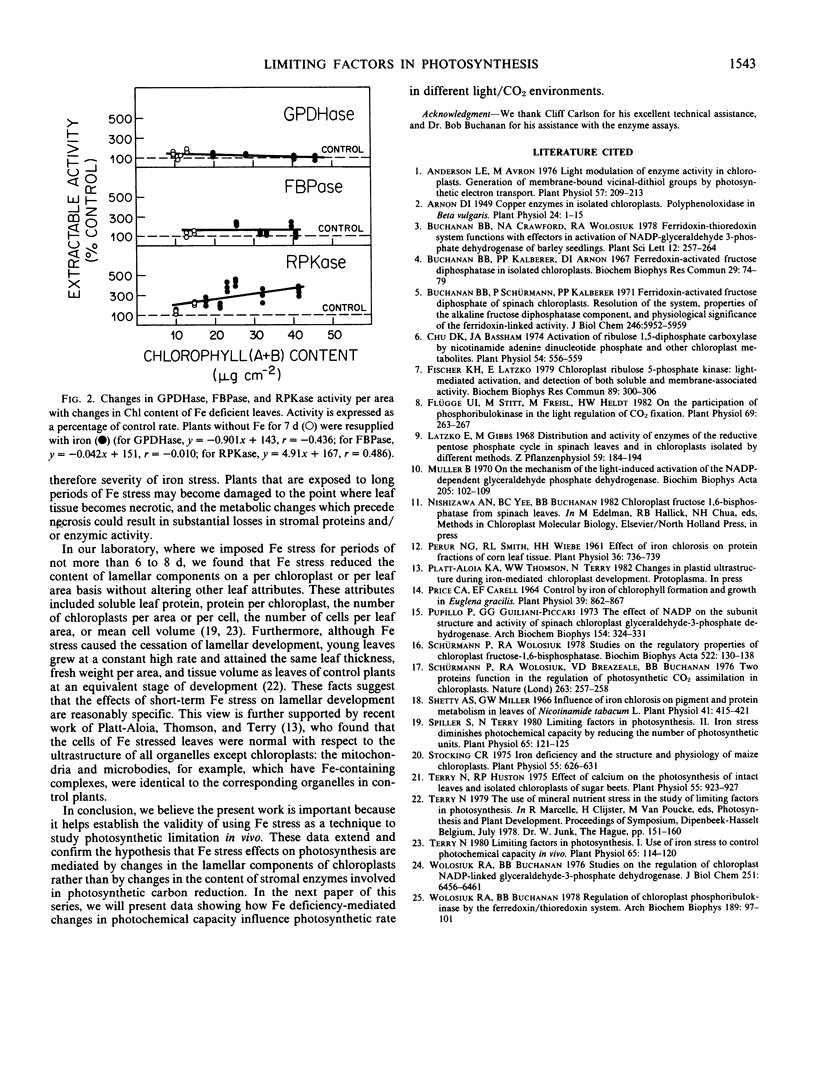
When Fe was withheld from sugar beets (Beta vulgaris L. CV F58-44H1), the activities of NADP-glyceraldehyde-3-phosphate dehydrogenase, fructose 1,6-bisphosphatase, and ribulose 5-phosphate kinase were not diminished, while chlorophyll per area was decreased by 75%. On resupplying Fe, chlorophyll per area increased to control levels within 5 days, whereas the activities of the three enzymes remained approximately constant. These results support the view advanced earlier (Terry 1980 Plant Physiol 65: 114-120) that the photosynthetic effects of Fe deprivation are mediated by changes in the lamellar components of chloroplasts and not by changes in stromal enzymes involved in photosynthetic carbon reduction.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., Avron M. Light Modulation of Enzyme Activity in Chloroplasts: Generation of Membrane-bound Vicinal-Dithiol Groups by Photosynthetic Electron Transport. Plant Physiol. 1976 Feb;57(2):209–213. doi: 10.1104/pp.57.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B. B., Kalberer P. P., Arnon D. I. Ferredoxin-activated fructose diphosphatase in isolated chloroplasts. Biochem Biophys Res Commun. 1967 Oct 11;29(1):74–79. doi: 10.1016/0006-291x(67)90543-8. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P., Kalberer P. P. Ferredoxin-activated fructose diphosphatase of spinach chloroplasts. Resolution of the system, properties of the alkaline fructose diphosphatase component, and physiological significance of the ferredoxin-linked activation. J Biol Chem. 1971 Oct 10;246(19):5952–5959. [PubMed] [Google Scholar]
- Chu D. K., Bassham J. A. Activation of ribulose 1,5-diphosphate carboxylase by nicotinamide adenine dinucleotide phosphate and other chloroplast metabolites. Plant Physiol. 1974 Oct;54(4):556–559. doi: 10.1104/pp.54.4.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K. H., Latzko E. Chloroplast ribulose-5-phosphate kinase: light-mediated activation, and detection of both soluble and membrane-associated activity. Biochem Biophys Res Commun. 1979 Jul 12;89(1):300–306. doi: 10.1016/0006-291x(79)90978-1. [DOI] [PubMed] [Google Scholar]
- Flügge U. I., Stitt M., Freisl M., Heldt H. W. On the Participation of Phosphoribulokinase in the Light Regulation of CO(2) Fixation. Plant Physiol. 1982 Jan;69(1):263–267. doi: 10.1104/pp.69.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller B. On the mechanism of the light-induced activation of the NADP-dependent glyceraldehyde phosphate dehydrogenase. Biochim Biophys Acta. 1970 Apr 7;205(1):102–109. doi: 10.1016/0005-2728(70)90066-6. [DOI] [PubMed] [Google Scholar]
- Perur N. G., Smith R. L., Wiebe H. H. Effect of iron chlorosis on protein fractions of corn leaf tissue. Plant Physiol. 1961 Nov;36(6):736–739. doi: 10.1104/pp.36.6.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C. A., Carell E. F. Control by Iron of Chlorophyll Formation and Growth in Euglena gracilis. Plant Physiol. 1964 Sep;39(5):862–868. doi: 10.1104/pp.39.5.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pupillo P., Piccari G. G. The effect of NADP on the subunit structure and activity of spinach chloroplast glyceraldehyde-3-phosphate dehydrogenase. Arch Biochem Biophys. 1973 Jan;154(1):324–331. doi: 10.1016/0003-9861(73)90064-7. [DOI] [PubMed] [Google Scholar]
- Schürmann P., Wolosiuk R. A. Studies on the regulatory properties of chloroplast fructose-1,6-bisphosphatase. Biochim Biophys Acta. 1978 Jan 12;522(1):130–138. doi: 10.1016/0005-2744(78)90329-7. [DOI] [PubMed] [Google Scholar]
- Shetty A. S., Miller G. W. Influence of Iron Chlorosis on Pigment and Protein Metabolism in Leaves of Nicotiana tabacum L. Plant Physiol. 1966 Mar;41(3):415–421. doi: 10.1104/pp.41.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller S., Terry N. Limiting Factors in Photosynthesis: II. IRON STRESS DIMINISHES PHOTOCHEMICAL CAPACITY BY REDUCING THE NUMBER OF PHOTOSYNTHETIC UNITS. Plant Physiol. 1980 Jan;65(1):121–125. doi: 10.1104/pp.65.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocking C. R. Iron deficiency and the structure and physiology of maize chloroplasts. Plant Physiol. 1975 Apr;55(4):626–631. doi: 10.1104/pp.55.4.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry N., Huston R. P. Effects of calcium on the photosynthesis of intact leaves and isolated chloroplasts of sugar beets. Plant Physiol. 1975 May;55(5):923–927. doi: 10.1104/pp.55.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry N. Limiting Factors in Photosynthesis: I. USE OF IRON STRESS TO CONTROL PHOTOCHEMICAL CAPACITY IN VIVO. Plant Physiol. 1980 Jan;65(1):114–120. doi: 10.1104/pp.65.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosiuk R. A., Buchanan B. B. Regulation of chloroplast phosphoribulokinase by the ferredoxin/thioredoxin system. Arch Biochem Biophys. 1978 Jul;189(1):97–101. doi: 10.1016/0003-9861(78)90119-4. [DOI] [PubMed] [Google Scholar]


