Abstract
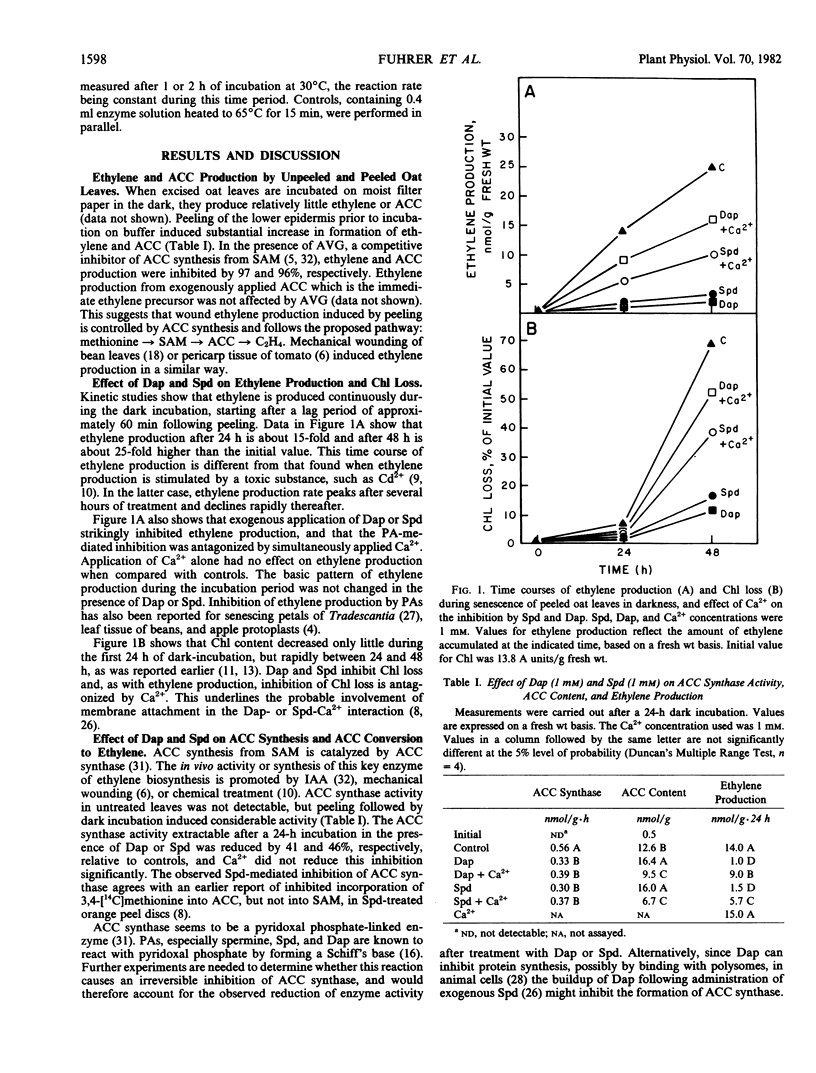
The effects of the polyamines spermidine and 1,3-diaminopropane on ethylene biosynthesis and chlorophyll (Chl) loss were studied in peeled leaves of oat (Avena sativa L., var. Victory) incubated in the dark. Peeling off the epidermal cells induces an increase in 1-aminocyclopropane-1-carboxylate (ACC) synthase activity, resulting in an enhanced ACC and ethylene formation. Both polyamines inhibit ethylene biosynthesis from methionine by inhibiting ACC synthase activity and, more effectively, the conversion of ACC to ethylene. They also inhibit Chl loss occurring between 24 and 48 h of dark incubation; but, as shown by inhibitor experiments, inhibition of Chl loss does not result from inhibition of ethylene formation. Ethylene production and Chl loss, both associated with senescence, require membrane integrity; thus, treatments which promote deterioration of membranes inhibit both processes. Ca2+ in the incubation medium competitively reduces the polyamine-mediated inhibition of ACC conversion and Chl loss. The data suggest that polyamines initially attach to membranes, thereby inducing changes which, in turn, lead to inhibition of ethylene biosynthesis and retardation of senescence.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Yang S. F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman A., Kaur-Sawhney R., Galston A. W. Stabilization of Oat Leaf Protoplasts through Polyamine-mediated Inhibition of Senescence. Plant Physiol. 1977 Oct;60(4):570–574. doi: 10.1104/pp.60.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelbaum A., Burgoon A. C., Anderson J. D., Lieberman M. Polyamines inhibit biosynthesis of ethylene in higher plant tissue and fruit protoplasts. Plant Physiol. 1981 Aug;68(2):453–456. doi: 10.1104/pp.68.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Even-Chen Z., Mattoo A. K., Goren R. Inhibition of ethylene biosynthesis by aminoethoxyvinylglycine and by polyamines shunts label from 3,4-[C]methionine into spermidine in aged orange peel discs. Plant Physiol. 1982 Feb;69(2):385–388. doi: 10.1104/pp.69.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepstein S., Thimann K. V. The role of ethylene in the senescence of oat leaves. Plant Physiol. 1981 Aug;68(2):349–354. doi: 10.1104/pp.68.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath R. L., Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968 Apr;125(1):189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Papahadjopoulos D. Phase transitions and phase separations in phospholipid membranes induced by changes in temperature, pH, and concentration of bivalent cations. Biochemistry. 1975 Jan 14;14(1):152–161. doi: 10.1021/bi00672a026. [DOI] [PubMed] [Google Scholar]
- Johnson W. T., Nordlie R. C. Stimulation of glucose-6-phosphatase by polyamines is a membrane-mediated event. Life Sci. 1980 Jan 28;26(4):297–302. doi: 10.1016/0024-3205(80)90341-0. [DOI] [PubMed] [Google Scholar]
- Lizada M. C., Yang S. F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979 Nov 15;100(1):140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Popovic R. B., Kyle D. J., Cohen A. S., Zalik S. Stabilization of Thylakoid Membranes by Spermine during Stress-induced Senescence of Barley Leaf Discs. Plant Physiol. 1979 Nov;64(5):721–726. doi: 10.1104/pp.64.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih L. M., Kaur-Sawhney R., Fuhrer J., Samanta S., Galston A. W. Effects of exogenous 1,3-diaminopropane and spermidine on senescence of oat leaves : I. Inhibition of protease activity, ethylene production, and chlorophyll loss as related to polyamine content. Plant Physiol. 1982 Dec;70(6):1592–1596. doi: 10.1104/pp.70.6.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomi K., Raina A., Mäntyjärvi R. 1,3-Diaminopropane rapidly inhibits protein synthesis and virus production in BKT-1 cells. FEBS Lett. 1980 Mar 10;111(2):329–332. doi: 10.1016/0014-5793(80)80820-9. [DOI] [PubMed] [Google Scholar]
- Wolff J., Cook G. H. Charge effects in the activation of adenylate cyclase. J Biol Chem. 1975 Sep 10;250(17):6897–6903. [PubMed] [Google Scholar]
- Yu Y. B., Adams D. O., Yang S. F. 1-Aminocyclopropanecarboxylate synthase, a key enzyme in ethylene biosynthesis. Arch Biochem Biophys. 1979 Nov;198(1):280–286. doi: 10.1016/0003-9861(79)90420-x. [DOI] [PubMed] [Google Scholar]
- Yu Y. B., Yang S. F. Auxin-induced Ethylene Production and Its Inhibition by Aminoethyoxyvinylglycine and Cobalt Ion. Plant Physiol. 1979 Dec;64(6):1074–1077. doi: 10.1104/pp.64.6.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]


