Abstract
Background:
Invasive mold infections (IMIs) such as aspergillosis, mucormycosis, fusariosis, and lomentosporiosis are associated with high morbidity and mortality, particularly in immunocompromised patients, with mortality rates as high as 40% to 80%. Outcomes could be substantially improved with early initiation of appropriate antifungal therapy, yet early diagnosis remains difficult to establish and often requires multidisciplinary teams evaluating clinical and radiological findings plus supportive mycological findings. Universal digital high resolution melting analysis (U-dHRM) may enable rapid and robust diagnosis of IMI. This technology aims to accomplish timely pathogen detection at the single genome level by conducting broad-based amplification of microbial barcoding genes in a digital polymerase chain reaction (dPCR) format, followed by high-resolution melting of the DNA amplicons in each digital reaction to generate organism-specific melt curve signatures that are identified by machine learning.
Methods:
A universal fungal assay was developed for U-dHRM and used to generate a database of melt curve signatures for 19 clinically relevant fungal pathogens. A machine learning algorithm (ML) was trained to automatically classify these 19 fungal melt curves and detect novel melt curves. Performance was assessed on 73 clinical bronchoalveolar lavage (BAL) samples from patients suspected of IMI. Novel curves were identified by micropipetting U-dHRM reactions and Sanger sequencing amplicons.
Results:
U-dHRM achieved an average of 97% fungal organism identification accuracy and a turn-around-time of 4hrs. Pathogenic molds (Aspergillus, Mucorales, Lomentospora and Fusarium) were detected by U-dHRM in 73% of BALF samples suspected of IMI. Mixtures of pathogenic molds were detected in 19%. U-dHRM demonstrated good sensitivity for IMI, as defined by current diagnostic criteria, when clinical findings were also considered.
Conclusions:
U-dHRM showed promising performance as a separate or combination diagnostic approach to standard mycological tests. The speed of U-dHRM and its ability to simultaneously identify and quantify clinically relevant mold pathogens in polymicrobial samples as well as detect emerging opportunistic pathogens may provide information that could aid in treatment decisions and improve patient outcomes.
Keywords: IMI, dPCR, HRM, machine learning
INTRODUCTION
Invasive mold infections (IMI) cause millions of infections globally and account for an estimated 1.6 million deaths annually(Bongomin et al. 2017). Patients at risk from IMIs, including both severely immunocompromised and also more immunocompetent individuals(Jenks et al. 2023), are increasing. IMIs in more immunocompetent persons/those receiving systemic corticosteroids are characterized by early tissue invasive growth in the lungs with bloodstream invasion potentially occurring later although not universally, while early angioinvasive growth is more common in severely immunocompromised persons (Jenks and Hoenigl 2018). Histopathologic examination and culture of tissue or bronchoalveolar lavage fluid (BALF) is considered the reference standard for IMI diagnosis, but is slow, with histopathology often available only at autopsy, while culture has poor sensitivity (Antinori, Corbellino, and Parravicini 2018). Incubation of fungal cultures for four weeks is considered best practice to maximize the recovery of slow growing species, with most detected by day 14 (Zhu and Qi, n.d.). BAL antigen tests, such as galactomannan (GM), can be helpful but are only positive for a limited number of specific mold organisms, and are further limited by variable turnaround times and lower sensitivity for individuals on mold-active antifungal prophylaxis or treatment (Jenks et al. 2023). The absence of rapid and accessible fungal diagnostics often results in empiric utilization of systemic antifungals, mostly targeted against Aspergillus spp., some of which are lacking activity against other molds (Denning et al. 2017). As a result, IMIs are often diagnosed and treated too late, leading to high mortality rates of 40-80%. It is estimated that 80% of patients could be saved with rapid diagnostics to inform early and targeted treatment (“Invasive Fungal Infections: A Creeping Public Health Threat” 2018).
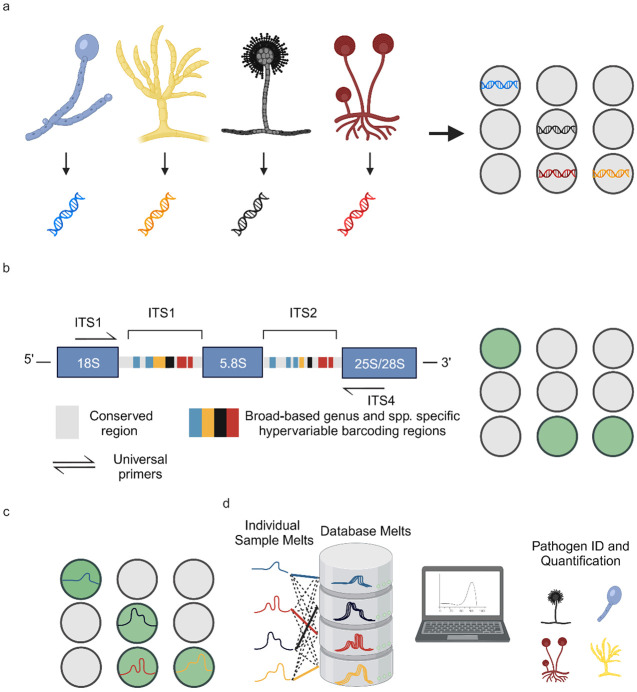
Universal digital High-Resolution Melt (U-dHRM) to detect mold pathogens in BALF may be a promising hypothesis free, unbiased diagnostic approach that could achieve rapid and near point-of-care diagnosis to inform treatment decisions and improve patient outcomes. This approach consists of a single closed-tube test that integrates universal amplification of pathogen barcoding sequences in a digital polymerase chain reaction (dPCR) format with high resolution melting of DNA and machine learning (Fig. 1) (Langouche et al. 2021; Aralar et al. 2020; Sinha, Mack, et al. 2018; Velez et al. 2017). The integration and advancement of these techniques promises a unique combination of advantages: speed and breadth of detection, sensitivity and absolute quantification, and pathogen identification in polymicrobial samples(Sinha, Jupe, et al. 2018).
Figure 1. U-dHRM technology overview.
a) Extraction of genomic DNA and digital loading. b) Universal amplification of fungal ITS barcoding region leading to a fluorescence increase in each positive reaction well. c) Barcode sequence-defined melt curve signatures. d) Automatic identification of each known pathogen melt curve and detection of novel melt curves using machine learning. Created with BioRender.com
Here, we advanced the U-dHRM assay and database for detection of IMI pathogens, advanced the machine learning algorithm to recognize database organism curves and also flag novel organism melt curves, and developed a dPCR reaction recovery method to Sanger sequence novel melt curves and expand the pathogen panel. We applied these advancements to test 75 clinical BALF samples, assessing the utility of this approach for IMI diagnosis compared to gold standard tests.
METHODS
ITS-Asp PCR
ITS1 (5’- TCCGTAGGTGAACCTGCGG -3’) and ITS4 (5’- TCCTCCGCTTATTGATATGC -3’) universal primers multiplexed with Asp 1 (5’- CGGCCCTTAAATAGCCCGGTC -3’) and Asp 2 (5’- ACCCCCCTGAGCCAGTCCG -3’) were used to amplify the Internal Transcribed Spacer (ITS) universal region for all fungi, and an Aspergillus specific region of 18S gene. ITS-Asp PCR was amplified in triplicates using the following protocol: each 15 μl reaction mixture contained 0.1 μM of each primer (IDT, Coralville, IA), 0.2 mM deoxynucleoside triphosphate (dNTP) (Invitrogen, Carlsbad, CA), 1X Phusion GC PCR buffer (Thermo Scientific, Waltham, MA), 2.5X EvaGreen (Biotium, Fremont, CA), 0.02 U/μl Phusion polymerase (New England Biolabs, Ipswich, MA), ultrapure water (Quality Biological, Gaithersburg, MD), and 3 μl of genomic DNA. Thermocycling for qPCR/dPCR and subsequent melt analysis were performed on a Quantstudio 3D real time PCR system and a ProFlex 2 x Flat Block Thermal Cycler (Applied Biosystems, Waltham, MA) using the QuantStudio 3D Digital PCR Chip (Applied Biosystems, Foster City, CA). The cycling conditions were as follows: hold at 98°C for 30 s, followed by 75 cycles of 98°C for 10 s, 61°C for 30 s, and 72°C for 60 s. At the end of cycling, there was a final extension step at 72°C for 5 min. PCR amplification was followed by a melt cycle of an initial denaturation at 95°C for 15 s, then heating from 65°C to 95°C.
Control human β-actin PCR
Human beta actin primers F (5’- CGGCCTTGGAGTGTGTATTAAGTA -3’) and R (5’- TGCAAAGAACACGGCTAAGTGT -3’) were used to amplify the human β-actin gene. The PCR was amplified in triplicates using the following protocol: each 15 μl reaction mixture contained 0.1 μM each primers (IDT, Coralville, IA), 0.2 mM deoxynucleoside triphosphate (dNTP) (Invitrogen, Carlsbad, CA), 1X Phusion GC PCR buffer (Thermo Scientific, Waltham, MA), 2.5X EvaGreen (Biotium, Fremont, CA), 0.02 U/μl Phusion polymerase (New England Biolabs, Ipswich, MA), ultrapure water (Quality Biological, Gaithersburg, MD), and 3 μl of direct BALF sample liquid. Thermocycling for qPCR and subsequent melt analysis were performed on a Quantstudio 3D real time PCR system (Applied Biosystems, Waltham, MA). The cycling conditions were as follows: hold at 98°C for 30 s, followed by 55 cycles of 98°C for 10 s, 66°C for 30 s, and 72°C for 45 s. At the end of cycling, there was a final extension step at 72° for 5 min. PCR amplification was followed by a melt cycle of an initial denaturation at 95°C for 15 s, then heating from 65°C fo 95°C.
Target DNA isolation
Melt curve database generation
The following fungal strains were provided as clinical isolates by Dr. Nathan Weiderhold at the Department of Pathology University of Texas Health Science Center, San Antonio, TX: Aspergillus terreus, Aspergillus nidulans, Aspergillus versicolor, Mucor circinelloides, Mucor velutinosus, Mucor plumbeus, Rhizopus arrhizus var. delemar, Rhizopus microsporus, Lomentospora prolificans, Scedosporium apiospermum, Scopulariopsis brevicalus, Scopulariopsis candida, and Scopulariopsis gosspii. Aspergillus fumigatus, Aspergillus flavus, Aspergillus niger, Fusarium oxysporum, Cryptococcus neoformans, Candida krusei, Candida glabrata, and Candida albicans were provided as clinical isolates from Dr. Sanjay Mehta at the San Diego VA Clinical Microbiology Laboratory. Candida auris was provided as a clinical isolate by Dr. Sharon Reed at the UCSD Center for Advanced Laboratory Medicine (CALM). For database generation, DNA was extracted using Lucigen MasterPure Yeast DNA Purification Kit (Lucigen, Middleton, WI, USA). DNA concentration was measured by bio-spectrophotometer absorbance readings and diluted to desired target concentrations.
Clinical samples host depletion and PCR inhibitor removal
Prior to DNA isolation, direct PCR β-actin was run to assess lavage quality as described previously. Clinical BALF sample DNA was isolated using Molysis Complete 5 Small Size Sample DNA Isolation (≤1ml Liquid) protocol (Molzym, Bremen, Germany). Each BALF sample was run in U-dHRM with Asp-ITS PCR conditions as described previously.
Control pig BALF and analytical validation
For control and analytical spike-in experiments pig BALF was collected from euthanized pigs previously treated with antibiotics and anesthetized with ketamine/xylene/atropine. Ambu® aScope™ 4 Broncho single-use bronchoscopes (Ambu® A/S, Ballerup, Denmark) were used with 50 mL sterile isotonic irrigation 0.9% saline (NDC 0990-6138-22) for lavages. Prior to analytical validation, pig BALF was screened to be negative for target organisms by U-dHRM. Target organism spores were counted and plated, and six 10-fold serial dilutions were conducted to achieve concentrations down to 1 CFU/mL, with concurrent no spike controls. A. fumigatus and C. albicans spores from each concentration were spiked into 2 mL of pig BALF to achieve the final concentrations of 10k, 1k, 100, 10, 1 and 1 spores (CFU)/mL of BAL.
DNA sequencing
The PCR products were prepared using ExoSAP-IT (Applied Biosystems, Foster City, CA) with the supplied protocol and then sent for Sanger sequencing (GENEWIZ, San Diego, USA) using the same respective forward primers in the 18S and ITS amplifications.
Image processing and data analysis
A sequence of raw fluorescence images was captured during the heating and melting procedure for a single chip. Subsequently, these images underwent a sequence of image processing steps to identify and extract the individual wells within them along with their corresponding average intensity values. This procedure resulted in the translation of the average intensity measurements for each well across the entire set of images into a chronological array of values, thus creating a time series representation.
The original fluorescence time series, recognized as melt curves, underwent a two-fold transformation: initially, they were converted into their respective derivatives, after which they were subjected to a smoothing process using a Savitzky-Golay filter. Furthermore, these smoothed derivative time series were classified as 'Positive' if they exhibited a peak or local maxima beyond a temperature threshold of 85°C and with a minimum height of 4 units. In this context, a 'Positive' melt curve designates an instance where the presence of a particular fungal target is anticipated, whereas the remaining instances are categorized as 'Negatives'.
Leveraging these identified 'Positive' melt curves, a dataset for machine learning purposes was constructed. Each time series within this dataset represented a derivative melt curve that was smoothed using a Savitzky-Golay filter with specific parameters: a window length of 9 and a polynomial order of 3. These time series were then normalized using Area Under the Curve Normalization to take care of scaling differences.
Machine learning
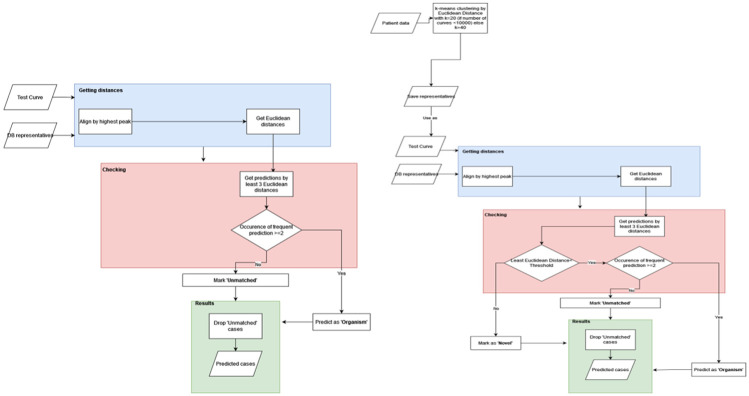
We constructed a model based on our established database of organisms. This approach unfolds through a two-step procedure. The dataset we employed comprises a comprehensive set of 12,000 melt curves attributed to each distinct organism. Within this dataset, a subset of 10%, equating to 1,200 random melt curves, were selected and subjected to a time series DTW distance based K-means clustering process, yielding a culmination of up to 50 representatives (Lloyd n.d.) (MacQueen 1966) (“A Global Averaging Method for Dynamic Time Warping, with Applications to Clustering” 2011). Clusters housing fewer than 10 melt curves were excluded from consideration due to their susceptibility to noise-related interference. Owing to the substantial variability and inherent noise within the melt curves, we employed the K-means clustering technique as the initial step to disentangle pivotal clusters of variation, thereby yielding corresponding cluster centers that serve as robust and condensed representations of signals. These cluster centers are referred to as “DB representatives” in the flow charts in Fig. 3.
Figure 3. Machine learning process.
a) Flowchart for database sample testing b) Flowchart of patient samples testing differentiating between database classification and novelty detection
Another point to note is that instead of using the usual Euclidean distance-based K-means, we use Dynamic Time Warping (DTW) for both the cluster assignment as well as the averaging step of K-means. Temporal distortions (or shift) along the temperature (or time) axis causing well-to-well as well as chip-to-chip variations in melt curves is something inherent in HRM (Gundry et al. 2008) (Sinha, Mack, et al. 2018) (Langouche et al. 2021) and can be dealt with by using the various elastic distance measures for time series – amongst which the most popular one is Dynamic Time Warping (DTW) (Langouche et al. 2021; Sakoe and Chiba n.d.) and its variations (Rabiner, Rabiner, and Juang 1993) (“Weighted kNN and Constrained Elastic Distances for Time-Series Classification” 2020). More specifically, as we use DTW distance, we employ a more suitable DTW-based Barycenter Averaging (DBA) technique, as proposed by François et. al (“A Global Averaging Method for Dynamic Time Warping, with Applications to Clustering” 2011), for the K-means averaging step.
Subsequently, the second phase (Fig. 3) entailed the development of a classifier grounded in a 3 nearest neighbor (3NN) framework, leveraging the Euclidean distance as the defining metric. In this step, each test curve underwent alignment with every representative curve curated from the database (see blue boxes Fig. 3 a-b). Consequently, the KNN model was executed to discern the three nearest neighbors for each aligned test curve (see pink boxes Fig. 3 a-b) (Fix and Hodges 1951). The alignment procedure was deemed necessary to account for the potential shift-based discrepancies present among melt curves.
The outcome of this model furnishes predictions wherein concordance among the majority of neighbors designates a high-confidence classification. Conversely, instances in which all three nearest neighbors correspond to dissimilar organisms are categorized as low-confidence and consequently disregarded. Low-confidence instances can originate from either noisy signals or from novel curves that remain un-represented within the existing database. The performance of classification was quantified through the assessment of accuracy for each organism.
Although in literature the techniques novelty detection (ND), anomaly detection (AD) and outlier detection (OD) have been used interchangeably (“A Review of Novelty Detection” 2014). However, unlike AD or OD which usually refer to noisy or erroneous signals, ND usually has a positive learning attitude where the novel point is treated as a resource for potential future use (“A Review of Novelty Detection” 2014; Ruff et al. n.d.) (Pang et al. 2020). Currently AD, ND and OD are being studied under the common framework of Generalized Out of Distribution Detection (OOD) (Yang et al. 2021). Specifically for time series data, there is a significant amount of literature on AD but this research primarily focuses on finding point or subsequence anomalies within a large time series (Darban et al. 2022). As we have a larger number of smaller length time series we consider each time series (melt curve) as a separate data point. We then use a distance based OOD methodology for novelty detection (see section 5.3 of (Yang et al. 2021)) where the test curve is checked if it is outside of a certain standard deviations (threshold) away from each of the nearest three DB representatives (class cluster centers) obtained via 3NN step described previously. If this check is successful then the test point is certified as out of distribution and labeled as ‘novel’.
Furthermore, when dealing with patient samples, their time series were initially clustered utilizing the Euclidean-based K-means method (top left Fig. 3b). The resultant cluster centers were then subjected to classification leveraging the pre-constructed 3NN-based classifier designed for the database curves.
Patients and Samples
In this retrospective case control study, banked BALF samples originated from patients with various underlying diseases and clinical suspicion of invasive pulmonary aspergillosis (IPA) or IMI, and galactomannan (GM) and Aspergillus spp. culture testing ordered between 2015 and 2019 at the University of California San Diego (UCSD). IMI was classified according to the revised European Organization for Research and Treatment of Cancer (EORTC)/Mycoses Study Group (MSG) criteria(Donnelly et al. 2019) and slightly modified AspICU criteria(Blot et al. 2012) (i.e., including positive BALF fluid GM of 1.0 ODI as entry criterion(Koehler et al. 2021)) (Meersseman et al. 2008) for patients in the intensive care unit (ICU) who did not fulfill EORTC/MSG host criteria. GM testing with the Platelia enzyme-linked immunosorbent assay (ELISA) (Bio-Rad Laboratories, Marnes-la-Coquette, France) was routinely and prospectively performed in all BALF samples before samples were stored at −70°C for up to 8 years. Based on classification we retrospectively tested 75 patient BALF samples from 30 patients classified as having suspected IPA with proven (n = 1), probable (n = 25), or putative (n = 4) IPA infections, and from 45 patients classified with limited evidence or as not having IPA (n=10 not classifiable, n = 4 possible IPA, n = 31 classified as no IPA). Not classifiable samples tested positive for mycological evidence and came from patients with clinical suspicion of IMI who did, however, not fulfill host factor criteria and/or did not present with typical radiological signs and were not admitted in the ICU. Direct β-actin PCR was used to access lavage quality according to previously published methods (Springer et al. 2018) (Lagier et al. 2016) (Fréalle et al. 2009). Two samples (n=1 possible and n=1 no IPA) were excluded due to no human DNA being detected.
Novelty detection and Micromanipulator interrogation
Novel curves that were un-represented within the existing database were identified with ML as described previously. These curves’ physical X-Y on-chip coordinates were then identified using Melio Melt Inspector software (MelioLabs, Inc., Santa Clara, CA, USA). A custom micromanipulator setup then sampled the target amplicons from individual or clusters of wells using a glass capillary. Sampled amplicons were either reamplified with Asp-ITS primers or sent directly for Sanger sequencing. Reamplified Asp-ITS dPCR chips were used to demonstrate the process of adding novel organisms to the established database.
RESULTS
Fungal U-dHRM Assay Development and Analytical Validation
To develop a universal PCR assay for fungal detection, we first selected primers targeting conserved sequence regions flanking the ITS1-ITS4 barcoding region of the fungal genome (Supplementary Fig. 1) and tested their ability to amplify 21 clinically relevant organisms (Table 1). We started with Aspergillus spp., since it is the most prevalent IMI pathogen worldwide, and Candida spp., the most prevalent commensal genus, and began testing the ITS primers.
Table 1.
Clinically relevant fungi, including rare molds, used to develop universal assay.
| Organism | Prevalence (US, Global) | Galacto- mannan Reactivity |
References |
|---|---|---|---|
| Aspergillus fumigatus | Most common mold infection; Estimated 50k cases of invasive pulmonary aspergillosis (IPA) per year in US alone; During COVID-19 10-15% of call COVID-19 patients in ICU; A. fumigatus listed as critical priority pathogen by WHO. | Yes | (Heldt et al. 2018; Jenks et al. 2021; Hoenigl, Seidel, Sprute, et al. 2022; ”WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action” 2022a) https://emedicine.medscape.com/article/296052-overview, May 2021 |
| Aspergillus flavus | Yes | ||
| Aspergillus terreus | Yes | ||
| Aspergillus nidulans | Yes | ||
| Aspergillus niger | Yes | ||
| Aspergillus versicolor | Yes | ||
| Candida albicans | Most common organism causing Candidemia and invasive Candidiasis; Estimated 700k cases per year of IC and candidemia; most common cuse of fungal endocarditis; Listed as critical priority pathogen by WHO. | No | (Hoenigl, Salmanton-García, et al. 2023; Pappas et al. 2018; ”WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action” 2022a; Bongomin et al. 2017) (Thompson et al. 2023) |
| Candida glabrata (Nakaseomyces glabratus) (de Hoog et al. 2023) | Second most common organism causing Candidemia; Often resistant to azoles and sometimes also echinocandins; Listed as high priority pathogen by WHO list. | No | (Aigner et al. 2019; Pappas et al. 2018; Hoenigl, Salmanton-García, et al. 2023; ”WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action” 2022b; Arendrup et al. 2023) |
| Candida parapsilosis | 3rd-4th most common organism causing Candidaemia; Recent emergence of outbreaks of fluconazole resistant strains; Listed as high priority pathogen by WHO. | No | (Daneshnia et al. 2023; Pappas et al. 2018) |
| Candida krusei (Pichia kudriavzevii) (de Hoog et al. 2023) | 5th-6th most common Candida spp. Causing candidemia; Often multiresistant. | No | (Hoenigl, Salmanton-García, et al. 2023; Pappas et al. 2018) |
| Candida auris | Emerging pathogen; multiresistant causing ICU outbreaks; Listed as critical priority pathogen by WHO. | No | (”WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action” 2022b; Pappas et al. 2018) |
| Cryptococcus neoformans | Listed as critical priority pathogen in WHO list. | No | (”WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action” 2022b) |
| Fusarium solani complex | Listed as high priority pathogen in WHO list; Third most common mold infection (after Aspergillosis and Mucormycosis); Incidence and prevalence of Fusarium spp. infections vary depending on the underlying disease and geographical region, reaching 20 per 1000 recipients of a allogeneic haematopoietic stem cell transplantation in Brazil and the USA. Cause of 2022/2023 fungal meningitis outbreaks in Mexico. | Yes | (Tortorano et al. 2012; Hoenigl et al. 2021; Nucci et al. 2014; ”WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action” 2022b); Hoenigl, Jenks, et al. 2023) |
| Lomentospora prolificans | Listed as a medium priority pathogen in WHO list. In the U.S. accounts for 6-35% of non-Aspergillus mold infections but prevalence and incidence largely unknown. | No | (Hoenigl et al. 2021; Jenks et al. 2020, 2018; Seidel et al. 2019) |
| Scedospoirum apiospermum | Listed as a medium priority pathogen in WHO list. In one U.S. study accounted for 11% of IMI and 19% of non-Aspergillus mold infections in SOT recipients. | No | (Hoenigl et al. 2021; Seidel et al. 2019) |
| Scopulariopsis spp. | Unknown | No | (Hoenigl et al. 2021) |
| Mucor circinelloides | Mucormycosis is the second- or third-most common mold infection; Worldwide occurrence; average annual incidence rate about 1/1 million population (although variable by geographic location and generally higher in eg India and Iran); Prevalence in India during COVID-19: 1.6% in COVID-19 ICU patients; Worldwide Mucor spp. and Rhizopus spp. are most common pathogens; Mucorales listed as high priority pathogen in WHO list. | No | (Cornely et al. 2019; Hoenigl, Seidel, Sprute, et al. 2022; ”WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action” 2022b) |
| Mucor velutinosus | No | (Cornely et al. 2019) | |
| Mucor plumbeus | No | (Cornely et al. 2019) | |
| Rhizopus arrhizus | No | (Cornely et al. 2019; Hoenigl, Seidel, Sprute, et al. 2022) | |
| Rhizopus microsporus | No | (Cornely et al. 2019) |
However, Aspergillus spp. were not consistently amplified by our ITS primers and the efficiency of this region for detection of Aspergillus spp. isolates is not optimal. Furthermore, the ITS1-4 region is not sufficient for discriminating between many individual Aspergillus spp. And has been shown to not amplify in certain isolates (Fraczek et al. 2019; “Target Genes, Primer Sets, and Thermocycler Settings for Fungal DNA Amplification” 2022, “Evaluation of Nucleic Acid Sequencing of the D1/D2 Region of the Large Subunit of the 28S rDNA and the Internal Transcribed Spacer Region Using SmartGene IDNS Software for Identification of Filamentous Fungi in a Clinical Laboratory” 2012) (Supplementary Fig. 2). Since Aspergillus spp. is one of the most clinically relevant fungal pathogens in the US but also globally, we next selected an Aspergillus-specific primer set targeting the 18S rDNA gene, which harbors species-specific sequence differences (Supplemental Figure. 3).
This primer set was multiplexed with the ITS primer set and the assay was tested for its ability to amplify the 21 species in Table 1. Our Scopulariopsis spp. isolates were not consistently amplified which has been observed previously (“Evaluation of Nucleic Acid Sequencing of the D1/D2 Region of the Large Subunit of the 28S rDNA and the Internal Transcribed Spacer Region Using SmartGene IDNS Software for Identification of Filamentous Fungi in a Clinical Laboratory” 2012), while Scedosporium apiospermum isolates produced variable melts indicating multiple organisms (Supplementary Fig. 4), and neither of these were added to the final database. Now, 19 amplified and sequenced in qPCR and produced reliable melt curve signatures in U-dHRM. Figure 2 shows the digital melt curve signatures for each organism and their average curve in black.
Figure 2. U-dHRM curves for 19 organisms.
Asp and ITS primers were multiplexed in U-dHRM and the assay was tested for the detection and melt-based discrimination of 19 organisms: (A-E) Candida spp., (F) Cryptococcus spp., (G) Lomentospora spp., (H) Fusarium spp., (I-K) Mucor spp., (L-M) Rhizopus spp., (N-S) Aspergillus spp.. Yeasts are blue/green, molds are orange/red/black.
Next, we conducted analytical validation studies on Aspergillus spp. and Candida spp. to assess the overall detection capability of the assay in combination with sample preparation starting from a real sample matrix. Mock samples were created by spiking whole organisms into pig BALF over a concentration range of approximately 1x105-1x10° CFU/mL and no spike controls. Host DNA depletion and pathogen DNA extraction was carried out using MolYsis Complete5 per manufacturer's instructions. Then, the extracted DNA was loaded onto dPCR chips with the multiplexed Asp+ITS universal fungal assay and amplification was performed prior to dHRM analysis (Supplementary Fig. 5). Fungal melt curve counts showed good linearity of quantification (r2=0.99) for Candida and Aspergillus spp. (Supplementary Fig. 6A,B). However, Aspergillus spp. detection was 10-fold lower than expected and Candida detection was 10-fold higher than expected, based on spore counting and plating. To test if this difference could be attributed to Aspergillus spp. being more difficult to lyse or whether it reflected assay sensitivity differences, we conducted Aspergillus spp. DNA dilution series experiments. This showed that the assay alone maintained high linearity of detection down to ~10 copies/chip, or 25 pg/mL (Supplementary Fig. 6C).
Database generation and algorithm training
To determine whether fungal organism digital melt curves (Fig. 2) could be reliably and automatically recognized by a ML algorithm, a database of >196,000 curves comprising biological and technical replicates n ≧ 3 for each of the 19 pathogens was generated on dPCR chips. Fig. 3 A-B depicts the ML flowchart comparison for testing database curves versus clinical unknown or novel curves. The classification performance of a ML algorithm that combines dynamic time warping and Euclidean distance based metrics was assessed in cross-validation studies (”Weighted kNN and Constrained Elastic Distances for Time-Series Classification” 2020).
Recall was assessed and plotted as a confusion matrix in Fig. 4A. This revealed that Aspergillus spp. were not reliably discriminated within the genus, while all other species were reliably classified. Among Aspergillus spp., cross-validation showed that an overall accuracy (F-score, a combination of precision and recall) of about 60% was achieved (Supplementary Table 1). This can be explained visually by overlaying representative curves from each species, which are quite similar (Fig. 4B), due to few sequence differences (Supplementary Fig 4). An overall accuracy of 86% was achieved across the 19 organisms with Aspergillus spp. treated as separate classes (Supplementary Table 2). Grouping Aspergillus spp. into a single class (Fig. 4C) at the genus level resulted in a significant improvement in the F-score for Aspergillus spp. (90%, Supplementary Table 3), and an overall accuracy for all classes of 97% was achieved. The associated confusion matrix (Fig. 4D) shows only 3.4% misclassification overall (5059/150752), with the most occurring between M. circinelloides and Aspergillus spp. when Aspergillus genus is the true class (7.2%, 768/10657). Representative melt curves for each organism class are shown in Fig. 4E.
Figure 4. Machine Classification Performance on Fungal Melt Curve Database and Curves.
a) confusion matrix with individual Aspergillus spp. b) Aspergillus spp. average curves overlap c) Grouped Aspergillus spp. average curve overlap. d) confusion matrix with grouped Aspergillus. e) Average curves of Grouped Aspergillus genus and all average curves of 13 other spp.
Clinical BALF Sample Analysis
Overall performance for pathogenic mold detection
U-dHRM achieved an average of 97% fungal organism identification accuracy and a turn-around-time of 4hrs. In total, 73 remnant banked BALF samples that were collected due to suspicion of IMI were analyzed by U-dHRM and compared to clinical diagnostic classifications (Fig. 5). U-dHRM detected pathogenic molds (Aspergillus, Mucorales, Lomentospora and/or Fusarium spp.; ≧ curve or 11 CFU/mL) in 73% (53/73) of all the samples (Fig. 5A). In addition Candida spp. were detected in 88% (64/73) of all samples, while 12% (9/73) had non-Candida yeasts as well. We note that there was no apparent association between human β-actin Ct and concentration of fungi detected by U-dHRM or BALF sample volume and concentration of fungi detected by U-dHRM (Supplementary Fig. 7). In 19% (14/73) of samples, mixtures of pathogenic molds were detected (Fig. 5A). Examples of curve signatures detected by U-dHRM and identified by ML in the clinical BALF samples and their closest matching database curve are shown in Supplementary Fig. 8. Of the samples considered positive for IMI, U-dHRM detected pathogenic molds in 73% (1/1 proven; 17/25 probable; 4/4 putative). In samples that were not classifiable for IMI, U-dHRM detected pathogenic molds in 90% (9/10). However, in samples considered negative or without mycological evidence for IMI, U-dHRM detected pathogenic molds in 67% (1/3 possible; 21/30 no). These samples were considered negative for IMI predominantly because of GM and culture negativity as well as absence of host factors, but nonetheless, they were collected due to some clinical suspicion of IMI. These results suggest that U-dHRM has good sensitivity for IMI, as defined by current diagnostic criteria, when host risk factors are also considered. Specificity was optimized by requiring the number of pathogenic mold curves detected in a sample to be >8 and sample volume to be 1mL, which resulted in a subset of 43% detection in criteria-matching positives (6/14), 50% (5/10) in not classifiable, and 0% detection in negatives (0/21) .
Figure 5. U-dHRM Pathogen Detection Statistics in Patient Samples.
a) Pathogen distribution by IMI diagnosis classification. Others are defined as yeasts in the U-dHRM database or unknown novel organisms b) U-dHRM detection of Aspergillus in suspected IMI cases ordered left to right by decreasing confidence of suspicion by IPA classification. c) U-dHRM detection of Aspergillus in combined highest, medium, and low suspicion. d) Examples of Aspergillus Detection by U-dHRM in BALF. Concordant Aspergillus detection confidence and overlapping ML representatives depicts examples of Asp-ITS U-dHRM detection and ML classification in patients with no IPA, probable IPA (treated at the time of collection and culture negative, and untreated at the time of collection and culture positive) and proven IPA. Correlations with routine mycological test results show that more Aspergillus curves were detected in the patient with probable IPA who had both, positive BALF GM and positive culture versus the other patient with probable IPA who had only positive BALF GM.
Aspergillus detection by U-dHRM compared to culture and GM
A summary of Aspergillus spp. detection by U-dHRM compared to clinical diagnostic criteria is shown in Fig. 5B-C. Of all the samples that cultured Aspergillus spp., U-dHRM detected Aspergillus spp. melt curves in 61% of positives (1/1 proven; 4/9 probable; 3/3 putative), 0% of not-classifiable (0/2) cases or no IPA (0/1 no). Considering only samples from proven, probable and putative cases that were culture+, GM+, antifungal treatment-, U-dHRM detected Aspergillus spp. melt curves in 78% (7/9). Examples of Aspergillus spp. melt curves from patient samples that correlated with routine mycological test results are shown in Fig. 5D. The highest Aspergillus spp. load was detected in the patient with proven IPA, and the second highest load was detected in a patient with probable influenza-associated pulmonary aspergillosis (IAPA).
U-dHRM also detected Aspergillus spp. in some samples that did not culture Aspergillus spp.: 10% (2/19) probable; 12% (1/8) not classifiable; 28% (8/29) no IPA. In samples that did not culture Aspergillus spp., other pathogenic molds were often detected alone or in combination with Aspergillus spp.: other molds were detected in 71% of probable and putative cases (12/17); 70% (7/10) not classifiable cases; 67% (22/33) of possible and no IPA cases .
Differentiation between Aspergillus spp. and Fusarium spp. by U-dHRM in GM positive samples
Of all the GM+ samples, U-dHRM detected GM-producing organisms Aspergillus and/or Fusarium spp. in 54% (21/39). Mixtures of Aspergillus and Fusarium spp. were detected in 8% (3/39).
In GM+/Aspergillus spp. culture+ samples, Aspergillus spp. alone were detected in 36% (5/14), Fusarium spp. alone in 7% (1/14), while both were detected in 21% (3/14). In GM+/Aspergillus spp. culture- samples, Aspergillus spp. were detected in 12% (3/25), Fusarium spp. in 36% (9/25), while both were detected in 0% (0/25). These results are depicted in Fig. 6A-B. An example of multiple pathogen detection including Aspergillus and Fusarium spp. melt curves from a patient sample is shown in Fig. 6C.
Figure 6: Aspergillus, Fusarium multiple pathogen co-detection.
a) Aspergillus and Fusarium detection distribution by GM and culture positivity. Others are defined as yeasts in the U-dHRM database or unknown novel organisms b) U-dHRM detection of GM-producing spp. compared to clinical GM status. c) Aspergillus, Fusarium, and Mucor codetection example of representative raw clinical curves demonstrating patient BALF pathobiome. Curves are shown for Aspergillus, Mucor, Fusarium, and Candida for visualization purposes with the following quantification: 8.9x102 CFU/mL Aspergillus, 3.3x101 CFU/mL F. oxysporum, and 7.7x101 CFU/mL M veluntunsosis, 2.7x102 CFU/mL C..parapsilosis, 3.1x102 CFU/mL novel organisms (not shown).
Detection of pathogenic molds in the absence of Aspergillus
In samples where no Aspergillus spp. was detected by U-dHRM, other pathogenic molds were detected in putative 1/1 (100%), probable 11/19 (58%), not classifiable 89% (8/9) cases, possible 33% (1/3), and no IMI 55% (12/22) cases.
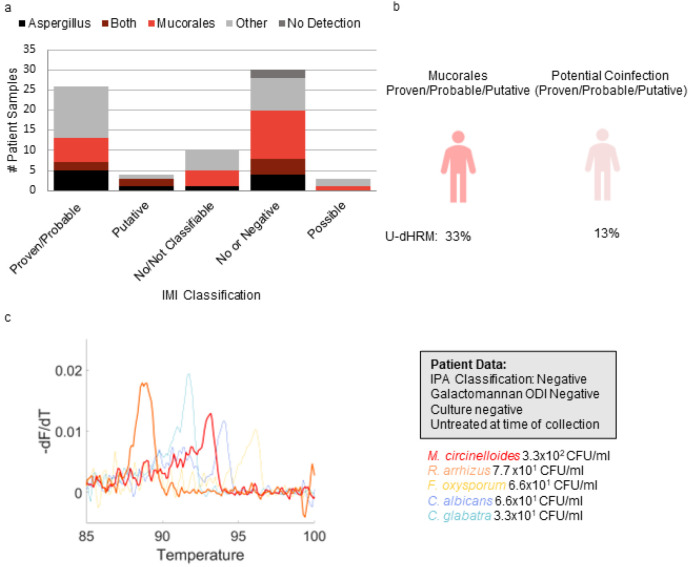
Mucorales Detection
Fungal pathogens in the Mucorales order were detected in 42% (31/73) of all samples. Mucorales was detected in 31% (8/26) of the proven/probable IMI, 50% (2/4) putative, 40% (4/10) not classifiable, 33% (1/3) of the possible cases, and 53% (16/30) of the samples classified as no IMI. Under optimal specificity criteria of pathogenic mold curves detected in a sample to be >8 and sample volume to be 1mL this subset of detection dropped to 15% (2/13) in proven/probable IMI cases, 100% (1/1) of putative cases, 30% (3/10) of not classifiable cases, and no detection in possible cases or samples classified as no IMI.
Co-detection of ≧1 curve for multiple Mucorales spp. occurred in 10% (7/73) of samples, with the highest rate in possible 33% (1/3), followed by not classifiable 20% (2/10), no IMI 10% (3/30), probable, probable 4% (1/25), with proven and possible at 0%. Co-detection of Mucorales and Aspergillus spp. occurred in 11% (8/73) of samples, with the highest rate in putative 50% (2/4) followed by samples classified as no IMI 13% (4/30), proven/probable 8% (2/26), with no co-detection in those classified as possible and those classified as not classifiable. These results are depicted in Fig. 7A-B. An example of co-detection of Mucorales spp. including melt curves from a patient sample representing discordant mold diagnosis is shown in Fig. 7C.
Figure 7. Mucorales detection.
a) Aspergillus and Mucorales detection distribution by IMI diagnosis classification. Others are defined as yeasts in the U-dHRM database or unknown novel organisms b) U-dHRM detection of mucorales and potential co-infection in suspected IMI cases. c) Discordant mold diagnosis example of representative raw clinical curves demonstrating patient BALF pathobiome. Curves are shown for. Mucor, Fusarium, and Candida for visualization purposes with the following quantifications: 6.6x101 CFU/mL C. albicans (blue) 3.3x10 CFU/mL C. glabrata (blue), 3.3x102 CFU/mL M. circinelloides (red), 7.7x10 CFU/mL R. arrhizus (orange), 6.6 x101 CFU/mL F. oxysporum, and 1.2x102 novel organisms (not shown)
Identification of Organisms Generating Novel Fungal Melt Curves
A unique feature of the U-dHRM trained ML algorithm is its ability to automatically detect novel organisms by their distinct melt curve shapes compared to common pathogen curves represented in the database (see Methods). 96% (70/73) of the BALF samples tested produced melt curves that confidently matched to the U-dHRM database of common pathogens. However, a few patient samples generated fungal melt curves that did not match the database and were called novel by the algorithm. To identify the organisms generating these curves, a micromanipulator was used to recover individual digital reactions and sequence their amplicons. Fig. 8 demonstrates the application of this new technique to a patient sample where novel melt curves dominated U-dHRM results (Fig. 8A). Custom software was used to determine the XY position of novel curve generating wells (Fig. 8B), and wells were sampled by using a micromanipulator (Fig. 8C) to position a micropipette into the target well (Fig. 8D) and extract the reaction containing novel amplicons (Fig. 8E). In this sample, Trichosporon asahii and Sacchromyces cerevisae (Fig. 8A, dark and light gray curves respectively) were identified. Using the recovered T. asahii amplicons as template, U-dHRM was conducted to generate database curves for training the ML algorithm to automatically identify this organism in future samples. Supplementary Table 4 describes other patient samples where novel amplicons were recovered and identified, including potentially causative pathogens and commensal yeasts Pneumonocystis jirovecii, Sporobolomyces salminocolor, Sacchromyces cerevisae, Epicoccum nigrum, and Candida inconspicua. This process avoids the need to culture amplify isolates, which is important considering the low sensitivity of BALF culture and potential fastidiousness of novel organisms. Additionally it will further expand the database while limiting the occurrence of future unidentifiable melt curves, thus minimizing the need for future sequencing, which in turn affects the turnaround time (TAT).
Figure 8. Novel Melt Curve Identification and Algorithm Retraining.

a) Novel fungal melt curves Trichosporon asahii (dark gray) and Sacchromyces cerevisae (light gray) identified by ML for patient sample IFI 004. Diagnostic information for this patient is shown in the adjacent gray box. b) Screenshot of the Melio Melt Inspector software used to find the specific XY location of the wells on-chip harboring novel amplicons. c-d) Schematic and photograph of the micromanipulator positioning a micropipette into the target well for novel amplicon collection. e) Fluorescent micrograph of chip after micropipette extraction of reaction from the target well. f) U-dHRM melt curves generated by re-amplification of the novel amplicon for database expansion and training of the ML algorithm.
Discussion
Evaluating the performance of new diagnostic tests for IMI is difficult due to the limitations of comparing these new modalities to imperfect gold standard clinical tests and the rarity of autopsy proven IMI, and the ongoing debate over the accuracy of diagnostic classifications (Arvanitis et al. 2014). U-dHRM results were not particularly well correlated with GM positivity or Aspergillus spp. culture results - neither of which correlated well with each other - but did demonstrate strong agreement with clinical mycology tests in general and showed good sensitivity for IMI, as defined by current diagnostic criteria, when host factors were also considered. A lack of concordance between GM positivity and U-dHRM detection (or Aspergillus spp. culture results) could arise from organism clearance when antigen levels are high or the presence of organisms before antigens are developed during active growth. Importantly, in cases where GM positivity did not correlate with Aspergillus spp. detection by culture, U-dHRM results occasionally provided potential explanations by detecting other GM producing organisms such as Fusarium and Trichosporon spp. (“Serology Anno 2021—fungal Infections: From Invasive to Chronic” 2021).
With BAL culture showing limited sensitivity for detecting pulmonary fusariosis (Hoenigl et al. 2021), Fusarium spp. infections resulting in GM positivity can lead to a false diagnosis of probable IPA and incorrect or inadequate antifungal treatment for these highly resistant pathogens. In San Diego Fusarium spp. Have been shown to be a frequent cause of rare mold infections (Jenks et al. 2018). U-dHRM had higher detection of Fusarium in GM+ samples that did not grow Aspergillus spp. in culture. Also, the ability of U-dHRM to detect multiple common pathogens, even in mixtures, has potential to identify mixed infections and improve treatment decisions. For example, in a patient classified as probable for IPA with positive GM and Aspergillus spp. Culture results, U-dHRM detected Aspergillus spp. in concordance with these results, but also detected F. oxysporum, and M. velutinosus at similar abundances (8.9x102 CFU/mL Aspergillus, 3.3x10^1 F. oxysporum, and 7.7x101 CFU/mL M. velutinosus, 2.7x102 CFU/mL C. parapsilosis). While at the time of BALF collection this patient had not received antifungal treatment, the patient subsequently received treatment for IPA with voriconazole (which likely covered F. oxysporum but not M. velutinosus) and passed away within a week, with no autopsy performed. In this case, U-dHRM results may have influenced treatment to include antifungals targeting M. velutinosus. In another example, U-dHRM detected a mixture of different Mucorales spp. in a patient with suspected IMI but negative GM and Aspergillus spp. culture results. Of note, one of the species detected, M. circinelloides, commonly shows higher MICs against isavuconazole and posaconazole, complicating therapy (Badali et al. 2021). While IPA can be diagnosed with the presence of host factors, clinical symptoms, radiological findings, and mycological evidence of Aspergillus either in culture or by detection of GM, other IMIs can mimic the clinical presentation of IPA, with mycological evidence mostly limited to insensitive culture or histology. As a prominent example, mucormycosis diagnostics is particularly challenging (Cornely et al. 2019). Pulmonary mucormycosis remains one of the most common non-Aspergillus mold infections in many US centers, and has been globally and particularly in India on the rise as a complication in COVID-19 patients(Hoenigl, Seidel, Carvalho, et al. 2022). There is hope on the horizon with Mucorales PCR now starting to be implemented in some clinical centers (Guegan et al. 2020).
While U-dHRM detected Aspergillus spp. in 61% (9/13) of culture positive samples from patients with IPA, the method also detected Aspergillus spp in 19% (11/58) of culture negative samples, The presence of viable but non-culturable organisms may explain the this finding. Molysis sample processing upstream of U-dHRM analysis utilizes selective lysis, DNase, and filtration steps to degrade host and cell-free DNA and enrich for intact organisms, which allows U-dHRM to detect organisms that are intact but may not grow in culture.
The ability of U-dHRM to detect novel fungal organisms also demonstrated diagnostic value in this patient cohort. Several patient samples contained more novel melt curves than curves from common pathogens. The ability to recover these amplicons for same-day Sanger sequencing enabled the fast identification of emerging pathogens of clinical significance. In one case, using this method resulted in the identification of T. asahii as the dominant organism in the BALF of a patient classified as probable for IPA with positive GM and negative culture who had already received 42 days of micafungin. U-dHRM did not detect Aspergillus spp. In that patient. T. asahii is resistant to micafungin and can cause positive GM. It is an emerging pathogen that is rarely identified in clinical practice but often causes fatal infections in immunocompromised individuals due to being misdiagnosed as other types of fungal infections and because of its resistance to many front-line antifungals (Li et al. 2020). This particular patient was never diagnosed with or treated for T. asahii and passed away, suggesting that U-dHRM could have provided critical diagnostic value with high impact for this patient.
Overall, the performance of U-dHRM suggests that it could represent a promising advance in molecular pathogen detection strategies for IMI. Previously, broad-based qPCR followed by sequencing has shown promise for improving the detection of rare molds, but this approach is recommended only when fungal elements are seen by histopathology due to sensitivity limitations (Donnelly et al. 2019). Also, the presence of multiple fungal species can lead to the detection of only the dominant species or failed detection altogether(Kidd et al. 2019; Zeller et al. 2017). U-dHRM distinguishes itself by implementing broad-based PCR in a higher sensitivity dPCR format. Implementation of melt analysis in a digital format enables identification and counting at the single genome level, even in polymicrobial samples, and eliminates template amplification competition and efficiency biases. Also, this format allows extensive melt curve training data to be rapidly generated, unlocking the power of machine learning through big data for automated melt curve identification to rapidly identify and quantify the sequences of all the common pathogens in the sample individually. Only novel organism curves of high abundance warrant interrogation by sequencing, saving time and expense. U-dHRM technology allows for a broader snapshot of the patient pathobiome, including more sensitively detecting and discriminating causative species. The quantitative nature of U-dHRM results also highlight the potential for monitoring over time to track mixed infections, measure effectiveness of therapies, and aid in discriminating between true infection (growth) versus colonization (stasis).
Limitations and Future Work
Based on total curve counts per chip and Poisson theory, we estimate that approximately 10% of samples (7/73) had 1.6% of total wells with double occupancy. So multiple organism curves could have overlapped in these wells, which may generate multiplexed curves that would be called novel. Running U-dHRM on a dilution of these samples overcomes this challenge. ML could also be potentially trained on combined melt curves in multiple occupancy wells. Also, Aspergillus spp. curves were not reliably differentiable, indicating that the sequence diversity of the Aspergillus specific amplicon generated by the selected primers was not sufficient. Future studies should re-engineer the assay to ensure sufficient sequence diversity to yield distinguishable melt curve shapes. A curve number cutoff may need to be implemented for better specificity, potentially establishing different cutoffs for specific pathogens or commensal organisms. The sample processing may have also contributed to some discrepancies, since culture was conducted at the time of sampling but U-dHRM was conducted after samples had been frozen and stored for up to 8 years. Freezing may have led to organism lysis prior to sample preparation, which could contribute to missed detections by U-dHRM, explaining some of the negative results in patients with prior Aspergillus detection by culture. Analytical study results also suggested that the lysis step prior to U-dHRM could also be improved to facilitate higher sensitivity for difficult to lyse organisms like Aspergillus spp.(Scharf et al. 2020).
In conclusion, the promising performance and speed of U-dHRM and its ability to simultaneously identify and quantify clinically relevant mold pathogens in polymicrobial samples as well as detect emerging opportunistic pathogens may provide information that could aid in treatment decisions and improve patient outcomes. Future studies would ideally be run on freshly obtained BALF samples instead of remnant banked samples, and would also have BALF PCR results as well as concurrent blood samples in order to provide a molecular comparator to help overcome the limitation when comparing against GM and culture, and to help discriminate angioinvasive infections (Mah et al. 2023). Sampling from timepoints before and after IMI classification would aid in evaluating U-dHRM’s diagnostic power compared to the gold standard tests, diagnostic classifications, and response to treatment. . While our study has shown the potential of this method to aid IMI diagnosis, all these measures could also help to establish a reliable cutoff for improving specificity for infection versus colonization and thereby accuracy.
Acknowledgements
We acknowledge and thank MelioLabs for the use of their MeltRead Platform and for their assistance in data analysis with their ML algorithm. We would like to thank Dr. Sarah Reed for providing fungal isolates. We would like to thank Dr. Pedro Cabrales from UCSD for use of his micromanipulator and Julian M. Jimenez from the Fraley lab for work on adapting the micromanipulator for reaction recovery.
Funding
This work was supported by grant funding from Astellas, award number ISR005824, and the National Institute for Allergy and Infectious Disease (NIAID), award number 1R01AI134982.
Footnotes
Conflict of Interest
MH and SIF received research funding from Astellas for a portion of this work. MH also received research funding from Gilead, MSD, Euroimmune, IMMY, Scynexis, Pulmocide, F2G and Pfizer, all outside the submitted work. SIF is a scientific cofounder, director, and advisor of MelioLabs, Inc., and has an equity interest in the company. JDJ received research funding from Astellas, F2G, and Pfizer – all outside of the submitted work. MS is co-founder and CEO of Melio and has equity interest. AK and AS are employees of Melio. NIAID award number R01AI134982 has been identified for conflict of interest management based on the overall scope of the project and its potential benefit to MelioLabs, Inc.; however, the research findings included in this particular publication may not necessarily relate to the interests of MelioLabs, Inc. The terms of this arrangement have been reviewed and approved by the University of California, San Diego, in accordance with its conflict of interest policies. PLW declares no conflicts of interests related to this work.
Ethics Statement
The study protocol and all study-related procedures were approved by the Human Research Protections Program at UCSD.
References
- “A Global Averaging Method for Dynamic Time Warping, with Applications to Clustering.” 2011. Pattern Recognition 44 (3): 678–93. [Google Scholar]
- Aigner M., Wanner M., Kreidl P., Lass-Flörl C., and Lackner M.. 2019. “In the Respiratory Tract Potentially Triggers Galactomannan Positivity in Nonhematological Patients.” Antimicrobial Agents and Chemotherapy 63 (6). 10.1128/AAC.00138-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori Spinello, Corbellino Mario, and Parravicini Carlo. 2018. “Challenges in the Diagnosis of Invasive Fungal Infections in Immunocompromised Hosts.” Current Fungal Infection Reports 12 (1): 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aralar April, Yuan Yixu, Chen Kevin, Geng Yunshu, Velez Daniel Ortiz, Sinha Mridu, Lawrence Shelley M., and Fraley Stephanie I.. 2020. “Improving Quantitative Power in Digital PCR through Digital High-Resolution Melting.” Journal of Clinical Microbiology 58 (6). 10.1128/JCM.00325-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendrup Maiken Cavling, Arikan-Akdagli Sevtap, Jørgensen Karin Meinike, Barac Aleksandra, Steinmann Jörg, Toscano Cristina, Arsenijevic Valentina Arsic, et al. 2023. “European Candidaemia Is Characterised by Notable Differential Epidemiology and Susceptibility Pattern: Results from the ECMM Candida III Study.” The Journal of Infection 87 (5): 428–37. [DOI] [PubMed] [Google Scholar]
- “A Review of Novelty Detection.” 2014. Signal Processing 99 (June): 215–49. [Google Scholar]
- Arvanitis Marios, Anagnostou Theodora, Fuchs Beth Burgwyn, Caliendo Angela M., and Mylonakis Eleftherios. 2014. “Molecular and Nonmolecular Diagnostic Methods for Invasive Fungal Infections.” Clinical Microbiology Reviews 27 (3): 490–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badali Hamid, Cañete-Gibas Connie, McCarthy Dora, Patterson Hoja, Sanders Carmita, David Marjorie P., Mele James, Fan Hongxin, and Wiederhold Nathan P.. 2021. “Epidemiology and Antifungal Susceptibilities of Mucoralean Fungi in Clinical Samples from the United States.” Journal of Clinical Microbiology 59 (9). 10.1128/JCM.01230-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blot Stijn I., Taccone Fabio Silvio, Van den Abeele Anne-Marie, Bulpa Pierre, Meersseman Wouter, Brusselaers Nele, Dimopoulos George, et al. 2012. “A Clinical Algorithm to Diagnose Invasive Pulmonary Aspergillosis in Critically Ill Patients.” American Journal of Respiratory and Critical Care Medicine, December. 10.1164/rccm.201111-1978OC. [DOI] [PubMed] [Google Scholar]
- Bongomin Felix, Gago Sara, Oladele Rita O., and Denning David W.. 2017. “Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision.” Journal of Fungi (Basel, Switzerland) 3 (4). 10.3390/jof3040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornely Oliver A., Alastruey-Izquierdo Ana, Arenz Dorothee, Chen Sharon C. A., Dannaoui Eric, Hochhegger Bruno, Hoenigl Martin, et al. 2019. “Global Guideline for the Diagnosis and Management of Mucormycosis: An Initiative of the European Confederation of Medical Mycology in Cooperation with the Mycoses Study Group Education and Research Consortium.” The Lancet Infectious Diseases 19 (12): e405–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneshnia Farnaz, de Almeida João N. Júnior, Ilkit Macit, Lombardi Lisa, Perry Austin M., Gao Marilyn, Nobile Clarissa J., et al. 2023. “Worldwide Emergence of Fluconazole-Resistant Candida Parapsilosis: Current Framework and Future Research Roadmap.” The Lancet Microbe 4 (6): e470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darban Zahra Zamanzadeh, Webb Geoffrey I., Pan Shirui, Aggarwal Charu C., and Salehi Mahsa. 2022. “Deep Learning for Time Series Anomaly Detection: A Survey.” http://arxiv.org/abs/2211.05244. [Google Scholar]
- Denning David W., Perlin David S., Muldoon Eavan G., Colombo Arnaldo Lopes, Chakrabarti Arunaloke, Richardson Malcolm D., and Sorrell Tania C.. 2017. “Delivering on Antimicrobial Resistance Agenda Not Possible without Improving Fungal Diagnostic Capabilities.” Emerging Infectious Diseases 23 (2): 177–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly J. Peter, Chen Sharon C., Kauffman Carol A., Steinbach William J., Baddley John W., Verweij Paul E., Clancy Cornelius J., et al. 2019. “Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium.” Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America 71 (6): 1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- “Evaluation of Nucleic Acid Sequencing of the D1/D2 Region of the Large Subunit of the 28S rDNA and the Internal Transcribed Spacer Region Using SmartGene IDNS Software for Identification of Filamentous Fungi in a Clinical Laboratory.” 2012. The Journal of Molecular Diagnostics: JMD 14 (4): 393–401. [DOI] [PubMed] [Google Scholar]
- Fix Evelyn, and Hodges J. L.. 1951. Discriminatory Analysis: Nonparametric Discrimination: Consistency Properties. [Google Scholar]
- Fraczek Marcin G., Zhao Can, Dineen Lauren, Lebedinec Ressa, Bowyer Paul, Bromley Michael, and Delneri Daniela. 2019. “Fast and Reliable PCR Amplification from Aspergillus Fumigatus Spore Suspension Without Traditional DNA Extraction.” Current Protocols in Microbiology 54 (1): e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fréalle E., Decrucq K., Botterel F., Bouchindhomme B., Camus D., Dei-Cas E., Costa J. M., Yakoub-Agha I., Bretagne S., and Delhaes L.. 2009. “Diagnosis of Invasive Aspergillosis Using Bronchoalveolar Lavage in Haematology Patients: Influence of Bronchoalveolar Lavage Human DNA Content on Real-Time PCR Performance.” European Journal of Clinical Microbiology & Infectious Diseases: Official Publication of the European Society of Clinical Microbiology 28 (3): 223–32. [DOI] [PubMed] [Google Scholar]
- Guegan Hélène, Iriart Xavier, Bougnoux Marie-Elisabeth, Berry Antoine, Robert-Gangneux Florence, and Gangneux Jean-Pierre. 2020. “Evaluation of MucorGenius® Mucorales PCR Assay for the Diagnosis of Pulmonary Mucormycosis.” The Journal of Infection 81 (2): 311–17. [DOI] [PubMed] [Google Scholar]
- Gundry Cameron N., Dobrowolski Steven F., Martin Y. Ranae, Robbins Thomas C., Nay Lyle M., Boyd Nathan, Coyne Thomas, Wall Mikeal D., Wittwer Carl T., and Teng David H-F. 2008. “Base-Pair Neutral Homozygotes Can Be Discriminated by Calibrated High-Resolution Melting of Small Amplicons.” Nucleic Acids Research 36 (10): 3401–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt Sven, Prattes Juergen, Eigl Susanne, Spiess Birgit, Flick Holger, Rabensteiner Jasmin, Johnson Gemma, et al. 2018. “Diagnosis of Invasive Aspergillosis in Hematological Malignancy Patients: Performance of Cytokines, Asp LFD, and Aspergillus PCR in Same Day Blood and Bronchoalveolar Lavage Samples.” The Journal of Infection 77 (3): 235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenigl Martin, Jenks Jeffrey D., Egger Matthias, Nucci Marcio, and Thompson George R. 3rd. 2023. “Treatment of Fusarium Infection of the Central Nervous System: A Review of Past Cases to Guide Therapy for the Ongoing 2023 Outbreak in the United States and Mexico.” Mycopathologia, August. 10.1007/s11046-023-00790-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenigl Martin, Salmanton-García Jon, Egger Matthias, Gangneux Jean-Pierre, Bicanic Tihana, Arikan-Akdagli Sevtap, Alastruey-Izquierdo Ana, et al. 2023. “Guideline Adherence and Survival of Patients with Candidaemia in Europe: Results from the ECMM Candida III Multinational European Observational Cohort Study.” The Lancet Infectious Diseases 23 (6): 751–61. [DOI] [PubMed] [Google Scholar]
- Hoenigl Martin, Salmanton-García Jon, Walsh Thomas J., Nucci Marcio, Neoh Chin Fen, Jenks Jeffrey D., Lackner Michaela, et al. 2021. “Global Guideline for the Diagnosis and Management of Rare Mould Infections: An Initiative of the European Confederation of Medical Mycology in Cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology.” The Lancet Infectious Diseases 21 (8): e246–57. [DOI] [PubMed] [Google Scholar]
- Hoenigl Martin, Seidel Danila, Carvalho Agostinho, Rudramurthy Shivaprakash M., Arastehfar Amir, Gangneux Jean-Pierre, Nasir Nosheen, et al. 2022. “The Emergence of COVID-19 Associated Mucormycosis: A Review of Cases from 18 Countries.” The Lancet. Microbe 3 (7): e543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenigl Martin, Seidel Danila, Sprute Rosanne, Cunha Cristina, Oliverio Matteo, Goldman Gustavo H., Ibrahim Ashraf S., and Carvalho Agostinho. 2022. “COVID-19-Associated Fungal Infections.” Nature Microbiology 7 (8): 1127–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoog Sybren, Walsh Thomas J., Ahmed Sarah A., Alastruey-Izquierdo Ana, Alexander Barbara D., Arendrup Maiken Cavling, Babady Esther et al. 2023. “A Conceptual Framework for Nomenclatural Stability and Validity of Medically Important Fungi: A Proposed Global Consensus Guideline for Fungal Name Changes Supported by ABP, ASM, CLSI, ECMM, ESCMID-EFISG, EUCAST-AFST, FDLC, IDSA, ISHAM, MMSA, and MSGERC.” Journal of Clinical Microbiology, October. 10.1128/jcm.00873-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- “Invasive Fungal Infections: A Creeping Public Health Threat.” 2018. ASM.org. August 24, 2018. https://asm.org/Articles/2018/September/Invasive-Fungal-Infections-A-Creeping-Public-Healt.
- Jenks Jeffrey D., and Hoenigl Martin. 2018. “Treatment of Aspergillosis.” Journal of Fungi (Basel, Switzerland) 4 (3). 10.3390/jof4030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenks Jeffrey D., Prattes Juergen, Frank Johanna, Spiess Birgit, Mehta Sanjay R., Boch Tobias, Buchheidt Dieter, and Hoenigl Martin. 2021. “Performance of the Bronchoalveolar Lavage Fluid Aspergillus Galactomannan Lateral Flow Assay With Cube Reader for Diagnosis of Invasive Pulmonary Aspergillosis: A Multicenter Cohort Study.” Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America 73 (7): e1737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenks Jeffrey D., Reed Sharon L., Seidel Danila, Koehler Philipp, Cornely Oliver A., Mehta Sanjay R., and Hoenigl Martin. 2018. “Rare Mould Infections Caused by Mucorales, Lomentospora Prolificans and Fusarium, in San Diego, CA: The Role of Antifungal Combination Therapy.” International Journal of Antimicrobial Agents 52 (5): 706–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenks Jeffrey D., Seidel Danila, Cornely Oliver A., Chen Sharon, van Hal Sebastiaan, Kauffman Carol, Miceli Marisa H., et al. 2020. “Clinical Characteristics and Outcomes of Invasive Lomentospora Prolificans Infections: Analysis of Patients in the FungiScope Registry.” Mycoses 63 (5): 437–42. [DOI] [PubMed] [Google Scholar]
- Jenks Jeffrey D., White P. Lewis, Kidd Sarah E., Goshia Tyler, Fraley Stephanie I., Hoenigl Martin, and Thompson George R. 3rd. 2023. “An Update on Current and Novel Molecular Diagnostics for the Diagnosis of Invasive Fungal Infections.” Expert Review of Molecular Diagnostics, October, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd Sarah E., Chen Sharon C-A, Meyer Wieland, and Halliday Catriona L.. 2019. “A New Age in Molecular Diagnostics for Invasive Fungal Disease: Are We Ready?” Frontiers in Microbiology 10: 2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler Philipp, Bassetti Matteo, Chakrabarti Arunaloke, Chen Sharon C. A., Colombo Arnaldo Lopes, Hoenigl Martin, Klimko Nikolay, et al. 2021. “Defining and Managing COVID-19-Associated Pulmonary Aspergillosis: The 2020 ECMM/ISHAM Consensus Criteria for Research and Clinical Guidance.” The Lancet Infectious Diseases 21 (6): e149–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagier J-C, Papazian L., Fenollar F., Edouard S., Melenotte C., Laroumagne S., Michel G., et al. 2016. “Tropheryma Whipplei DNA in Bronchoalveolar Lavage Samples: A Case Control Study.” Clinical Microbiology and Infection: The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases 22 (10): 875–79. [DOI] [PubMed] [Google Scholar]
- Langouche Lennart, Aralar April, Sinha Mridu, Lawrence Shelley M., Fraley Stephanie I., and Coleman Todd P.. 2021. “Data-Driven Noise Modeling of Digital DNA Melting Analysis Enables Prediction of Sequence Discriminating Power.” Bioinformatics 36 (22-23): 5337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Haitao, Guo Meihong, Wang Congmin, Li Yibo, Fernandez Anne Marie, Ferraro Thomas N., Yang Rongya, and Chen Yong. 2020. “Epidemiological Study of Infections over the Past 23 Years.” Epidemiology and Infection 148 (July): e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd S. n.d. “Least Squares Quantization in PCM.” Accessed November 5, 2023. https://ieeexplore.ieee.org/document/1056489. [Google Scholar]
- MacQueen James B. 1966. Some Methods for Classification and Analysis of Multivariate Observations. [Google Scholar]
- Mah Jordan, Nicholas Veronica, Tayyar Ralph, Moreno Angel, Murugesan Kanagavel, Budvytiene Indre, and Banaei Niaz. 2023. “Superior Accuracy of Aspergillus Plasma Cell-Free DNA PCR over Serum Galactomannan for the Diagnosis of Invasive Aspergillosis.” Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, July. 10.1093/cid/ciad420. [DOI] [PubMed] [Google Scholar]
- Meersseman Wouter, Lagrou Katrien, Maertens Johan, Wilmer Alexander, Hermans Greet, Vanderschueren Steven, Spriet Isabel, Verbeken Eric, and Van Wijngaerden Eric. 2008. “Galactomannan in Bronchoalveolar Lavage Fluid: A Tool for Diagnosing Aspergillosis in Intensive Care Unit Patients.” American Journal of Respiratory and Critical Care Medicine 177 (1): 27–34. [DOI] [PubMed] [Google Scholar]
- Nucci Marcio, Carlesse Fabianne, Cappellano Paola, Varon Andrea G., Seber Adriana, Garnica Marcia, Nouér Simone A., and Colombo Arnaldo L.. 2014. “Earlier Diagnosis of Invasive Fusariosis with Aspergillus Serum Galactomannan Testing.” PloS One 9 (1): e87784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Guansong, Shen Chunhua, Cao Longbing, and van den Hengel Anton. 2020. “Deep Learning for Anomaly Detection: A Review,” July. 10.1145/3439950. [DOI] [Google Scholar]
- Pappas Peter G., Lionakis Michail S., Arendrup Maiken Cavling, Ostrosky-Zeichner Luis, and Kullberg Bart Jan. 2018. “Invasive Candidiasis.” Nature Reviews Disease Primers 4 (1): 1–20. [DOI] [PubMed] [Google Scholar]
- Rabiner Lawrence, Rabiner Lawrence R., and Juang Biing-Hwang. 1993. Fundamentals of Speech Recognition. Prentice Hall. [Google Scholar]
- Ruff Lukas, Kauffmann Jacob R., Vandermeulen Robert A., Montavon Grégoire, Samek Wojciech, Kloft Marius, Dietterich Thomas G., and Müller Klaus-Robert. n.d. “A Unifying Review of Deep and Shallow Anomaly Detection.” Accessed November 5, 2023. https://ieeexplore.ieee.org/document/9347460. [Google Scholar]
- Sakoe H., and Chiba S.. n.d. “Dynamic Programming Algorithm Optimization for Spoken Word Recognition.” Accessed November 1, 2023. https://ieeexplore.ieee.org/abstract/document/1163055. [Google Scholar]
- Scharf Sebastian, Bartels Anna, Kondakci Mustafa, Pfeffer Klaus, Henrich Birgit, and Haas Rainer. 2020. “Introduction of a Bead Beating Step Improves Fungal DNA Extraction from Selected Patient Specimens.” International Journal of Medical Microbiology: IJMM 310 (6): 151443. [DOI] [PubMed] [Google Scholar]
- Seidel Danila, Meißner Arne, Lackner Michaela, Piepenbrock Ellen, Salmanton-García Jon, Stecher Melanie, Mellinghoff Sibylle, et al. 2019. “Prognostic Factors in 264 Adults with Invasive Scedosporium Spp. and Lomentospora Prolificans Infection Reported in the Literature and FungiScope.” Critical Reviews in Microbiology 45 (1): 1–21. [DOI] [PubMed] [Google Scholar]
- “Serology Anno 2021—fungal Infections: From Invasive to Chronic.” 2021. Clinical Microbiology and Infection: The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases 27 (9): 1230–41. [DOI] [PubMed] [Google Scholar]
- Sinha Mridu, Jupe Julietta, Mack Hannah, Coleman Todd P., Lawrence Shelley M., and Fraley Stephanie I.. 2018. “Emerging Technologies for Molecular Diagnosis of Sepsis.” Clinical Microbiology Reviews 31 (2). 10.1128/CMR.00089-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha Mridu, Mack Hannah, Coleman Todd P., and Fraley Stephanie I.. 2018. “A High-Resolution Digital DNA Melting Platform for Robust Sequence Profiling and Enhanced Genotype Discrimination.” SLAS Technology 23 (6): 580–91. [DOI] [PubMed] [Google Scholar]
- Springer Jan, White P. Lewis, Kessel Johanna, Wieters Imke, Teschner Daniel, Korczynski Daniel, Liebregts Tobias, et al. 2018. “A Comparison of Aspergillus and Mucorales PCR Testing of Different Bronchoalveolar Lavage Fluid Fractions from Patients with Suspected Invasive Pulmonary Fungal Disease.” Journal of Clinical Microbiology 56 (2). 10.1128/JCM.01655-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- “Target Genes, Primer Sets, and Thermocycler Settings for Fungal DNA Amplification.” 2022. October 13, 2022. https://www.cdc.gov/fungal/lab-professionals/settings-for-fungal-dna-amplification.html.
- Thompson George R. 3rd, Jenks Jeffrey D., Baddley John W., Lewis James S. 2nd, Egger Matthias, Schwartz Ilan S., Boyer Johannes, et al. 2023. “Fungal Endocarditis: Pathophysiology, Epidemiology, Clinical Presentation, Diagnosis, and Management.” Clinical Microbiology Reviews 36 (3): e0001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorano Anna Maria, Esposto Maria Carmela, Prigitano Anna, Grancini Anna, Ossi Cristina, Cavanna Caterina, and Cascio Giuliana Lo. 2012. “Cross-Reactivity of Fusarium Spp. in the Aspergillus Galactomannan Enzyme-Linked Immunosorbent Assay.” Journal of Clinical Microbiology 50 (3): 1051–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez Daniel Ortiz, Mack Hannah, Jupe Julietta, Hawker Sinead, Kulkarni Ninad, Hedayatnia Behnam, Zhang Yang, Lawrence Shelley, and Fraley Stephanie I.. 2017. “Massively Parallel Digital High Resolution Melt for Rapid and Absolutely Quantitative Sequence Profiling.” Scientific Reports 7 (February): 42326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- “Weighted kNN and Constrained Elastic Distances for Time-Series Classification.” 2020. Expert Systems with Applications 162 (December): 113829. [Google Scholar]
- “WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action.” 2022a. World Health Organization. October 25, 2022. https://www.who.int/publications/i/item/9789240060241. [Google Scholar]
- . 2022b. World Health Organization. October 25, 2022. https://www.who.int/publications/i/item/9789240060241.
- Yang Jingkang, Zhou Kaiyang, Li Yixuan, and Liu Ziwei. 2021. “Generalized Out-of-Distribution Detection: A Survey.” http://arxiv.org/abs/2110.11334. [Google Scholar]
- Zeller Iris, Schabereiter-Gurtner Claudia, Mihalits Verena, Selitsch Brigitte, Barousch Wolfgang, Hirschl Alexander M., Makristathis Athanasios, and Willinger Birgit. 2017. “Detection of Fungal Pathogens by a New Broad Range Real-Time PCR Assay Targeting the Fungal ITS2 Region.” Journal of Medical Microbiology 66 (10): 1383–92. [DOI] [PubMed] [Google Scholar]
- Zhu Alex, and Qi Chao. n.d. “Molecular Detection, Not Extended Culture Incubation, Contributes to Diagnosis of Fungal Infection.” 10.21203/rs.3.rs-55585/v2. [DOI] [PMC free article] [PubMed] [Google Scholar]