Abstract
One of the major barriers of fungal infections of mammals is the inability to grow and/or survive at mammalian body temperature, typically around 37°C. This has provided mammals an advantage over fungi. However, environmental fungi may soon adapt to persist at higher temperatures, consistent with mammalian body temperature, due to thermal selection pressures imposed by climate change, global warming, and increased frequency of extreme heat events. Consequently, there is a need for more updated information about the thermal tolerance range of fungi near humans, such as in urban areas. The heat island effect suggests that cities are up to 8°C warmer than their suburban counterparts because of increased heat production, asphalt coatings and reduced greenspace among other factors, and it is more common in lower income and marginalized urban communities. Thus, urban centers are at increased risk for the emergence of heat tolerant fungi. In this study, we developed a methodology to collect and archive fungal isolates from sidewalk and soil samples in both warmer and cooler neighborhoods in Baltimore, Maryland. We demonstrate a novel methodology for fungal sample collection from sidewalks, employing the use of standardized and commercially available taffy. Analysis of fungal isolates collected from warmer neighborhoods revealed greater thermal tolerance and lower pigmentation, suggesting local adaptation to heat. Lower pigmentation in hotter areas is consistent with the notion that fungi use pigmentation to help regulate their temperature. Further, we identified the robust presence of the polyextremotolerant fungus Aureobasidium pullalans from the warmest neighborhood in Baltimore, further showing that the extreme conditions of cities can drive proliferation of extremotolerant fungi. This study develops new techniques for environmental fungal collection and provides insight on the fungal census in an urban setting that can inform future work to study how urban environments may drive stress/thermotolerance in fungi, which could alter fungal interactions with humans and impact human health.
Keywords: Urban fungi, climate change, disaster microbiology, thermotolerance, pathogenic potential, heat island effect
Introduction
Natural and human-made disasters have been associated with adaptive changes in environmental that can lead to diseases in humans, plants, and animals [1]. Global warming is hypothesized to be associated with the observed emergence of new human fungal pathogens, such as Candida auris [2,3]. In this theory, global warming and extreme heat (including prolonged and more frequent heatwaves), will drive new thermotolerance of environmental fungi, leading to their ability to survive within mammalian hosts and those with pathogenic potential will acquire the capacity for human virulence by virtue of being able to grow at higher temperatures. Today, most fungi are unable to survive at or above human body temperature, and analyzes of yeast thermal tolerance has shown that there is a 6% reduction in the fungal species able to survive at each degree above 30°C [4,5]. In recent decades we have seen the sharp emergence of new fungal pathogens such as Candida auris and Sporothrix brasiliensis, a phenomenon that has been attributed to gained thermotolerance [2,6]. Analysis of environmental fungi deposited at the Westerdijk Fungal Diversity Institute shows that fungi deposited in recent decades have a higher maximum growth temperature than those in decades prior, potentially showing the shift of gaining thermotolerance [4].
To anticipate and understand future emerging fungal pathogens, it is important to know what fungi are found in areas conducive to emergence, for example, in areas where there is heavy environmental use of antifungals (i.e. agriculture), or where there is high thermal selective pressure. Environment with high thermal selective pressure around the globe are urban centers. Cities and urban centers across the globe are subject to the “heat island effect” where the city tends to be nearly 5–8°C warmer than rural or suburban neighboring areas [7–10]. This warmer environment in cities is predominantly due to reduced greenspaces, blocked airflow, high heat absorbance of buildings, and heat production by machines and cars. Within cities, there is a large temperature difference even between neighborhoods, where some experience more severe heat than others, predominantly in marginalized communities [10–12]. This includes Baltimore, Maryland, where cooler neighborhoods are about 8°C cooler than the warmest neighborhoods.
Currently, there is a lack of many studies investigating the mycobiome of urban centers, in part, some of the lack of mycobiome analysis in areas where there is ample bacterial microbiome analysis is due to technical difficulties in performing metagenomic analysis of fungal DNA and aligning sequences [13,14]. There are even fewer studies studying the heat-resistance and phenotypic profiles of urban fungi. A previous study has shown that filamentous fungi isolated from urban sites in Louisville, Kentucky have faster rates of growth and have higher enzymatic activity at higher temperatures than the fungi of the same species isolated from rural sites nearby Louisville [15]. This highlights the need for more functional characterization of urban mycobiome and a more detailed characterization of how heat affects urban fungal populations.
Here, we explore the urban fungal census from sidewalks across thermally diverse neighborhoods in Baltimore, Maryland using a new technique for collection, and show examples of genotypic and phenotypic characterization of the fungi present, including pigmentation variation and the presence of polyextremophilic yeasts.
Materials and Methods
Sample Site and Date Selection
To collect samples from thermally diverse neighborhoods in Baltimore, MD, we relied on publicly available data showing heat disparities across Baltimore City in 2018, in a study supported by the National Oceanographic and Atmospheric Administration (NOAA) [16]. For warm neighborhood sites, we chose the corner of N. Wolfe St and E. Fayette St. and the corner of N. Charles St. and Washington Park East; for an average neighborhood site we chose the corner of S. Wolfe St., and Fell St, and for a cool neighborhood we chose the corner of N. Charles St. and St. Paul St. We collected samples during sunny weather, at least 3 days after last precipitation on August 20th, 2023 and September 4th, 2023 between 12 P.M. and 2 P.M. Thermal images of each site were taken using an FLIR C2 IR camera, and images were processed using FIJI (ImageJ) IRimage plugin [17].
Sidewalk Sample Collection
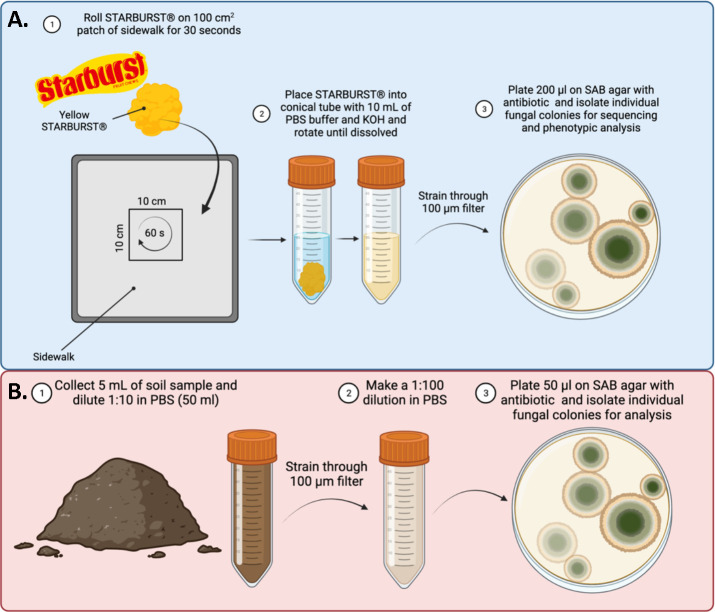
To collect fungal samples from sidewalks, we developed a collection protocol using Starburst taffy (Mars Corporation, VA, USA) (Figure 1A). Taffy is soft, sticky and easy adapts its contour to that of environmental surfaces. To collect environmental fungi found in the nooks and crannies of the sidewalk, we rolled a yellow Starburst taffy in an approximately 100 cm2 area along the sidewalk for 30 seconds, making sure to press the taffy into the pavement. We then added the taffy to 10 mL PBS containing 60 μl of 25% KOH solution to neutralize the citric acid in the candy upon dissolving. After the taffy was dissolved and all the sidewalk fungi were released into solution, we strained the solution through a 100 μm filter to remove debris. We then removed 200 μl aliquots and spread them on Sabouraud Dextrose Agar (SDA) with 2X Penicillin-Streptomycin solution. Samples were incubated at room temperature (22°C)
Figure 1. Sample Collection Methods.
A. Fungal sample collection from the sidewalk via Starburst. B. Fungal sample collection from dirt.
Dirt Sample Collection
Dirt samples were collected by scrapping the top layer of dirt in the tree or garden patches interspersed in the sidewalk, or dirt/grass adjacent to the curb. At least 5 mL of dirt was collected. The 5 mL of dirt was added to 45 mL PBS, solubilized at room temperature, and passed through a 100 μm strainer. Strained dirt was then diluted 1:100 in PBS and 50 μl of diluted dirt was added to SDA plates with antibiotic and incubated at room temperature (22°C). Isolation scheme summarized in Figure 1B.
Colony Isolation
Five plates from each sample were typically streaked, and grown room temperature. Each day, plates were examined for fungal growth, both filamentous and yeast like. Upon growth of a yeast-like colony (small, circular, non-filamentous), the colony was picked with a pipette tip and transferred to a Yeast Peptone Dextrose (YPD) agar plate. This was repeated daily for 7 days. Picked colonies were labelled according to location and source. Once the cultures grew on YPD plates at room temperature, they were observed microscopically to confirm they were of fungal origin by size, morphology, and appearance of intracellular organelles and not bacterial. Isolate appearance was catalogued.
Isolates were then transferred to a 96-well plate with freezing media (25% glycerol, 75% YPD broth), and stored at −80°C. Another plate was made using sterile PBS and stored at 4°C. All original plates were wrapped in parafilm and stored at 4°C.
Pigmentation Analysis
Pigmentation of the fungi was performed as previously described [18]. To measure the pigmentation of molds, each original sample plate was scanned using a Canon CanoScan9000F at 600 dpi. To measure the pigmentation of yeast isolates, the frozen stocks stored at −80°C were stamped onto YPD agar in an OmniWell agar plate using a flame-sterilized microplate replicator (Boekel Scientific) and grown at room temperature. Grown yeast colonies were scanned using the CanoScan9000F at 600 dpi. The mean gray value of mold and yeast colonies were measured using FIJI (ImageJ) measure tool.
Thermal Absorbance Analysis
Petri dishes with mold cultures were acclimated to room temperature for an hour (22°C). Petri dishes were unlidded and exposed to a pre-warmed 19W 4000K white light bulb at a distance of 50 cm for 10 minutes. Following the 10 min exposure, plates were imaged immediately using an FLIR E96 infrared camera. Images were processed using FIJI (ImageJ) IRimage plugin [17], and temperature measurements were recorded for each mold colony. Mean gray value measurements were taken using the visible light spectrum image taken by the FLIR E96 camera at time of experiment.
Internal Transcribed Spacer (ITS) Sequencing
Each isolate was touched lightly with a pipette tip and transferred to an empty PCR tube and deposited along the inside of the tube in a thin layer. The tubes were microwaved on high for 2 minutes as previously described [19]. DreamTaq Green PCR Master Mix (ThermoFisher) was added to each tube with 0.2 μM ITS4 and ITS5 primers to amplify the internal transcribed sequences. The sequences of these primers are as follows: ITS4 – 5′-TCCTCCGCTTATTGATATGC-3′ and ITS5 – 5′-GGAAGTAAAAGTCGTAACAAGG-3′. PCR products were amplified according to the manufacturer’s protocol; 3 min initial denaturation at 95°C, 35 cycles with 30 s denaturation at 95°C, 30 s annealing at 55°C, and 1 min extension at 78°C, with a final 5 min extension at 78°C. Samples were run on 1% agarose gel to confirm product amplification, and Sanger sequenced at the Johns Hopkins School of Medicine Sequencing & Synthesis Core facility. Resulting sequences were analyzed using 4Peaks software and NCBI Blast nucleotide feature.
Results
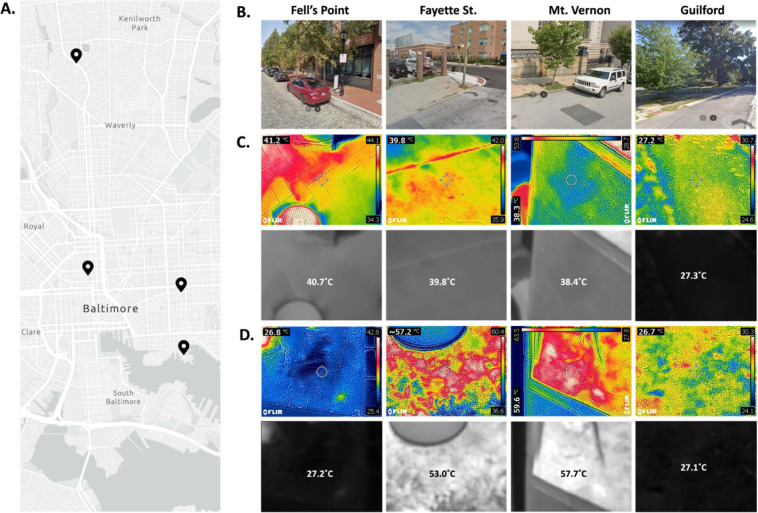
We sampled four locations across Baltimore City in late-summer afternoons, with an average outdoor temperature of 30°C. Our sample sites represented both warmer and cooler neighborhoods and were representative of the approximate environment of the neighborhoods (Figure 2A, B). As expected, the soil and sidewalk collection sites from N. Wolfe St and E. Fayette St (Fayette St.) and N. Charles St. and Washington Park East (Mt. Vernon) had the warmest environments, with the soil samples at either site reaching up to 55°C on average, with some spots reaching 60°C (Figure 2C). Both the sidewalk sites in these locations were slightly cooler at ~40°C (Figure 2D). S. Wolfe St. and Fell St. (Fell’s Point) had a cool soil around ambient temperature (27°C), while the sidewalk sample was warmer at ~40°C (Figure 2C, D). Both sidewalk and dirt samples from N. Charles St. and St. Paul St. (Guilford) were ~27°C.
Figure 2. Thermal Properties of Sampling Sites in Baltimore, MD.
A. Map of the four sites sampled in Baltimore, MD (counter-clockwise from the bottom: Fell’s Point, Fayette, Guilford, Mt. Vernon). B. Google StreetView images from the sampling sites taken during summer months in recent years. Infrared images of the sidewalks (C) and dirt (D) on August 20th, 2023 with visible light photo overlayed (top rows), and the raw temperature image standardized to 27°C (min) and 60°C (max) with the average temperature measured overlayed (bottom rows).
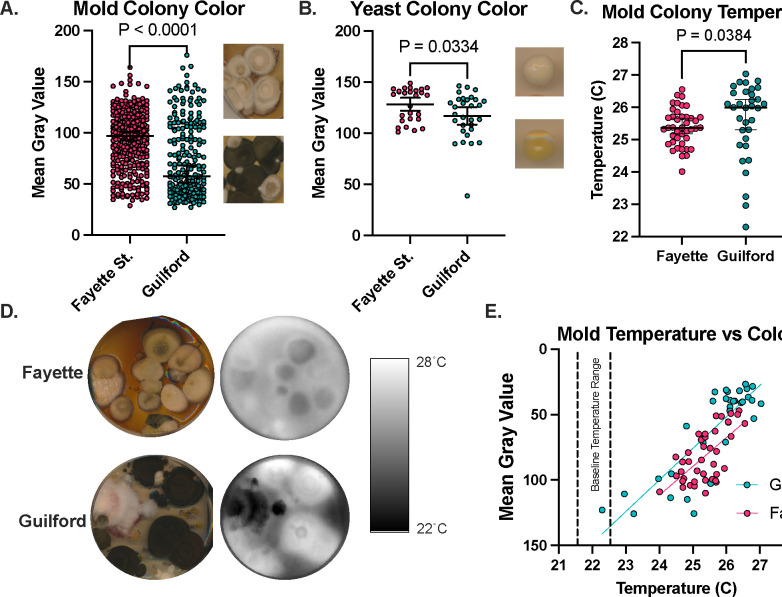
We successfully isolated fungi from all four sites, including the sidewalk and dirt from Fayette St. and Mt. Vernon despite the exceedingly high surface temperatures. We found that the mold and yeast isolates from Fayette St. were significantly less pigmented/lighter in color than those from Guilford from both isolation days (Figure 3A, B). Overall, the mold colonies found in Guilford absorbed more heat than the mold Fayette St. (Figure 3C, D). There was a correlation between temperature following exposure to 10 minutes of white light and the mean gray value of the colonies (Figure 3E) indicating some evidence of thermal melanism or thermal albinism, as these phenomena have been called, to regulate thermal properties of the fungi. This has been previously described as it relates to fungi of different latitudes, where fungi from arctic regions display enhanced pigmentation to promote head absorbance, while equatorial fungi have less pigmentation to avoid too much heat absorbance [18].
Figure 3. Fungi collected in thermally diverse neighborhoods exhibit thermal and pigment differences.
The mean gray value of mold (A) and yeast (B) isolated from Fayette St. is higher than the mean gray value of the fungi isolated from Guilford, indicating less pigmentation in the Fayette St. fungi. (C,D) Following exposure to light, the Guilford samples get warmer than the Fayette samples. (E) There is a correlation between colony temperature and the mean gray value, where the darker colonies get warmer. A,B show mean values with 95% CI and t-tests, while C shows median temperature and non-parametric Mann-Whitney test.
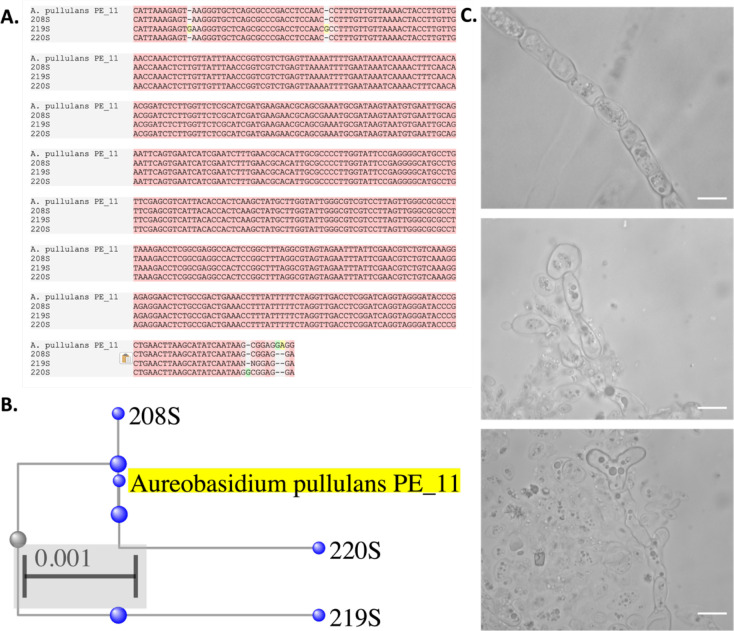
Of the 42 yeast-like isolates collected from Fayette St. in our study, 15 (35%) of them are identified as Aureobasidium pullulans, representing 45% of the total yeast-like fungi isolated from the August 20th sidewalk samples. The identity of this fungus was established using ITS sequencing, showing conserved ITS sequence to the A. pullulans PE_11 strain (Figure 4A, B). A. pullulans is notable as it is a polyextremotolerant fungus, including temperature, saline stress, and nutrient stress, having first been isolated in glacial ice [20]. Microscopically, we observed A. pullulans to have diverse morphology with hyphae, yeast-like structures, and other abnormal shapes (Figure 4C). Identification of this species in a neighborhood experiencing the most extreme heat in Baltimore City is thus a notable finding and places further context in this fungus’ role as a potential future human pathogen.
Figure 4. Identification of Aureobasidium pullulans from High-Temperature Environment.
A. alignment of three of the 15 A. pullulans cultures isolated from Fayette St. sidewalk (208S, 219S, 220S) to the A. pullulans PE_11 isolate, and their phylogenetic relationship (B). (C) Microscopic images of the A. pullulans cultures show hyphal, yeast-like, and irregular shaped. Scale bar represents 20 μm.
Also notable was the presence of Cystofilobasidium macerans, Rhodotorula spp., Cystobasidium spp., and other Cystobasidiumycetes at all the sampled sites (Supplementary File 1). This allows the comparison of properties of the same species of fungi when exposed to different environmental pressures. For example, Cystobasidium spp., a rare opportunistic fungal pathogen [21], was found at Fayette St., Mt. Vernon, and Guilford, but only the C. minutum isolate recovered from the 40°C sidewalk in Mt. Vernon (304S) was thermotolerant (able to grow at 37°C), while the C. lysinophilum isolate from Fayette St. (242D) and C. minutum isolate from Guilford (403S) were unable to grow at high temperatures (Figure 5).
Figure 5. Thermotolerance of Cystobasidium spp.
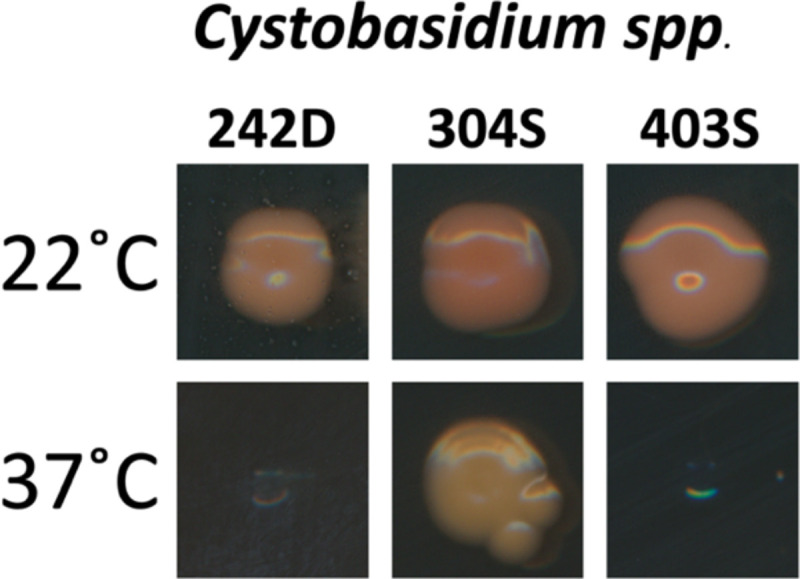
Cystobasidium spp. isolated from Fayette St. dirt (242D) and Guilford sidewalks (403S) were unable to grow at 37°C, but C. minutum isolated from Mt. Vernon sidewalk (304S) were thermotolerant at 37°C.
Discussion
As areas vulnerable to extreme heat conditions, cities are crucibles for the adaptation of fungi to warmer temperature, and consequently, are potential crucibles for the emergence of new human fungal pathogens. Here we showed a novel way to collect and culture environmental fungi from sidewalks in Baltimore, MD. We used this technique to demonstrate that fungi from warmer neighborhoods within cities exhibited different thermal and pigmentation difference. Dark pigments in fungi are often melanins and this pigments have been shown to have a remarkable capacity to absorb electromagnetic radiation and convert it to heat[18,22]. Differences in pigmentation have been previously demonstrated on a global scale, where equatorial fungi and polar fungi are lighter and darker respectively, and our study shows that the extreme ranges in heat in neighborhoods within urban centers may be enough to drive similar differences [18]. This finding suggests that the thermal microclimates of an urban environment have strong/varied selective pressures and are similar to those found on a global scale. The temperatures of the sidewalk and dirt in the warmer Fayette St. location were 40°C and 55°C, respectively, which are both above the typical temperature ranges of fungi, and above human body temperature (37°C), thus providing a selective pressure to survive at mammalian body temperature. Meanwhile, the temperatures at the cooler, more wooded and shaded, Guilford neighborhood were around 27°C, and thus did not provide a selective pressure to grow at or above mammalian body temperature.
A prior study suggested that city-dwelling fungi are better equipped at growing at higher temperatures and have better enzymatic function at higher temperatures compared with rural counterparts of the same fungal species [15]. Our findings are consistent with that study and suggest the need to consider heat islands in cities as sites for rapid fungal thermal evolution. It is hypothesized that global warming has led to the recent emergence of the fungal pathogen Candida auris [2],and our findings suggest the need for increased vigilance of urban fungi with pathogenic potential.
We also demonstrated the strong presence of the extremotolerant fungus A. pullulans in neighborhoods with high thermal stress. This fungus is capable of surviving saline, thermal, and nutrient-poor stresses, and has been shown to be a rare opportunistic human pathogen [23–25]. The presence of this fungus is thus notable as it indicates the selection for extremotolerant fungal populations, which may correlate to ability to tolerate other stressors including mammalian immune response. Gostinčar et. al. discuss the importance of A. pullulans as a potential emerging pathogen due to its ability to resist extreme conditions, hyperplasticity, and its presence within the indoor built environment [20].
The previous studies on urban fungi [15] along with our data indicate that studying fungi cities can prove valuable to our understanding of how fungi will continue to adapt to our changing climate. In addition, studying urban fungi and thermal properties of the species found may help identify rising threats to human health, including precursors to new pathogens. Many of these neighborhoods experiencing extreme heat vulnerability (and the risk of emerging thermotolerance in fungi), are also often marginalized communities [10,12,26]. This is particularly worrisome, as these communities are also the ones already most vulnerable to fungal infection due to healthcare disparities and other social determinants of health [27].
Following the proof-of-principle exploration of urban fungi demonstrated in this study, we will be able to better collect, understand, and characterize urban fungi and evaluate their stress and temperature resistances. This will enable and bolster future efforts to related to urban disaster mycology and performing surveillance for new fungi with pathogenic potential as they emerge in cities.
Supplementary Material
Acknowledgements:
We would like to acknowledge CJ, Gracen, and Jenna for the assistance and support in collecting the fungal samples, Dr. Radames Cordero for the guidance related to thermal melanism in the fungal isolates, and Dr. Madhura Kulkarni for writing edits and suggestions. Figure 1 was made using BioRender.com. A.C. and D.F.Q.S. were supported in part by NIH grants AI162381, AI152078, and HL059842. Funders played no role in the experimental design or outcome of the project.
References:
- 1.Smith DFQ, Casadevall A. Disaster Microbiology—a New Field of Study. mBio. 2022;13: e01680–22. doi: 10.1128/mbio.01680-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casadevall A, Kontoyiannis DP, Robert V. On the Emergence of Candida auris: Climate Change, Azoles, Swamps, and Birds. mBio. 2019;10. doi: 10.1128/mBio.01397-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Solache MA, Casadevall A. Global Warming Will Bring New Fungal Diseases for Mammals. mBio. 2010;1: e00061–10. doi: 10.1128/mBio.00061-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robert V, Cardinali G, Casadevall A. Distribution and impact of yeast thermal tolerance permissive for mammalian infection. BMC Biology. 2015;13: 18. doi: 10.1186/s12915-015-0127-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert VA, Casadevall A. Vertebrate Endothermy Restricts Most Fungi as Potential Pathogens. The Journal of Infectious Diseases. 2009;200: 1623–1626. doi: 10.1086/644642 [DOI] [PubMed] [Google Scholar]
- 6.Valdez AF, Corrêa-Junior D, Bonilla JJA, Zamith-Miranda D, Frases S, Freitas DFS, et al. A Review on Sporotrichosis and the Emergence of Sporothrix brasiliensis as a Pathogen. Curr Trop Med Rep. 2023. [cited 20 Oct 2023]. doi: 10.1007/s40475-023-00297-6 [DOI] [Google Scholar]
- 7.Learn About Heat Islands | US EPA. [cited 11 May 2022]. Available: https://www.epa.gov/heatislands/learn-about-heat-islands
- 8.Sharma R, Hooyberghs H, Lauwaet D, De Ridder K. Urban Heat Island and Future Climate Change—Implications for Delhi’s Heat. J Urban Health. 2019;96: 235–251. doi: 10.1007/s11524-018-0322-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sachindra DA, Ng AWM, Muthukumaran S, Perera BJC. Impact of climate change on urban heat island effect and extreme temperatures: a case-study. Quarterly Journal of the Royal Meteorological Society. 2016;142: 172–186. doi: 10.1002/qj.2642 [DOI] [Google Scholar]
- 10.Saverino KC, Routman E, Lookingbill TR, Eanes AM, Hoffman JS, Bao R. Thermal Inequity in Richmond, VA: The Effect of an Unjust Evolution of the Urban Landscape on Urban Heat Islands. Sustainability. 2021;13: 1511. doi: 10.3390/su13031511 [DOI] [Google Scholar]
- 11.Metzger KB, Ito K, Matte TD. Summer Heat and Mortality in New York City: How Hot Is Too Hot? Environmental Health Perspectives. 2010;118: 80–86. doi: 10.1289/ehp.0900906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Popovich N, Flavelle C. Summer in the City Is Hot, but Some Neighborhoods Suffer More. The New York Times. 9 Aug 2019. Available: https://www.nytimes.com/interactive/2019/08/09/climate/city-heat-islands.html. Accessed 5 Nov 2023.
- 13.Tessler M, Neumann JS, Afshinnekoo E, Pineda M, Hersch R, Velho LFM, et al. Large-scale differences in microbial biodiversity discovery between 16S amplicon and shotgun sequencing. Sci Rep. 2017;7: 6589. doi: 10.1038/s41598-017-06665-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nilsson RH, Anslan S, Bahram M, Wurzbacher C, Baldrian P, Tedersoo L. Mycobiome diversity: high-throughput sequencing and identification of fungi. Nat Rev Microbiol. 2019;17: 95–109. doi: 10.1038/s41579-018-0116-y [DOI] [PubMed] [Google Scholar]
- 15.McLean MA, Angilletta MJ, Williams KS. If you can’t stand the heat, stay out of the city: Thermal reaction norms of chitinolytic fungi in an urban heat island. Journal of Thermal Biology. 2005;30: 384–391. doi: 10.1016/j.jtherbio.2005.03.002 [DOI] [Google Scholar]
- 16.Detailed maps of urban heat island effects in Washington, DC, and Baltimore | NOAA Climate.gov. [cited 5 Nov 2023]. Available: http://www.climate.gov/news-features/features/detailed-maps-urban-heat-island-effects-washington-dc-and-baltimore
- 17.Pereyra Irujo G. IRimage: open source software for processing images from infrared thermal cameras. PeerJ Comput Sci. 2022;8: e977. doi: 10.7717/peerj-cs.977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cordero RJB, Robert V, Cardinali G, Arinze ES, Thon SM, Casadevall A. Impact of Yeast Pigmentation on Heat Capture and Latitudinal Distribution. Curr Biol. 2018;28: 2657–2664.e3. doi: 10.1016/j.cub.2018.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang MY, Joshi MB, Boucher MJ, Lee S, Loza LC, Gaylord EA, et al. Short homology-directed repair using optimized Cas9 in the pathogen Cryptococcus neoformans enables rapid gene deletion and tagging. Genetics. 2022;220: iyab180. doi: 10.1093/genetics/iyab180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gostinčar C, Grube M, Gunde-Cimerman N. Evolution of Fungal Pathogens in Domestic Environments? Fungal Biology. 2011;115: 1008–1018. doi: 10.1016/j.funbio.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 21.Inácio CP, Diniz MV, Araújo PSR, Barros MS, Andrade MCL, Lima-Neto RG, et al. Bloodstream Infection of a Cancer Patient by Cystobasidium minutum: A Case Report and Literature Review. Mycopathologia. 2020;185: 395–398. doi: 10.1007/s11046-019-00415-x [DOI] [PubMed] [Google Scholar]
- 22.Cordero RJB, Casadevall A. Functions of fungal melanin beyond virulence. Fungal Biology Reviews. 2017;31: 99–112. doi: 10.1016/j.fbr.2016.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolignano G, Criseo G. Disseminated Nosocomial Fungal Infection by Aureobasidium pullulans var. melanigenum: a Case Report. Journal of Clinical Microbiology. 2003;41: 4483–4485. doi: 10.1128/jcm.41.9.4483-4485.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawkes M, Rennie R, Sand C, Vaudry W. Aureobasidium pullulans infection: Fungemia in an infant and a review of human cases. Diagnostic Microbiology and Infectious Disease. 2005;51: 209–213. doi: 10.1016/j.diagmicrobio.2004.10.007 [DOI] [PubMed] [Google Scholar]
- 25.Pikazis D, Xynos ID, Xila V, Velegraki A, Aroni K. Extended fungal skin infection due to Aureobasidium pullulans. Clinical and Experimental Dermatology. 2009;34: e892–e894. doi: 10.1111/j.1365-2230.2009.03663.x [DOI] [PubMed] [Google Scholar]
- 26.Reckien D, Lwasa S, Satterthwaite D, McEvoy D, Creutzig F, Montgomery M, et al. Equity, Environmental Justice, and Urban Climate Change. 1st ed. In: Rosenzweig C, Solecki WD, Romero-Lankao P, Mehrotra S, Dhakal S, Ali Ibrahim S, editors. Climate Change and Cities. 1st ed. Cambridge University Press; 2018. pp. 173–224. doi: 10.1017/9781316563878.013 [DOI] [Google Scholar]
- 27.Jenks JD, Aneke CI, Al-Obaidi MM, Egger M, Garcia L, Gaines T, et al. Race and ethnicity: Risk factors for fungal infections? PLOS Pathogens. 2023;19: e1011025. doi: 10.1371/journal.ppat.1011025 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.