Abstract
Background:
Overlapping symptoms from cardiomyopathy, respiratory insufficiency, and skeletal myopathy confound assessment of heart failure in Duchenne Muscular Dystrophy. We developed an ordinal scale of multiorgan clinical variables that reflect cumulative disease burden—the Major Adverse Dystrophinopathy Event (MADE) Score. We hypothesized that a higher MADE score would be associated with increased mortality in boys with Duchenne Muscular Dystrophy. The Cooperative International Neuromuscular Research Group Duchenne Natural History Study dataset was utilized for validation.
Methods:
Duchenne Natural History Study variables were selected based on clinical relevance to prespecified domains: Cardiac, Pulmonary, Myopathy, Nutrition. Severity points (0–4) were assigned and summed for study visits. MADE score for cohorts defined by age, ambulatory status, and survival were compared at enrollment and longitudinally.
Associations between MADE score and mortality were examined.
Results:
Duchenne Natural History Study enrolled 440 males, 12.6 ±6.1 years old, with 3,559 visits over 4.6 ±2.8 years, 45 deaths. MADE score increased with age and nonambulatory status. Mean MADE score per visit was 19 ±10 for those who died vs. 9.8 ±9.3 in survivors p=0.03. Baseline MADE score >12 predicted mortality independent of age (78% sensitivity, CPE.70). Rising MADE score trajectory was associated with mortality in models adjusted for enrollment age, follow-up time, and ambulatory status, all p<.001.
Conclusion:
A multiorgan severity score, MADE, was developed to track cumulative morbidities that impact heart failure in Duchenne muscular dystrophy. MADE score predicted Duchenne Natural History Study mortality. MADE score can be used for serial heart failure assessment in males and may serve as an endpoint for Duchenne muscular dystrophy clinical research.
Keywords: Duchenne muscular dystrophy, heart failure, dystrophinopathy, mortality risk predictor
Introduction:
Duchenne muscular dystrophy (DMD) is a progressive neuromuscular disorder due to X-linked mutation in the gene encoding dystrophin. The resulting dystrophinopathy causes progressive myocyte injury in skeletal, smooth and cardiac muscles. This results in loss of ambulation, respiratory insufficiency and cardiomyopathy manifesting during childhood into adolescence [1–5]. Heart failure typically manifests in the non-ambulatory phase of DMD, often presenting as late stage with acute decompensation and multiple comorbidities as adolescents [6–9]. Heart failure is the most common cause of death in the current era, accounting for 30–50% of mortality [7,8]. Earlier identification of heart failure, which can have diverse manifestations, can lead to more timely applications of therapeutic interventions to improve disease outcomes. However multisystem interactions and overlapping symptoms of DMD morbidities make accurate assessments of heart failure severity challenging [7]. Additionally, traditional heart failure assessments [10] (ex. New York Heart Association Class, exercise stress tests), are in context of exertional ability and are confounded by severe muscle weakness. Currently there is no objective method to assess heart failure progression for the non-ambulatory population. Unique assessment tools to determine interactions of cardiopulmonary pathology and functional status are needed to optimize cardiac care and to advance DMD cardiomyopathy research [11–13]. The objective of this study is to describe and validate a multisystem score developed to reflect cumulative burden of progressive dystrophinopathy that affects heart failure in DMD males, the Major Adverse Dystrophinopathy Event (MADE) Score.
METHODS
Score development
Initial domain development was by multidisciplinary DMD clinicians. The Major Adverse Dystrophinopathy Event (MADE) Score is comprised of four clinical DMD conditions that occur consistently, are progressive in severity, and impact both functional status and heart failure symptoms: Cardiomyopathy, Respiratory Insufficiency, Myopathy, and Nutritional status alteration (Table 1). These conditions, or domains, will be referred to as Major Adverse Dystrophinopathy Events. Each MADE has components representing clinical events, treatments, diagnostic test measurements, and patient reported symptoms and outcome measures (PROM). An ordinal scale based on severity (0–4) was assigned to each MADE component utilizing Common Terminology Criteria for Adverse Events (CTCAE) v 5 [14] where applicable, or categorized and ranked by expert clinician opinion [4,15]. Total MADE Score is determined at any point in time, a follow-up clinic visit for example, by assessing for the presence of each Score variable since last assessment and assigning the pre-specified severity points per the Clinical MADE Template Table 1. One score (0–4) should be assigned per variable that corresponds to highest severity present. To compute MADE Score for that visit, the points for all variables, across all 4 domains are added. A higher MADE score represents a higher burden of DMD morbidities. (Clinical examples, Table 2) Established cardiovascular adverse clinical event scores: MACE -major adverse cardiovascular event [16–17], MATE – major adverse transplant event [18], and pediatric acute heart failure symptom score [19], were used as paradigms for MADE Score development.
TABLE 1.
Clinical Template MADE Score
| MADE DOMAIN
Variables Total 92 pts max |
MADE Points by Severity Category | ||||||
|---|---|---|---|---|---|---|---|
| Variable | None =0 | Mild =1 | Moderate=2 | Severe=3 | Critical=4 | Variable Score 0–4 | |
| VAD Surgery | No | Yes | |||||
| Congestive heart failure hospitalization | No | Yes | |||||
| Congestive heart failure - outpatient management (Diuretic titration) | No | Yes | |||||
| Syncope | No | Yes | |||||
| Arrhythmia | No | -Sinus tachycardia OR -Ventricular couplets or triplets |
-NSVT OR -Asymptomatic Atrial tachycardia |
-Sustained VT (HD stable)
OR- Symptomatic Atrial tachycardia |
-Cardiac Arrest OR -Vfib OR -VT w/HD compromise |
||
| Ejection Fraction or Shortening Fraction (Echo/MRI) | EF ≥55%or SF≥26% |
EF 45–54% SF 20–25% |
EF 35–44% SF 15–19% |
EF <35% SF<15% |
|||
| Cardiac MRI | Delayed Enhancement or other abnormality | ||||||
| IV Inotropic medication (milrinone, dopamine) | No | Intermittent/transient | Inotrope Dependent | ||||
| ACEI/ARB/ARNI use | No | prophylactic | Symptomatic therapy (LV dilation, MR) | ||||
| Beta Blocker medication use | No | prophylactic | Symptomatic therapy (arrhythmia) | ||||
| Chest pain | No | Yes | |||||
| Palpitations/Racing heart beat | No | Yes | |||||
| Dizziness | No | Yes | |||||
| Clinical Variable | None or Not reported =0 | Mild =1 | Moderate=2 | Severe=3 | Critical=4 | Variable Score 0–4 | |
| Ambulatory or wheelchair full time (nonambulatory) | Ambulatory | Nonambulatory (wheelchair full time) | |||||
| Age at full time wheelchair use (years) | >13 yrs old | 10–12 yrs old | <9 yrs old | ||||
| Vignos Scale for lower extremity function* | Vignos 1–2 Walks, Climbs stairs | Vignos 3–4 Toe Walks, no stairs or chair rise | Vignos 5–7 Nonambulatory, wheelchair dependent | Vignos 8 Bed bound | |||
| Brooke Scale for upper extremity function* | Brooke 1 Raises arms above head no limits | Brooke 2 Limited arm raise , bent elbows above head | Brooke 3–4 Can raise cup to mouth, feed self | Brooke 5–6 5/can drive wheelchair
OR 6/No useful function of hands |
|||
| Dysphagia | none | Minimal/rare | Choking/rest rictions on intake | Can’t handle secretions | |||
| Fatigue | -Upright all day (if nonamb
ulatory) -Full day activities tolerated |
-Fatigue after activity relieved by rest | -Fatigue not relieved by rest limiting instrumental ADLs (school, work, recreation) | -Bed bound 24/7 OR -Fatigue limiting self care ADLs (feeding, bathing, toileting) |
|||
| Clinical Variable | None or Not reported =0 | Mild =1 | Moderate=2 | Severe=3 | Critical=4 | Variable Score 0–4 | |
| Significant problem with weight loss (malnutrition) | No | Yes | |||||
| Significant problem with weight gain(obesity) | No | Yes | |||||
| Formula/caloric enteral supplements | No | As needed | Daily | ||||
| Parenteral Nutrition | No | Yes | |||||
| Poor appetite | No | Yes | |||||
| Early satiety | No | Yes | |||||
| Vomiting | No | Yes | |||||
| Abdominal Pains | No | Yes | |||||
| Clinical Variable | None or Not reported =0 | Mild =1 | Moderate=2 | Severe=3 | Critical=4 | Variable Score 0–4 | |
| Pneumonia hospitalization (without intubation or escalation of baseline support beyond supplemental oxygen) | No | Yes | |||||
| Respiratory failure hospitalization | No | Yes | |||||
| FVC % Predicted value* | >75% | 61–75% | 51–60% | 31–50% | ≤30% | ||
| Cough-assistance therapies used | No | Yes | |||||
| Ventilatory Assistance | None | CPAP occasion al/willness | Nocturnal CPAP or Mouthpiece intermittent vent/BiPAP/VentTrach occasional w/illness | Nocturnal BiPap or Full time CPAP or intermit tent mouthpiece vent | Full time BiPAP or ventilation via trach | ||
| Supplemental Oxygen use | No | Occasional/with illness | Nocturnal | Full time | |||
| Asthma | No | Yes | |||||
| Problems snoring | No | Yes | |||||
| Excessive daytime sleepiness | No | Yes | |||||
| Difficulty breathing when lying flat | No | Yes | |||||
| TOTAL | |||||||
Abbreviations: ADL activities daily living, EF ejection fraction, ICD implantable cardioverter defibrillator, MRI magnetic resonance imaging, PPV positive pressure ventilation, TPN total parenteral nutrition, VT ventricular tachycardia
Brooke and Vignos standardized scales for upper and lower extremity strength1
Instructions: Assess for presence of variable since last assessment, or historically if initial evaluation. Assign one score per variable, highest category. MADE Score = total points for all variables
Table 2.
Clinical Examples for MADE Score determination
| 6 yo new diagnosis DMD, muscle cramps, toe walking, HR 90, echo normal, negative ROS | ||||||||
|---|---|---|---|---|---|---|---|---|
| CARDIAC | PULMONARY | MYOPATHY | NUTRITION | TOTAL MADE | ||||
| none | 0 | none | 0 | Toe walking | 1 | none | 0 | |
| Domain Points | 0 | 0 | 1 | 0 | 1 | |||
| 12 yo HR 110, echo normal, CMRI delayed enhancement, snores w OSA no therapy, ambulates in house, no stairs, limited arm raise, fatigue after activity, negative ROS | ||||||||
| CARDIAC | PULMONARY | MYOPATHY | NUTRITION | TOTAL MADE | ||||
| tachycardia | 1 | Snores (OSA) | 1 | Limited arm raise | 1 | none | 0 | |
| abnormal CMRI | 1 | Walks No stairs | 1 | |||||
| Fatigue improves with rest | 1 | |||||||
| Domain Points | 2 | 1 | 3 | 0 | 6 | |||
| 18 yo palpitations, HR 120, EF 30%, worsening dyspnea, BIPAP use 24 hours, last forced vital capacity 39%, anorexia, severe fatigue in bed, heart failure hospitalization for diuresis, milrinone | ||||||||
| CARDIAC | PULMONARY | MYOPATHY | NUTRITION | TOTAL MADE | ||||
| tachycardia | 1 | dyspnea at rest | 2 | confined to bed | 3 | anorexia | 2 | |
| palpitations | 2 | 24hour BIPAP | 4 | no use of hands | 3 | |||
| EF 30% | 4 | FVC 39% | 3 | |||||
| transient inotropes | 3 | |||||||
| CHF hospitalization | 4 | |||||||
| Domain Points | 14 | 9 | 6 | 2 | 31 | |||
Abbreviations: CHF congestive heart failure, CMRI cardiac magnetic resonance imaging, DMD Duchenne muscular dystrophy, HR heart rate, echo echocardiog ram, EF ejection fraction, FVC forced vital capacity, ROS review of systems, yo years old
MADE Score Validation
The MADE score was applied to the deidentified dataset from the Cooperative International Neuromuscular Research Group Duchenne Natural History Study (CINRG DNHS)[20] for initial validation and demonstration of clinical utility. Standardized data collection from DMD males was conducted at 22 international centers between 2006–2016. Study visits were every 3 months for a year, every 6 months up to 2 years, and then annually for up to 7 years. Data collected included: medical and surgical history, symptom report, and functional evaluations of muscle strength, pulmonary, and cardiac function obtained for clinical care[20]. Myopathy status was defined using the Brooke (upper extremity) and Vignos (lower extremity), well-validated standardized metrics used in patients with progressive neuromuscular disease [1]. All participating institutions obtained IRB protocol approval and informed consent from all participants.
Variables from DNHS were mapped to the four prespecified MADE domains and grouped accordingly. Case report form responses for the selected DNHS variable were assigned severity points, 0–4 mild to severe, at each study visit Table 3. Total MADE score for the subject’s study visit was the sum of all domain variable severity points (as modeled in Table 2).
TABLE 3.
MADE Points assigned to DNHS variables
| MADE DOMAIN DNHS Variables | MADE Points by Severity Category | |||||
|---|---|---|---|---|---|---|
| DNHS Variable | None or Not reported =0 | Mild =1 | Moderate=2 | Severe =3 | Critical=4 | |
| Cardiac surgery | No | Yes | ||||
| Congestive heart failure hospitalization | No | Yes | ||||
| Other cardiac health issue | No | Yes | ||||
| Congestive heart failure requiring medications | No | Yes | ||||
| Diuretic use | No | prophylactic | Symptomatic therapy | |||
| Inotropic medication (oral or intravenous) | No | prophylactic | Symptomatic therapy | |||
| Anti-arrhythmic medication | No | prophylactic | Symptomatic therapy | |||
| ACE inhibitor use | No | prophylactic | Symptomatic therapy | |||
| Beta Blocker medication use | No | prophylactic | Symptomatic therapy | |||
| Ejection Fraction or Shortening Fraction | EF ≥55%or SF≥26% |
EF 45–54% SF 20–25% |
EF 35–44% SF 15–19% |
EF <35% SF<15% |
||
| Chest pain | No | Yes | ||||
| Racing heart beat | No | Yes | ||||
| Dizziness | No | Yes | ||||
| DNHS Variable | None or Not reported =0 | Mild =1 | Moderate=2 | Severe =3 | Critical=4 | |
| Significant fatigue | No | Yes | ||||
| Ambulatory or wheelchair full time (nonambulatory) | Ambulatory | Nonambulatory | ||||
| Age at full time wheelchair use (years) | >13 yrs old | 10–12 yrs old | <9 yrs old | |||
| Vignos Scale for lower extremity function* | Vignos 1–2 | Vignos 3–4 | Vignos 5–7 | Vignos 8 | ||
| Brooke Scale for upper extremity function* | Brooke 1 | Brooke 2 | Brooke 3–4 | Brooke 5–6 | ||
| Difficulty swallowing | No | Yes | ||||
| DNHS Variable | None or Not reported =0 | Mild =1 | Moderate=2 | Severe =3 | Critical=4 | |
| Gastrointestinal surgery(feeding tube) | No | Yes | ||||
| gastrointestinal tract issues | No | Yes | ||||
| Formula/caloric supplements | No | As needed | Daily | |||
| Nasogastric tube use | No | As needed | Daily | |||
| Poor appetite | No | Yes | ||||
| Early satiety | No | Yes | ||||
| Nausea | No | Yes | ||||
| Indigestion | No | Yes | ||||
| Significant problem with weight loss | No | Yes | ||||
| Significant problem with weight gain | No | Yes | ||||
| Poor growth | No | Yes | ||||
| PULMONARY | DNHS Variable | None or Not reported =0 | Mild =1 | Moderate=2 | Severe =3 | Critical=4 |
| Pneumonia hospitalization | No | Yes | ||||
| Respiratory failure hospitalization | No | Yes | ||||
| FVC % Predicted value* | >75% | 61–75% | 51–60% | 31–50% | <30% | |
| Cough-assistance therapies used | No | Yes | ||||
| Any ventilatory assistance used | No | Yes | ||||
| Full time ventilatory assistance | No | Yes | ||||
| BiPAP use | No | Occasional/with illness | Nocturnal | Full time | ||
| C PAP use | No | Occasional/with illness | Nocturnal | Full time | ||
| Mouthpiece Intermittent ventilation | No | Occasional/with illness | Nocturnal | Full time | ||
| Negative pressure ventilation | No | Occasional/with illness | Nocturnal | Full time | ||
| Ventilation via tracheostomy | No | Occasional/with illness | Nocturnal | Full time | ||
| Supplemental Oxygen use | No | Occasional/with illness | Nocturnal | Full time | ||
| Report significant problems with pneumonia | No | Yes | ||||
| Asthma | No | Yes | ||||
| Weak cough | No | Yes | ||||
| Shortness of breath | No | Yes | ||||
| Morning headaches | No | Yes | ||||
| Diminished voice quality | No | Yes | ||||
| Problems snoring | No | Yes | ||||
| Difficulty sleeping | No | Yes | ||||
| Excessive daytime sleepiness | No | Yes | ||||
| Night sweats | No | Yes | ||||
| Difficulty breathing when lying flat | No | Yes | ||||
| Difficulty breathing when exercising | No | Yes | ||||
Abbreviations:ACE angiotensin converting enzyme, BIPAP bilevel positive airway pressure, CPAP continuous positive airway DNHS Duchenne Natural History Study, EF ejection fraction, FVC forced vital capacity, SF shortening fraction
Imputation strategies were applied for missing data. Variables for clinically relevant events were imputed with 0 when not present, such as cardiovascular surgery or heart failure hospitalization. Time-varying progressive variables such as Vignos score, Brooke score, and forced vital capacity percent predicted value (FVC %), were imputed using last observation carried forward (LOCF).
Statistical analysis
Participants were grouped based on age at study enrollment and survival status at end of DNHS. Mean and standard deviation for continuous variables, subject number and percentages for categorical variables, are reported. Baseline standardize mean differences (SMD) of deceased and surviving subjects were calculated using the “tableone” package in R. (SMD 0.2 small, 0.5 medium, and 0.8 large [21]). Subjects’ MADE severity points were calculated at each study visit. Descriptive analysis was performed of total MADE score as well as individual MADE domains at enrollment and serial visits. Cohort stratification and age-adjusted statistical models were applied to account for variable enrollment age and known increased mortality risk with older age. As a goal of the MADE Score is to create a tool to identify and track disease burden for the nonambulatory DMD population, cohort analyses was performed based on reported ambulatory status at initial study visit [11,20].
Main outcome
The primary endpoint of this validation study was all-cause mortality. Cross sectional and longitudinal analyses were performed. The optimal cut-point for total MADE score to predict mortality in this population was determined utilizing “OptimalCutpoints” package in R’ [22] maximizing the Youden function, the difference between true positive rate and false positive rate over all possible cut-point values. High-risk (>cut-point) vs low-risk (≤cut-point) for mortality category was assigned and Cox logistic regressions, unadjusted and adjusted by baseline age and subsequently ambulatory status, performed. Corresponding Kaplan-Meier curves were generated and model performance was evaluated based on the concordance probability estimate (CPE) statistic. [23]
Secondary analyses of the longitudinal data of the DNHS dataset to evaluate MADE Score trajectories over time for cohorts based on age, ambulatory status, and study survival was performed with three mixed effect models. The first model adjusted by follow-up time, participant’s survival status, as well as an interaction term between survival status and follow-up time. These models were subsequently adjusted by baseline age, and baseline ambulatory status.
Results:
Longitudinal clinical data was collected by DNHS for 440 males, 12.6 ±6.13 years old, for 3,559 visits over mean 4.6 ± 2.8 years. One third were non-ambulatory at enrollment. Deaths occurred in 45 subjects. Cause of death was: pulmonary 22(49%), cardiac 14(31%), and unknown 9(20%). Patient characteristics at first visit are shown in Table 4. At study enrollment, subjects who died during the study period compared to survivors were on average: older (mean age = 16.40 vs 10.02, SMD 1.16), more likely to be non-ambulatory (77.8% vs 28.6%, SMD 1.13) and have abnormal Forced Vital Capacity % (SMD 1.0). Ejection fraction did not differ significantly. Details of the DNHS have been reported in full. [1–3, 11]
Table 4:
Demographics and clinical characteristics for all participants at their first study visit stratified by survival
| Overall | Survivors | Deceased | *SMD | |
|---|---|---|---|---|
| n | N=440 | N=395 | N=45 | |
| Participant .age at visit (mean (SD)) | 10.67 (5.74) | 10.02 (5.39) | 16.40 (5.56) | 1.164 |
| Angiotensin converting enzyme | ||||
| inhibitor or angiotensin-receptor blocker = Yes/Prophylactic (%) | 50 (11.4) | 38 (9.6) | 12 (26.7) | 0.454 |
| Diuretics use = No (%) | 440 (100.0) | 395 (100.0) | 45 (100.0) | <0.001 |
| Cardiac inotropic agents use = To treat signs and/or symptoms (%) | 8 (1.8) | 5 (1.3) | 3 (6.7) | 0.279 |
| Anti-arrhythmic use = To treat signs and/or symptoms (%) | 1 (0.2) | 0 (0.0) | 1 (2.2) | 0.213 |
| Beta-blockers use = Yes/Prophylactic (%) | 14 (3.2) | 6 (1.5) | 8 (17.8) | 0.573 |
| LVEF/SF (%) | 0.503 | |||
| LVEF % >= 55, SF % >=26 | 397 (90.2) | 363 (91.9) | 34 (75.6) | |
| LVEF % 45–54, SF % 20–25 | 30 (6.8) | 24 (6.1) | 6 (13.3) | |
| LVEF % 35–44, SF % 15–19 | 10 (2.3) | 8 (2.0) | 2 (4.4) | |
| LVEF % <= 35 %, SF % <=14 | 3 (0.7) | 0 (0.0) | 3 (6.7) | |
| Currently on steroid = Yes (%) | 260 (59.1) | 240 (60.8) | 20 (44.4) | 0.331 |
| Currently taking steroid drug = Prednisone (%) | 98 (22.3) | 92 (23.3) | 6 (13.3) | 0.26 |
| Functional tests - lower extremity (Vignos score) (%) | 1.579 | |||
| 1 | 248 (56.4) | 246 (62.3) | 2 (4.4) | |
| 2–7 | 33 (7.5) | 28 (7.1) | 5 (11.1) | |
| 8 | 158 (35.9) | 121 (30.6) | 37 (82.2) | |
| 9 | 1 (0.2) | 0 (0.0) | 1 (2.2) | |
| Functional tests - upper extremity (Brooke score) (%) | 1.151 | |||
| 1 | 265 (60.2) | 257 (65.1) | 8 (17.8) | |
| 2 | 51 (11.6) | 45 (11.4) | 6 (13.3) | |
| 3–4 | 43 (9.8) | 34 (8.6) | 9 (20.0) | |
| 5–6 | 81 (18.4) | 59 (14.9) | 22 (48.9) | |
| Ambulatory status = Nonambulatory (%) | 148 (33.6) | 113 (28.6) | 35 (77.8) | 1.132 |
| Formula/caloric supplements = As needed (%) | 15 (3.4) | 13 (3.3) | 2 (4.4) | 0.06 |
| gastrostomy tube = Daily (%) | 10 (2.3) | 6 (1.5) | 4 (8.9) | 0.336 |
| Calculated FVC % Predicted value | (%) | 1.065 | ||
| Not specified | 268 (60.9) | 258 (65.3) | 10 (22.2) | |
| 61–75% | 62 (14.1) | 56 (14.2) | 6 (13.3) | |
| 51–60% | 26 (5.9) | 20 (5.1) | 6 (13.3) | |
| 31–50% | 36 (8.2) | 27 (6.8) | 9 (20.0) | |
| <=30% | 48 (10.9) | 34 (8.6) | 14 (31.1) | |
| Ventilatory assistance used = Yes (%) | 36 (8.2) | 25 (6.3) | 11 (24.4) | <0.001 |
| Required full-time ventilatory assistance = Yes (%) | 7 (1.6) | 5 (1.3) | 2 (4.4) | 0.192 |
| Bi-PAP / Mask use (%) | 0.265 | |||
| Not specified | 420 (95.5) | 379 (95.9) | 41 (91.1) | |
| Occasionally or with illness | 2 (0.5) | 2 (0.5) | 0 (0.0) | |
| Nocturnal | 17 (3.9) | 13 (3.3) | 4 (8.9) | |
| Full-time | 7 (1.6) | 1 (0.3) | 0 (0.0) | |
| BiPAP / Nasal pillows use = No (%) | 0.088 | |||
| Not specified | 432 (98.2) | 388 (98.2) | 44 (97.8) | |
| Occasionally or with illness | 1 (0.2) | 1 (0.3) | 0 (0.0) | |
| Nocturnal | 7 (1.6) | 6 (1.5) | 1 (2.2) | |
| CPAP use = Occasionally or with illness (%) | 3 (0.7) | 1 (0.3) | 2 (4.4) | 0.279 |
| Mouthpiece IPPV use = Fulltime(%) | 2 (0.5) | 1 (0.3) | 1 (2.2) | 0.179 |
| Negative pressure use = Fulltime (%) | 1 (0.2) | 1 (0.3) | 0 (0.0) | 0.071 |
| Invasive (with tracheotomy) use = No (%) | 0.236 | |||
| Not Specified | 437 (99.3) | 393 (99.5) | 44 (97.8) | |
| Nocturnal | 1 (0.2) | 0 (0.0) | 1 (2.2) | |
| Full-time | 2 (0.5) | 2 (0.5) | 0 (0.0) | |
| Supplemental oxygen use = No (%) | 0.176 | |||
| Not Specified | 434 (98.6) | 389 (98.5) | 45 (100.0) | |
| Nocturnal | 3 (0.7) | 3 (0.8) | 0 (0.0) | |
| Full-time | 3 (0.7) | 3 (0.8) | 0 (0.0) | |
| Age Group (%) | 1.621 | |||
| 0–4 | 51 (11.6) | 51 (12.9) | 0 (0.0) | |
| 5–8 | 170 (38.6) | 170 (43.0) | 0 (0.0) | |
| 9–12 | 89 (20.2) | 72 (18.2) | 17 (37.8) | |
| 13–16 | 57 (13.0) | 48 (12.2) | 9 (20.0) | |
| 17+ | 73 (16.6) | 54 (13.7) | 19 (42.2) |
Abbreviations: BIPAP bilevel positive airway pressure, CPAP continuous positive airway pressure, EF ejection fraction, FVC forced vital capacity, IPPV intermittent positive pressure ventilation, SF shortening fraction
SMD standardized mean difference: <0.2 = small, 0.2– 0.5 medium, and >0.8 large difference between means
MADE Score Trends by Subject Characteristics
Total MADE score across all 3559 visits had a median (interquartile range (IQR) of 8 (3–15). MADE score was higher in older subjects (Figure 1). Mean (SD) MADE score at initial visit for age 0–4 years was 3.2 ±3.3 (n=51) compared to 26.7 ±13 (n= 73) for subjects ≥17 years. MADE score was also higher in non-ambulatory subjects for all age groups (Figure 1). For example, nonambulatory subjects 9–12 years old at enrollment had MADE 15.3 ±6 (n=37) compared to 9.5 ±5.9 (n=52) for ambulatory subjects of same age.
Figure 1.
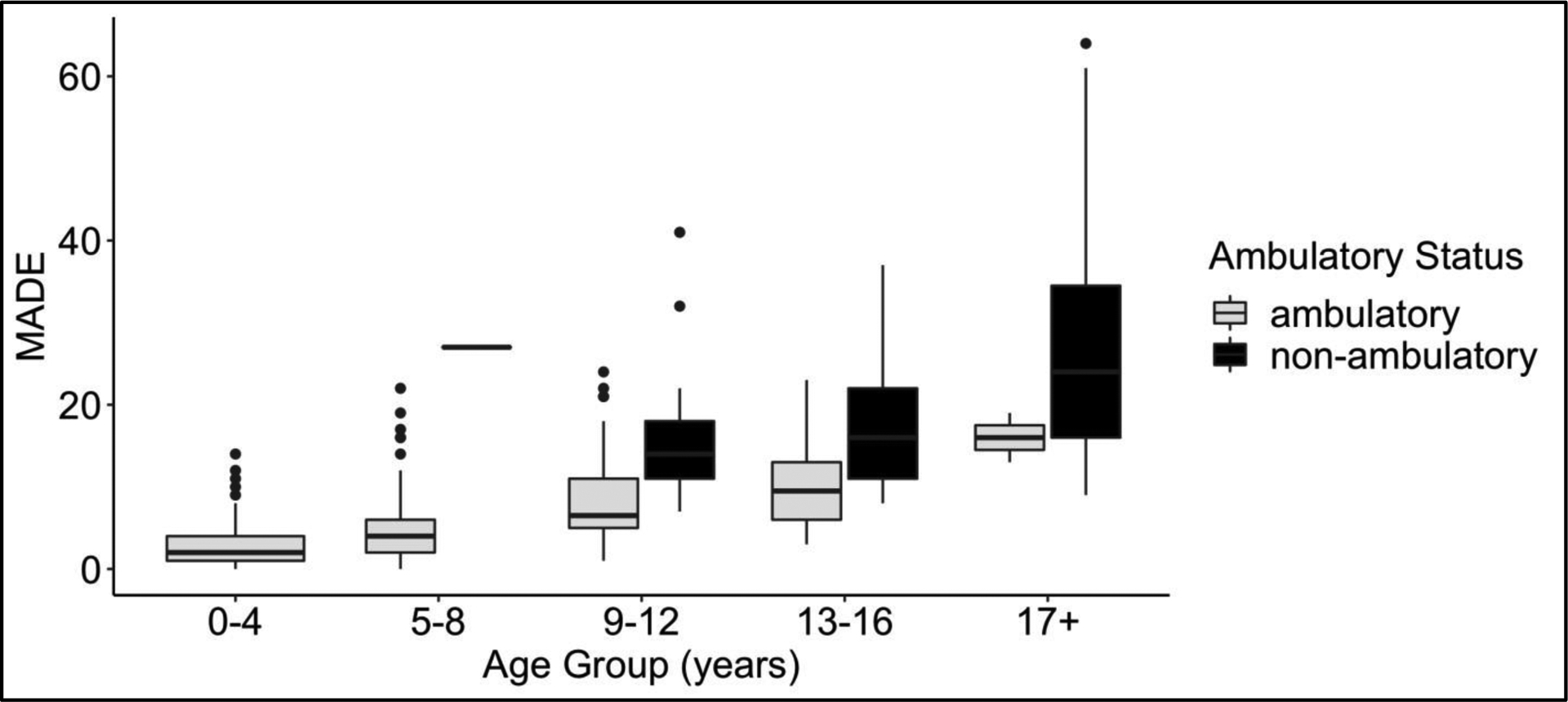
MADE score at enrollment by age group and ambulatory status
Each MADE domain increased over time and significantly contributed to total Score, independent of length of follow-up and baseline age. On cohort analysis, the contribution of the Cardiac domain to total MADE Score (effect size) was doubled in the non-ambulatory population(n=148) compared to the ambulatory population 0.2 vs 0.1 respectively, and tripled in the Pulmonary domain 0.6 vs 0.2 for non-ambulatory and ambulatory patients respectively. (Table 4). Nutritional status alterations (gastrointestinal symptoms) had less reported severity in the non-ambulatory cohort over time.
MADE Score and MORTALITY
MADE Score was significantly higher in those who died during DNHS (n=45). Mean MADE score per visit was 19 ±10 for those who died vs. 9.8 ±9.3 in survivors p=0.03. Baseline MADE >12 predicted DNHS mortality independent of age with sensitivity 0.78, specificity 0.70 and AUC = 0.71. Of the 440 subjects, 300 (68%) were categorized as low-risk for mortality by baseline MADE ≤12 and 140 (32%) as having a baseline high-risk MADE score. On age adjusted Cox regression model, baseline MADE >12 significantly predicted mortality with HR =2.8 (95% CI, 1.2–6.9) CPE 0.69. Effect was attenuated when baseline ambulatory status and steroid use added to models (Figure 2). Of note, steroid use was not significantly related to mortality on univariate analysis.
Figure 2.
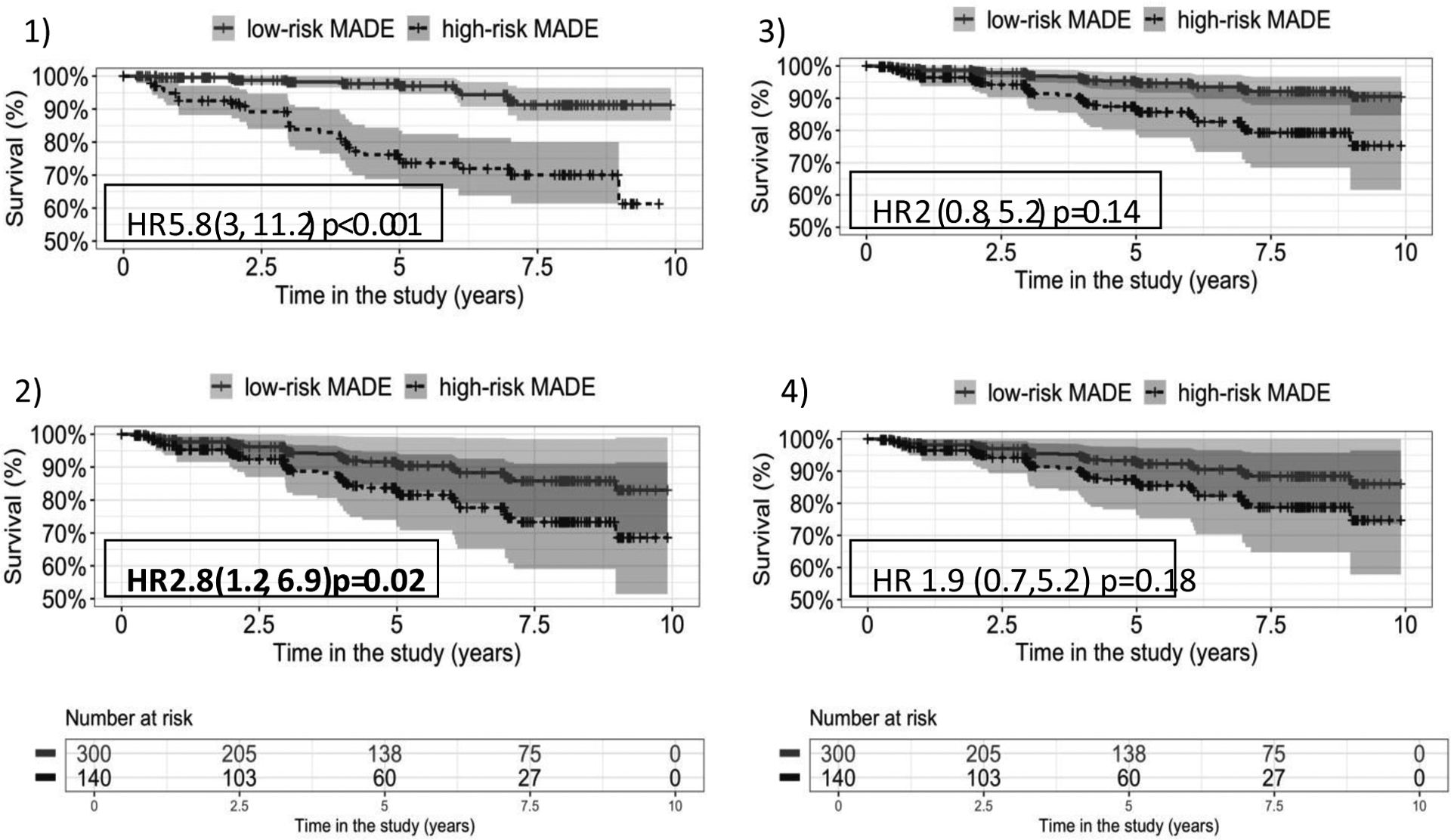
Kaplan-Meier curves corresponding to each of the Cox models for baseline MADE score risk category (high-risk = MADE>12)
1)Unadjusted 2) Adjusted by baseline age 3)Adjusted by age and ambulatory status at baseline 4)Adjusted by age, ambulatory status and steroid use, all at baseline
Longitudinal analysis (Supplemental Table 1) also found MADE score significantly associated with mortality, independent of age and ambulatory status. Deceased subjects had larger increase in MADE score over time – more than twice as much as survivors: for participants who died, MADE score increased 1.7 points per year while survivors increased 0.8 points per year (p<.001). This difference in trajectory remained significant in models adjusted for baseline age and ambulatory status (p<0.001). (Figure 3)
Figure 3.
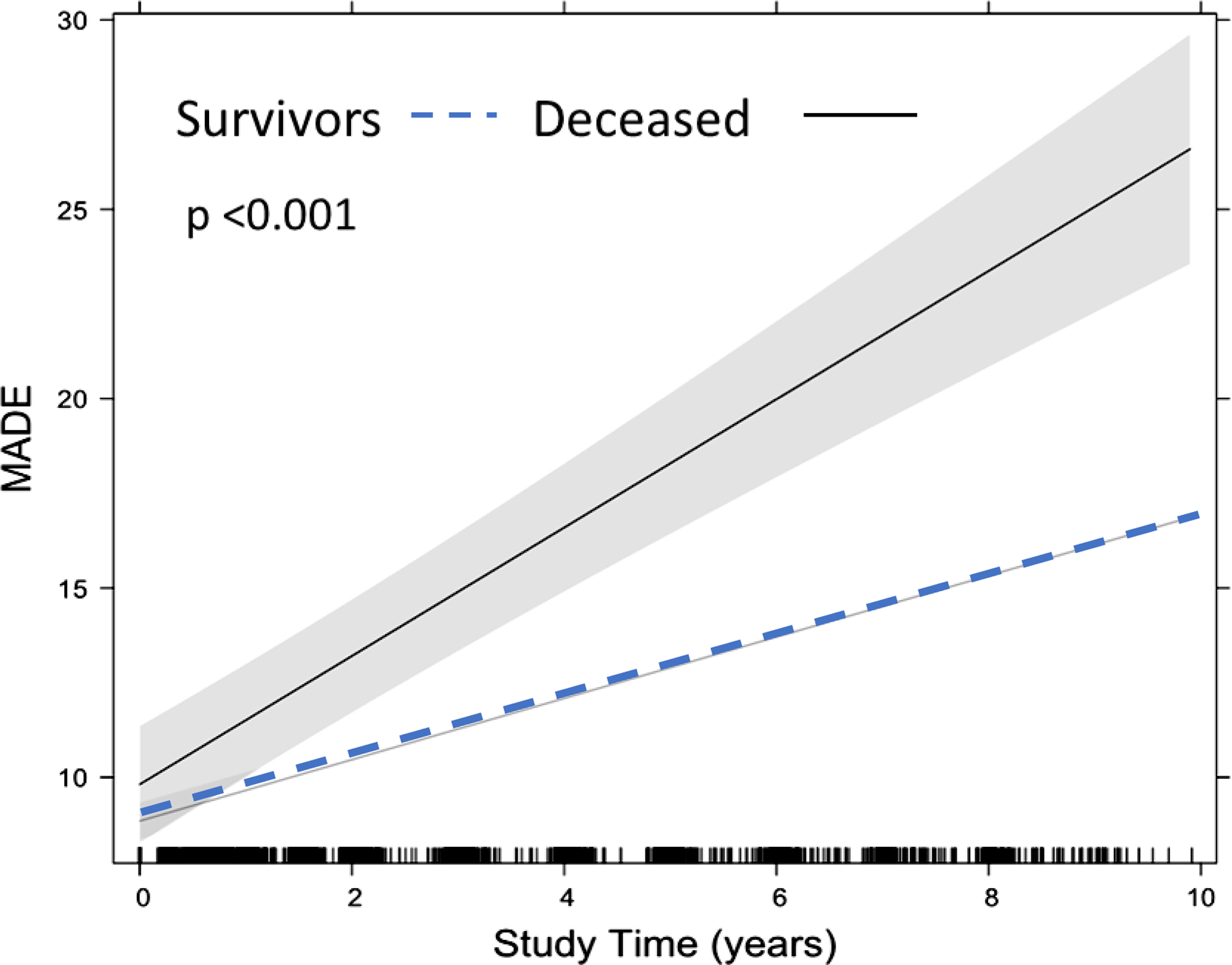
Longitudinal MADE Score trajectory adjusted for baseline age and ambulatory status
DISCUSSION
This paper presents the development and initial validation of the Major Adverse Dystrophinopathy Events (MADE) Score with the longitudinal CINRG DNHS. The intent of MADE Score is to incorporate easily obtained factors in a typical DMD clinic visit to identify a boy at increased risk for heart failure and DMD related mortality.
MADE score clinical correlation:
MADE score increased with age, as expected. All MADE domains increased over time, representing worsening disease burden, even when correcting for age at study enrollment. This demonstrates clinical validity, as MADE score correlated with expected disease progression patterns, and appears to represent the multisystem disease burden of DMD in a single intuitive score.
MADE score predicts mortality
Importantly, MADE score was associated with mortality that occurred during the DNHS study period, independent of age at enrollment and steroid use. A MADE score >12 at enrollment identified a cohort at increased mortality risk with 78% sensitivity and hazard ratio of 3 on age-adjusted model. In addition, changes in MADE score over time in those who died during DNHS were double that of survivors. This association persisted in mixed effect models adjusted for baseline age and ambulatory status, illustrating how MADE score determination at serial visits can be helpful. Increasing MADE score over consecutive visits could trigger closer clinical surveillance, subspecialty consults, and augmentation of therapeutic interventions.
The multidimensional MADE score is unique from other reported predictors of death in DMD such as LV systolic dysfunction[24], cardiac MRI biomarkers [25,26], arrhythmias [27,28], or thresholds of FVC% on pulmonary function tests(PFTs)[3], as these are all single system assessments that do not represent adverse cardiopulmonary and other multi-system interactions. The MADE Cardiac and Pulmonary domains contain ejection fraction and FVC%, respectively. Additionally they include: cardiac and pulmonary related hospitalizations, cardiac medications, respiratory support type and indication, and patient reported symptoms, to serve as a more robust marker of cardiopulmonary status than echocardiogram or PFT measurements alone.
MADE Score application to nonambulatory population
As pattern of disease progression in DMD is well established, the nonambulatory population would be expected to have a higher disease burden, a higher myopathy domain score, and therefore a higher total MADE score [11,12]. Ambulatory status cohort was assigned based on DNHS definition [20] and included in mixed effect models. We found that when analyzing MADE domain changes longitudinally over the study period (mean 4.6 ± 2.8 years), the effect size of the cardiac and pulmonary scores in the nonambulatory population were double and triple that of the ambulatory cohort, respectively. This dynamic risk relationship illustrates utility of MADE Score to reflect increasing cardiac and pulmonary disease burden for those who are at increased risk for heart failure, yet have limited access to exertional-based cardiac functional assessments due to wheelchair dependency. Heart failure in DMD often presents clinically at a late stage due to the body’s ability to accommodate to slowly progressive cardiac dysfunction, as demands for cardiac output are reduced by decreasing physical demands from being nonambulatory. As rates of decline vary, assessing longitudinal changes are important. MADE Score tracking could serve as a screening tool for early heart failure in those with later stage DMD, an important need identified by Spurney et al. in previous investigations of cardiomyopathy in CINRG DNHS [9,11]. Longitudinal MADE analysis in the nonambulatory cohort also revealed decreases in the Nutritional status alteration domain. The intent of the Nutritional status domain is to capture gastrointestinal manifestations of heart failure, (abdominal pains, anorexia, failure-to-thrive) that are often observed in pediatric dilated cardiomyopathy [29]. However the DNHS nutritional status/GI related variables were more representative of steroid adverse effects and gastrointestinal dysmotility, that might have confounded the MADE Nutrition domain scores. Utility of the Nutritional Status alteration domain will be reexamined on future MADE validation studies utilizing a heart failure-specific dataset.
Nonetheless, this analysis demonstrates how incorporation of changing severity scores of each domain into a total MADE Score can track interrelated and cumulative disease burden, as well as potential response to therapeutic interventions, which are typically not isolated to a single organ system.
Cardiac-related death is most common in nonambulatory males in the current era [5,8]. MADE Score performance to identify increased mortality risk in the nonambulatory cohort of DNHS was robust and bodes well for application as a risk assessment tool specifically for nonambulatory DMD populations in subsequent studies.
Multidomain scores and composite endpoints
The concept of the MADE score, an additive model of multiple ordinal scores that represent progressive severity of systemic disease and occurrence of interrelated adverse events, is based on the paradigm of the widely used MACE composite endpoint for adults with cardiovascular disease[16,17], and the more recently developed major adverse transplant event (MATE) score that predicts graft loss for pediatric heart transplant[18]. MATE consists of summation of severity scores assigned to degree of graft coronary disease, rejection, infection, renal insufficiency and malignancies. The state of being immunosuppressed is the common factor which affects the incidence of these progressive adverse transplant related events. Similarly, the state of having a dystrophinopathy is the common factor that affects the incidence of MADE in the DMD population.
Similar to MATE score development described by Almond et al [18], while we do not presume that a 1 point difference in severity score from the Cardiac domain has equivalent impact on mortality risk for those with DMD as a 1 point severity change in Pulmonary, Nutritional, or Myopathy domain score, the simple summation of points assigned to each severity category performs well to identify a high risk cohort and to predict mortality in this retrospective analysis. While we explored de novo statistical models, we focused on development of the simple additive model for this initial analysis to explore utility of MADE as a tool based on routinely available clinical data rather than requiring advanced diagnostics or testing calculations. Future weighting strategies may be assigned to each domain to adjust for variations in strength of associations with heart failure and /or mortality in future score iterations.
Utility of MADE Score for Research
A tracker of heart failure symptoms in context of progressive neuromuscular disease does not currently exist and would be of significant utility as a possible surrogate endpoint for cardiac clinical trials. While there has been significant progress in drug development targeting skeletal muscle weakness[30–34] utilizing timed motor function tests as outcome variables[35,36], interventions to modify cardiac dysfunction is lagging. Cardiovascular trial design for neuromuscular disorders is challenged by infrequent late onset cardiac events and limited utility of traditional heart failure outcome measures of exertional based symptom scores [37] and exercise test performance. As it is often impossible to isolate respiratory, cardiac, and neuromuscular interactions, or impact of disease modifiers such as nutritional status and psychological well-being, a multiorgan system score more accurately represents disease state for people with dystrophinopathy. Our study demonstrated ability for MADE Score to identify a cohort at increased mortality risk both at DNHS study enrollment as well as longitudinally, independent of age and ambulatory status effects. As such, MADE Score could be utilized to identify cohorts for clinical trial enrollments of subjects with similar disease burden (similar to NYHA class inclusion criteria), as well as a surrogate endpoint to track overall impact of interventions on changes in MADE Score longitudinally.
Future Directions for Score development
Our goal is for MADE Score to serve as a predictor of heart failure in DMD. Further validation and optimization of our primary MADE Score model (Table 1) for clinical and research use will be performed utilizing current era DMD heart failure assessments and biomarkers including MRI data and PROM of fatigue and quality of life (QOL), which were not extensively available in the DNHS data set. This can be accomplished utilizing the Advanced Cardiac Therapies Improving Outcomes Network (ACTION) DMD Heart Failure Registry [8,39] that collects heart failure-related data and events in DMD males with cardiac dysfunction. Subsequently MADE Score will be evaluated prospectively as a potential surrogate endpoint for clinical trials [38] targeting cardiac and pulmonary function in DMD.
Limitations:
This initial effort to demonstrate clinical and construct validity of the MADE Score utilized retrospective application to the DNHS dataset. DNHS was designed as an observational study without specified study procedures other than case report forms. Consequentially, missing data variables resulted in limitations in statistical analysis. Extent of missing data related to PROM of fatigue and QOL precluded inclusion of a Fatigue domain in this analysis. Definitions of “heart failure exacerbations” and “cause of death” were not specified. As it was unknown whether the “pulmonary” or “unknown cause” deaths had cardiac components, which would be expected and difficult to isolate in this population, all-cause mortality was chosen as primary endpoint, rather than only cardiac, to maximize statistical power for this pilot study of MADE Score.
Conclusion
A multiorgan severity score, MADE, was created to track cumulative morbidities of DMD that impact heart failure. MADE score independently predicted mortality, and identified a high-risk cohort within a nonambulatory population with progressive cardiac and respiratory disease burden, demonstrating clinical utility. With further development, MADE score can be utilized to assess heart failure progression for at-risk populations and serve as a surrogate endpoint for cardiovascular trials in DMD.
Supplementary Material
Table 5.
MADE Domain contributions to Total MADE score (effect size) and ambulatory cohorts, adjusting for length of followup and baseline age.
| Ambulatory | Non-ambulatory | |||
|---|---|---|---|---|
| MADE Domain | Effect size | P-value | Effect size | P-value |
| CARDIAC | ||||
| Years in the study | 0.1 | <0.001 | 0.2 | <0.001 |
| Baseline age | 0.1 | <0.001 | 0.2 | <0.001 |
| MYOPATHY | ||||
| Years in the study | 0.5 | <0.001 | 0.09 | <0.001 |
| Baseline age | 0.3 | <0.001 | 0.07 | 0.002 |
| NUTRITION | ||||
| Years in the study | 0.02 | 0.007 | −0.0005 | 0.98 |
| Baseline age | 0.08 | <0.001 | 0.1 | <0.001 |
| PULMONARY | ||||
| Years in the study | 0.2 | <0.001 | 0.6 | <0.001 |
| Baseline age | 0.2 | <0.001 | 0.6 | <0.001 |
Highlights:
The Major Adverse Dystrophinopathy Event (MADE) Score for Duchenne Muscular Dystrophy
MADE Score is a clinical tool for DMD to represent multiorgan system morbidities
MADE score predicted mortality in DMD Natural History Study
Serial MADE assessments identify a high risk nonambulatory DMD cohort
Future utility of MADE score as a heart failure endpoint for DMD research
Acknowledgments:
Cooperative International Neuromuscular Research Group (CINRG) DNHS (DNHS) Chairs: Craig M. McDonald1 Erik K. Henricson1Richard T. Abresch1
CINRG DNHS Investigators: Nanette Joyce 1 , V. Vishwanathan2 ,S. Chidambaranathan2 , W. Douglas Biggar3,Laura C. McAdam3,Jean K. Mah4,Mar Tulinius5,Avital Cnaan6,Lauren P. Morgenroth6,21, Robert Leshner6 ,Carolina Tesi-Rocha6 ,Mathula Thangarajh6,Tina Duong6,22,Andrew Kornberg7,Monique Ryan7 ,Yoram Nevo8,Alberto Dubrovsky9,Paula R. Clemens10,Hoda Abdel-Hamid10,Anne M. Connolly11,Alan Pestronk11,Jean Teasley12,Tulio E. Bertorini13,Richard Webster14,Hanna Kolski15,Nancy Kuntz16,Sherilyn Driscoll16,John B. Bodensteiner16,Jose Carlo17,Ksenija Gorni18,Timothy Lotze19,John W. Day20,and Peter Karachunski20
University of California, Davis, Sacramento,California,USA 1 Sundaram Medical Foundation and Apollo Children’s Hospital, Chennai, India2 Holland Bloorview Kids Rehab Hospital, Toronto, Ontario, Canada3 Alberta Children’s Hospital, Calgary, Alberta, Canada4 Queen Silvia Children’s Hospital, Göteborg, Sweden5 Children’s National Medical Center, Washington DC, USA6 Royal Children’s Hospital, Melbourne, Victoria, Australia7 Hadassah Hebrew University Hospital, Jerusalem, Israel8 Instituto de Neurosciencias Fundacion Favaloro, Buenos Aires, Argentina9 University of Pittsburgh and Department of Veterans Affairs, Pittsburgh, Pennsylvania, USA10 Washington University in St Louis, St Louis, Missouri, USA11 Children’s Hospital of Virginia, Richmond, Virginia, USA12 University of Tennessee, Memphis, Tennessee,USA13 Children’s Hospital at Westmead, Sydney, New South Wales, Australia14 University of Alberta, Edmonton, Alberta, Canada15 Mayo Clinic, Rochester, Minnesota, USA16 University of Puerto Rico, San Juan, Puerto, Rico17 University of Pavia and Niguarda Ca’ Granda Hospital, Milan, Italy18 Texas Children’s Hospital, Houston, Texas, USA19 University of Minnesota, Minneapolis, Minnesota, USA20 Therapeutic Research in Neuromuscular Disorders Solutions (TRiNDS), Pittsburgh, Pennsylvania, USA21 Stanford University, Palo Alto, California, USA22
CINRG Funding:
U.S. Department of Education/NIDRR (#H133B031118, #H133B090001), U.S. Department of Defense (#W81XWH-09-1-0592), National Institutes of Health (#UL1RR031988, U54HD053177, #UL1RR024992, #U54RR026139, #2U54HD053177, #G12RR003051),Parent Project Muscular Dystrophy
CINRG Funding did not support the analysis reported in this manuscript. Only CINRG DNHS investigators specifically named as authors, or included in cited references, contributed to this manuscript.
Declarations of Interest:
B Kaufman, A Garcia, Z He, M Buu, C Almond: No potential conflicts of interest to disclose Tina Duong: Served on medical advisory boards and or consultant for Scholar Rock, Genentech, F Hoffman La Roche, Biogen, Sarepta, Novartis, Solid Biosciences, Dynacure, Dyne, Audentes. Consultancy also through ATOM International and Trinds (Biomarin, Pfizer, Solid Biosicences, Sarepta, Astellas). She has received research grant support from Ionis.
David Rosenthal: Consultant to Audentes and Medtronic
Carolina Tesi-Rocha: Consultant: Sarepta, Biogen, Avexis/Novartis, Genentech, Roche Site investigator for clinical trials: Sarepta, Roche, PTC, Biogen, Avexis, Scholar Rock, Pfizer, Scholar Rock, Genzyme, Cytokinetics.
Heather Gordish-Dressman: Consultant-Agada Biosciences, Solid BioSciences, Audentes Therapeutics INC., TRiNDS LLC
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethical Publication Statement: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Henricson EK, et al. , The cooperative international neuromuscular research group DNHS: glucocorticoid treatment preserves clinically meaningful functional milestones and reduces rate of disease progression as measured by manual muscle testing and other commonly used clinical trial outcome measures. Muscle Nerve, 2013. 48(1): p. 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDonald CM, et al. , Long-term effects of glucocorticoids on function, quality of life, and survival in patients with DMD: a prospective cohort study. Lancet, 2018. 391(10119): p. 451461. [DOI] [PubMed] [Google Scholar]
- 3.McDonald CM, et al. , Longitudinal pulmonary function testing outcome measures in DMD: Long-term natural history with and without glucocorticoids. Neuromuscul Disord, 2018. 28(11): p. 897–909. [DOI] [PubMed] [Google Scholar]
- 4.Birnkrant DJ, Bushby K, Bann CM, et al. for the DMD Care Considerations Working Group Diagnosis and management of DMD, part 2: respiratory, cardiac, bone health, and orthopaedic management Lancet Neurol 2018; 17: 347–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Ruiten HJ, Marini Bettolo C, Cheetham T, Eagle M, Lochmuller H, Straub V, Bushby K, Guglieri M (2016) Why are some patients with DMD dying young: An analysis of causes of death in North East England. Eur J Paediatr Neurol 20:904–909 [DOI] [PubMed] [Google Scholar]
- 6.Mavrogeni SI, et al. , Cardiac Involvement in DMD and Related Dystrophinopathies. Methods Mol Biol, 2018. 1687: p. 31–42. [DOI] [PubMed] [Google Scholar]
- 7.Feingold B, Mahle WT, Auerbach S, Clemens P, Domenighetti AA, Jefferies JL, Judge DP, Lal AK, Markham LW, Parks WJ, Tsuda T, Wang PJ, Yoo SJ, American Heart Association Pediatric Heart Failure Committee of the Council on Cardiovascular Disease in the Y, Council on Clinical C, Council on Cardiovascular R, Intervention, Council on Functional G, Translational B, Stroke C (2017) Management of cardiac involvement associated with neuromuscular diseases: a scientific statement From the American Heart Association. Circulation 136:e200–e231 [DOI] [PubMed] [Google Scholar]
- 8.Villa C, Auerbach SR, Bansal N, Birnbaum BF, Conway J, Esteso P, Gambetta K, Hall EK, Kaufman BD, Kirmani S, Lal AK, Martinez HR, Nandi D, O’Connor MJ, Parent JJ, Raucci FJ, Shih R, Shugh S, Soslow JH, Tunuguntla H, Wittlieb-Weber CA, Kinnett K, Cripe L. Current Practices in Treating Cardiomyopathy and Heart Failure in DMD (DMD): Understanding Care Practices in Order to Optimize DMD Heart Failure Through ACTION. Pediatr Cardiol. 2022. Jun;43(5):977–985. doi: 10.1007/s00246-021-02807-7. Epub 2022 Jan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spurney CF, Cardiomyopathy of DMD: current understanding and future directions. Muscle Nerve, 2011. 44(1): p. 8–19. [DOI] [PubMed] [Google Scholar]
- 10.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A,Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC,Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College ofCardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895–e1032. doi: 10.1161/CIR.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 11.Spurney C, Shimizu R, Morgenroth LP, Kolski H, Gordish‐Dressman H, Clemens PR and (2014), Cooperative international neuromuscular research group DNHS demonstrates insufficient diagnosis and treatment of cardiomyopathy in DMD. Muscle Nerve, 50: 250–256. 10.1002/mus.24163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNally EM et al. , Contemporary cardiac issues in DMD. Working Group of the National Heart, Lung, and Blood Institute in collaboration with Parent Project Muscular Dystrophy. Circulation. 2015. May 5;131(18):1590–8. doi: 10.1161/CIRCULATIONAHA.114.015151. Erratum in: Circulation. 2015 Jun 23;131(25):e539. Groh, William J [added]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birnkrant DJ, Bello L, Butterfield RJ, Carter JC, Cripe LH, Cripe TP, McKim DA, Nandi D, Pegoraro E. Cardiorespiratory management of DMD: emerging therapies, neuromuscular genetics, and new clinical challenges. Lancet Respir Med. 2022. Apr;10(4):403–420. doi: 10.1016/S2213-2600(21)00581-6. [DOI] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services. “Common Terminology Criteria for Adverse Events. Version 5.0. Published November 27, 2017.” (2020).
- 15.Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation—a consensus conference report. Chest 1999; 116: 521–34. DOI: 10.1378/chest.116.2.521 [DOI] [PubMed] [Google Scholar]
- 16.Poudel I, Tejpal C, Rashid H, Jahan N. Major Adverse Cardiovascular Events: An Inevitable Outcome of ST-elevation myocardial infarction? A Literature Review. Cureus. 2019. Jul 30;11(7):e5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai IT, Wang CP, Lu YC, Hung WC, Wu CC, Lu LF, Chung FM, Hsu CC, Lee YJ, Yu TH The burden of major adverse cardiac events in patients with coronary artery disease. BMC Cardiovasc Disord. 2017. Jan 4; 17(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Almond CA Development and validation of a major adverse transplant event (MATE) score to predict late graft loss in pediatric heart transplantation J HeartLungTransplant 2018;37:441–450 [DOI] [PubMed] [Google Scholar]
- 19.Almond C, Chen S, Dykes J, Kwong J, Burstein D, Rosenthal D, Kipps A,Teuteberg J, Murray J, Kaufman B, Hollander S,Profita E,Yarlagadda V, Sacks L,Chen C The Stanford Acute Heart Failure Symptom Score For Patients Hospitalized with Heart Failure Journal of Heart and Lung Transplantat. 2020. Aug 8:S10532498(20)31691–0. [DOI] [PubMed] [Google Scholar]
- 20.McDonald CM, et al. , The cooperative international neuromuscular research group DNHS--a longitudinal investigation in the era of glucocorticoid therapy: design of protocol and the methods used. Muscle Nerve, 2013. 48(1): p. 32–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peter Austin (2009) Using the Standardized Difference to Compare the Prevalence of a Binary Variable Between Two Groups in Observational Research, Communications in Statistics - Simulation and Computation, 38:6, 1228–1234, DOI: 10.1080/03610910902859574 [DOI] [Google Scholar]
- 22.Lopez-Raton M, Rodriguez-Alvarez MX, Cadarso-Suarez C and Gude-Sampedro F (2014). OptimalCutpoints: An R Package for Selecting Optimal Cutpoints in Diagnostic Tests. Journal of Statistical Software 61(8), 1–36. URL http://www.jstatsoft.org/v61/i08/. [Google Scholar]
- 23.Gonen M Heller G Concordance probability and discriminatory power in proportional hazards regression. Biometrica. 2005; 92: 965–970 [Google Scholar]
- 24.Angelini C, Prevention of cardiomyopathy in DMD. Lancet Neurol, 2015. 14(2): p. 127–8. [DOI] [PubMed] [Google Scholar]
- 25.Florian A, Ludwig A, Engelen M, Waltenberger J, Rösch S, Sechtem U, Yilmaz A. Left ventricular systolic function and the pattern of late-gadolinium-enhancement independently and additively predict adverse cardiac events in muscular dystrophy patients. J Cardiovasc Magn Reson. 2014. Sep 25;16(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magrath P, et al. , Cardiac MRI biomarkers for DMD. Biomark Med, 2018. 12(11): p. 1271–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheeran D, Khan S, Khera R, Bhatt A, Garg S, Grodin JL, Morlend R, Araj FG, Amin AA, Thibodeau JT, Das S, Drazner MH, Mammen PPA. Predictors of Death in Adults With DMD-Associated Cardiomyopathy. J Am Heart Assoc. 2017. Oct 17;6(10):e006340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corrado G, Lissoni A, Beretta S, Terenghi L, Tadeo G, Foglia-Manzillo G, Tagliagambe LM, Spata M, Santarone M. Prognostic value of electrocardiograms, ventricular late potentials, ventricular arrhythmias, and left ventricular systolic dysfunction in patients with DMD. Am J Cardiol. 2002. Apr 1;89(7):838–41 [DOI] [PubMed] [Google Scholar]
- 29.Hollander SA, Addonizio LJ, Chin C, Lamour JM, Hsu DT, Bernstein D, Rosenthal DN. Abdominal complaints as a common first presentation of heart failure in adolescents with dilated cardiomyopathy. Am J Emerg Med. 2013. Apr;31(4):684–6. doi: 10.1016/j.ajem.2012.12.009. Epub 2013 Feb 4. [DOI] [PubMed] [Google Scholar]
- 30.Manzur AY, et al. , Glucocorticoid corticosteroids for DMD. Cochrane Database Syst Rev, 2008(1): p. CD003725. [DOI] [PubMed] [Google Scholar]
- 31.Mendell JR, et al. , Randomized, double-blind six-month trial of prednisone in Duchenne’s muscular dystrophy. N Engl J Med, 1989. 320(24): p. 1592–7. [DOI] [PubMed] [Google Scholar]
- 32.Larkindale J, et al. , Duchenne Regulatory Science Consortium Meeting on Disease Progression Modeling for DMD. PLoS Curr, 2017. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDonald CM, et al. , Ataluren in patients with nonsense mutation DMD (ACT DMD): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet, 2017. 390(10101): p. 1489–1498. [DOI] [PubMed] [Google Scholar]
- 34.Mendell JR, et al. , Longitudinal effect of eteplirsen versus historical control on ambulation in DMD. Ann Neurol, 2016. 79(2): p. 257–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duong T, et al. , The Minimal Clinical Important Difference (MCID) in Annual Rate of Change of Timed Function Tests in Boys with DMD. J Neuromuscul Dis, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steffensen B, et al. , Validity of the EK scale: a functional assessment of non-ambulatory individuals with DMD or spinal muscular atrophy. Physiother Res Int, 2001. 6(3): p. 119–34. [DOI] [PubMed] [Google Scholar]
- 37.Dolgin M, Association NYH, Fox AC, Gorlin R, Levin RI, New York Heart Association. Criteria Committee. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. 9th ed. Boston, MA: Lippincott Williams and Wilkins; March 1, 1994. [Google Scholar]
- 38.Gilbert PB, Gabriel EE, Huang Y, and Chan IS, Surrogate Endpoint Evaluation: Principal Stratification Criteria and the Prentice Definition J Causal Inference. 2015. September 1; 3(2): 157–175. doi: 10.1515/jci-2014-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lorts A, Smyth L, Gajarski RJ, VanderPluym CJ, Mehegan M, Villa CR, Murray JM, Niebler RA, Almond CS, Thrush P, O’Connor MJ, Conway J, Sutcliffe DL, Lantz JE, Zafar F, Morales DLS, Peng DM, Rosenthal DN. The Creation of a Pediatric Health Care Learning Network: The ACTION Quality Improvement Collaborative. ASAIO J. 2020. Apr;66(4):441–446. doi: 10.1097/MAT.0000000000001133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


