Abstract
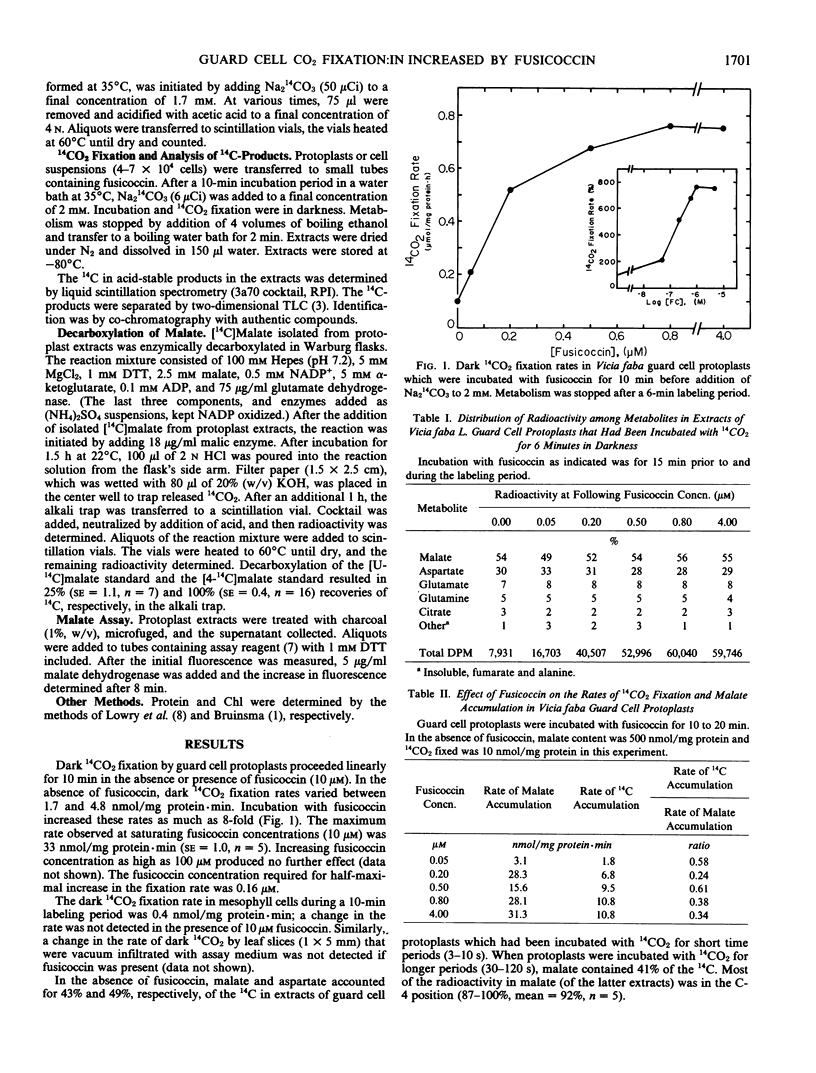
When Vicia faba guard cell protoplasts were treated with fusicoccin, dark 14CO2 fixation rates increased by as much as 8-fold. Rate increase was saturated with less than 1 micromolar fusicoccin. Even after 6 minutes of dark 14CO2 fixation, more than 95% of the incorporated radioactivity was in stable products derived from carboxylation of phosphoenolpyruvate (about 50% and 30% in malate and aspartate, respectively). The relative distribution of 14C among products and in the C-4 position of malate (initially more than 90% of [14C]malate) was independent of fusicoccin concentration. After incubation in the dark, malate content was higher in protoplasts treated with fusicoccin. A positive correlation was observed between the amounts of 14CO2 fixed and malate content.
It was concluded that (a) fusicoccin causes an increase in the rate of dark 14CO2 fixation without alteration of the relative fluxes through pathways by which it is metabolized, (b) fusicoccin causes an increase in malate synthesis, and (c) dark 14CO2 fixation and malate synthesis are mediated by phosphoenolpyruvate carboxylase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davies D. D. Control of and by pH. Symp Soc Exp Biol. 1973;27:513–529. [PubMed] [Google Scholar]
- Feige B., Gimmler H., Jeschke W. D., Simonis W. Eine Methode zur dünnschichtchromatographischen Auftrennung von 14C- und 32P-markierten Stoffwechselprodukten. J Chromatogr. 1969 Apr 22;41(1):80–90. doi: 10.1016/0021-9673(64)80099-6. [DOI] [PubMed] [Google Scholar]
- Fischer R. A., Hsiao T. C. Stomatal Opening in Isolated Epidermal Strips of Vicia faba. II. Responses to KCl Concentration and the Role of Potassium Absorption. Plant Physiol. 1968 Dec;43(12):1953–1958. doi: 10.1104/pp.43.12.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humble G. D., Hsiao T. C. Light-dependent Influx and Efflux of Potassium of Guard Cells during Stomatal Opening and Closing. Plant Physiol. 1970 Sep;46(3):483–487. doi: 10.1104/pp.46.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Rayle D. L. Enhancement of CO(2) Uptake in Avena Coleoptiles by Fusicoccin. Plant Physiol. 1976 May;57(5):806–811. doi: 10.1104/pp.57.5.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Outlaw W. H., Kennedy J. Enzymic and substrate basis for the anaplerotic step in guard cells. Plant Physiol. 1978 Oct;62(4):648–652. doi: 10.1104/pp.62.4.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw W. H., Lowry O. H. Organic acid and potassium accumulation in guard cells during stomatal opening. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4434–4438. doi: 10.1073/pnas.74.10.4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw W. H., Manchester J., Dicamelli C. A. Histochemical Approach to Properties of Vicia faba Guard Cell Phosphoenolpyruvate Carboxylase. Plant Physiol. 1979 Aug;64(2):269–272. doi: 10.1104/pp.64.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw W. H., Manchester J. Guard cell starch concentration quantitatively related to stomatal aperture. Plant Physiol. 1979 Jul;64(1):79–82. doi: 10.1104/pp.64.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw W. H., Manchester J., Zenger V. E. The relationship between protein content and dry weight of guard cells and other single cell samples of Vicia faba L. leaflet. Histochem J. 1981 Mar;13(2):329–334. doi: 10.1007/BF01006886. [DOI] [PubMed] [Google Scholar]
- Outlaw W. H., Mayne B. C., Zenger V. E., Manchester J. Presence of Both Photosystems in Guard Cells of Vicia faba L: IMPLICATIONS FOR ENVIRONMENTAL SIGNAL PROCESSING. Plant Physiol. 1981 Jan;67(1):12–16. doi: 10.1104/pp.67.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmer C., Kanai R., Pallas J. E., Jr, Black C. C., Jr Detection of high levels of phosphoenolpyruvate carboxylase in leaf epidermal tissue and its significance in stomatal movements. Life Sci II. 1973 Feb 22;12(4):155–159. [PubMed] [Google Scholar]
- Zeiger E., Hepler P. K. Light and stomatal function: blue light stimulates swelling of guard cell protoplasts. Science. 1977 May 20;196(4292):887–889. doi: 10.1126/science.196.4292.887. [DOI] [PubMed] [Google Scholar]


