Abstract
There is a lack of studies comparing the risk of cardio-cerebrovascular disease between angiotensin receptor blockers (ARBs) of different half-lives. We aimed to compare the risks of myocardial infarction (MI), heart failure (HF), and cerebrovascular disease with the use of valsartan, losartan, irbesartan, and telmisartan with different half-lives in a national claim-based retrospective cohort of patients aged ≥ 40 years with hypertension. To establish a cohort exposed to valsartan, losartan, irbesartan, or telmisartan, we performed propensity score (PS) matching and used an as-treated approach to evaluate exposure. The Cox regression model was employed to calculate hazard ratios, which were based on the incidence rate for each newly occurring event of MI, heart failure, or cerebrovascular disease. These hazard ratios were calculated to compare the risk of MI, heart failure, and cerebrovascular disease associated with valsartan, losartan, and irbesartan in comparison to telmisartan. A PS-matched cohort of 148,229 patients was established for each of valsartan, losartan, irbesartan, or telmisartan. The matched cohort analysis showed that the adjusted hazard ratio (aHRs, 95% confidence interval) for MI was higher for valsartan use (1.39, 1.33–1.45) and losartan use (1.10, 1.05–1.15) but lower for irbesartan use (0.90, 0.86–0.94) compared with the reference (telmisartan). The aHRs for HF were not different among these ARBs (angiotensin receptor blockers). The aHR for cerebrovascular disease was lower for valsartan use (0.85, 0.83–0.87) and losartan use (0.80, 0.78–0.82) but higher for irbesartan use (1.11, 1.09–1.13) compared with the reference. We found differences in the risk of MI and cerebrovascular disease with the use of different ARBs compared to telmisartan use. Valsartan, and losartan with a short half-life, which showed a higher risk of MI, had a lower risk of cerebrovascular disease. Conversely, irbesartan with a long half-life, which showed a lower risk of MI, had a higher risk of cerebrovascular disease.
Keywords: angiotensin receptor blockers, cerebrovascular disorders, half-life, heart failure, myocardial infarction
1. Introduction
Cardiovascular and cerebrovascular diseases remain the leading global causes of death,[1] and hypertension is widely recognized as a major risk factor for these diseases.[2,3] The overall lifetime risk of cardiovascular disease at 30 years of age is 63.3% in patients with hypertension, which is approximately 17.2% higher than that of patients without hypertension. In addition, cardiovascular disease is diagnosed 5 years earlier in patients with hypertension than in those without hypertension.[4]
Numerous clinical trials and observational studies have shown that lowering blood pressure reduces the risk of myocardial infarction (MI), heart failure (HF), stroke, and mortality from other vascular diseases. A meta-analysis of randomized controlled trials on the effects of lowering blood pressure on cardiovascular disease and mortality has shown that every 10 mm Hg reduction in systolic blood pressure significantly reduces the risk of major cardiovascular disease events (relative risk 0.80, 95% confidence interval [CI] 0.77–0.83), coronary heart disease (0.83, 0.78–0.88), stroke (0.73, 0.68–0.77), and HF (0.72, 0.67–0.78). These reductions consequently led to a significant (13%) reduction in all-cause mortality in the studied population (0.87, 0.84–0.91).[5]
Angiotensin receptor blockers (ARBs), the most commonly prescribed class of antihypertensive drugs in South Korea,[6] are recognized for their efficacy, tolerability, and additional therapeutic benefits, such as cardiovascular disease risk reduction. The blood pressure-lowering effect and cardiovascular benefits of ARBs have been extensively studied. However, there is a lack of comparative studies between different ARBs.
A meta-analysis that indirectly compared the blood pressure-lowering effects between ARBs has suggested that all ARBs exert similar blood pressure-lowering effects.[7] However, studies that directly compared the blood pressure-lowering effects between ARBs have revealed differences in the degree of blood pressure lowering or ability to maintain a consistent blood pressure throughout the day. One study has shown that the degree of blood pressure reduction is greatest with telmisartan, followed by valsartan, candesartan, and losartan,[8] whereas another study has shown that the degree of blood pressure reduction is greatest with olmesartan, followed by irbesartan, losartan, and valsartan.[9] A ratio of morning blood pressure to evening blood pressure (M/E ratio) close to 1 indicates consistent blood pressure maintenance. The a ratio of morning blood pressure to evening blood pressure was 0.92 for telmisartan, 0.78 for valsartan, 0.71 for candesartan, and 0.47 for losartan in 1 study.[8] Considering these results, ARBs with long half-lives such as telmisartan seem to have relatively large blood pressure-lowering and maintenance effects compared to those with short half-lives such as losartan.
In this study, we aimed to compare the risks of MI, HF, and cerebrovascular disease with valsartan, losartan, irbesartan, and telmisartan use in a nationwide retrospective cohort of patients with hypertension. Additionally, we examined whether these risks were associated with the half-lives of the ARBs.
2. Methods
2.1. Data source
This retrospective cohort study was conducted using a database built by the Health Insurance Review and Assessment Service (HIRA) by collecting the insurance claims data of hospitals, excluding clinics, throughout South Korea. In South Korea, social security-type health insurance was initiated in 1977, a medical expense review agency was established in 1979, and national medical insurance was implemented in 1989.[10] The HIRA evaluates medical expense claims from hospitals that provide public healthcare services and subsequently share the results with the National Health Insurance Service, which handles insurance claims and reimbursements for eligible healthcare institutions. Additionally, the HIRA provides specific data on patient details, treatment details, and outpatient prescriptions.[10] Both agencies utilize the International Classification of Diseases Tenth Revision (ICD-10) to assign codes for diagnosis and the Anatomical Therapeutic Chemical Classification to assign codes for active drug ingredients. An 82% degree of concordance between the information provided by hospitals and insurance claims data generated by the HIRA has been reported.[11]
2.2. Study population
Patients aged ≥ 40 years with hypertension exposed to losartan, valsartan, irbesartan, or telmisartan between January 1, 2008 and December 31, 2016 were included in the study. Patients who were exposed to ARBs or were diagnosed with cardio- and cerebrovascular diseases, such as MI, HF, stroke, or cerebral infarction, within a year before the cohort entry were excluded. The index date was defined as the date on which a study drug (losartan, valsartan, irbesartan, or telmisartan) was first prescribed. New users were defined as patients with hypertension (ICD-10, I10) who received their first prescription of losartan, valsartan, irbesartan, or telmisartan in an inpatient or outpatient setting. The reference standard comprised patients with hypertension (ICD-10, I10) who were initially prescribed telmisartan in an inpatient or outpatient setting. Patient follow-up was conducted from the index date until the earliest occurrence of an outcome, a switch to other hypertensive drugs, the discontinuation of a prescription, or the end of the study (December 31, 2016).
2.3. Exposure
The data of patients who were administered several ARBs, including losartan, valsartan, irbesartan, and telmisartan, either alone or in combination with other medications, were analyzed in this study. An analysis of data from the National Health Insurance Service in 2013 has shown that the prescription rates for valsartan, losartan, irbesartan, and telmisartan among 0.2 million people were 13.8%, 32.0%, 5.7%, and 16.5%, respectively.[12] In the present study, an as-treated approach was utilized, and censoring was performed when the study drug was changed to another drug, including another ARB, during the follow-up period. Furthermore, treatment continuation was considered only if a second prescription was issued within 45 days (grace period, 15 days) after the first prescription of the study drug. If the time interval exceeded 45 days, the treatment was considered discontinued.
2.4. Outcome
The primary outcome was the hospital diagnosis of MI, HF, or cerebrovascular disease based on ICD-10 codes, following the initial prescription of a study drug. The secondary outcomes were cases in which each of the aforementioned events, with the addition of cerebral infarction, occurred.
The ICD-10 diagnostic codes for MI are I21–I23, I25.0, and I25.1,[13] with a reported diagnostic accuracy of 82% to 92%.[14] The diagnostic codes for HF are I11.0, I13.0, I13.2, and I50, with a diagnostic accuracy of 82%.[11,15] Lastly, the diagnostic codes for cerebrovascular disease are I60 to I66, with a diagnostic accuracy of 82% to 83%.[16]
2.5. Potential confounders
Demographic and socioeconomic factors, including age, sex, and insurance type, were assessed on the index date. To identify potential clinical confounders, comorbidities (hyperlipidemia, MI, congestive HF, cerebrovascular disease, diabetes mellitus, dementia, renal failure, liver disease, psychiatric disorder, chronic pulmonary disease, peripheral vascular disease, rheumatologic disease, hemiplegia, paraplegia, malignant leukemia, malignant lymphoma, and metastatic solid tumor) present the year before the index date were assessed. In addition, the use of concomitant drugs (calcium channel blockers, angiotensin-converting enzyme inhibitors, β-blockers, diuretics, statins, digoxin, tricyclic antidepressants, selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, gabapentinoids, antipsychotics, non-steroidal anti-inflammatory drugs, insulin, anticoagulants, metformin, sulfonylurea, dipeptidyl peptidase 4 inhibitors, sodium–glucose co-transporter 2 inhibitors, glucagon-like peptide 1 agonists, and other antidiabetic drugs) during the same period was evaluated. The Charlson comorbidity index (CCI) score was calculated to reflect the severity of a comorbidity using a previously validated algorithm (Supplemental table 1, http://links.lww.com/MD/K681).[17]
2.6. Statistical analysis
To limit potential confounding factors and improve comparability among valsartan, losartan, irbesartan, and telmisartan (as the reference standard), a matched cohort with a 1:1 ratio was established. A propensity score (PS), calculated using a logistic regression model, was used to match the patients in the cohort. An area under the curve of between 0.5 and 0.8 ensured the appropriateness of the matched cohort. A standardized mean difference of ≤ 0.1 confirmed that there were no significant differences among the groups.
Demographic and clinical characteristics, including age, sex, insurance type, CCI score, comorbidities, and concomitant medications were described using means and standard deviations, as well as frequencies and percentages based on categorical criteria. The incidence rate for each newly occurring event of MI, heart failure, or cerebrovascular disease was calculated separately and presented as the number of occurrences per 1000 person-years. Furthermore, hazard ratios (HRs) and 95% CIs were calculated using multivariate Cox proportional regression hazard model analysis, with correction factors for the aforementioned characteristics. The cumulative incidence of each outcome was estimated using Kaplan–Meier plots and log-rank tests. All statistical analyses were performed using R software on the server provided by the HIRA.
2.7. Subgroup and sensitivity analysis
A subgroup analysis based on factors such as age (<65 and ≥ 65 years), was performed. The grace period was modified to either 7 or 30 days for the as-treated approach. Finally, the effects of unmeasured confounding factors were evaluated using the E-value.[18]
2.8. Ethics approval
This study was approved by the Institutional Review Board of Sungkyunkwan University (No: SKKU-IRB-2022-06-004, June 17, 2022). The requirement for informed consent was waived by the Board.
3. Results
3.1. Patient characteristics
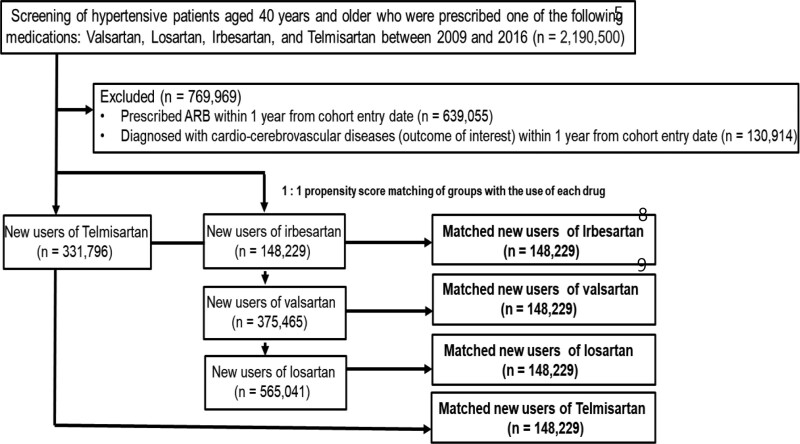
The study cohort of 1.29 million people with valsartan, losartan, irbesartan, or telmisartan use was derived from a HIRA database of 2.19 million people. A PS-matched cohort of 148,229 patients was established for each drug (Fig. 1). After PS matching, the standardized mean difference decreased to ≤ 0.016 for all demographic and clinical characteristics, except for the daily drug utilization rate and CCI score, with an standardized mean difference of 0.076 and 0.030, respectively. PS-matched cohort area under the curve values were calculated to be between 0.512 and 0.515. The median follow-up period ranged from 85 days to 118 days, and the mean number of ARBs administered per day ranged from 1.02 to 1.04 (Table 1).
Figure 1.
Flowchart of the selection criteria for patients with hypertension who were initially prescribed either valsartan, losartan, irbesartan, or telmisartan.
Table 1.
Patient demographics and clinical characteristics.
| Overall cohort | Matched Cohort by propensity score | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Valsartan (n = 375,465) | Losartan (n = 565,041) | Irbesartan (n = 148,229) | Telmisartan (n = 331,796) | SMD | Valsartan (n = 148,229) | Losartan (n = 148,229) | Irbesartan (n = 148,229) | Telmisartan (n = 148,229) | SMD | |
| AUC (95% CI) | 0.599 (0.597~0.60) | 0.60 (0.598~0.602) | - | 0.582 (0.580~0.584) | 0.512 (0.510~0.514) | 0.515 (0.513~0.517) | - | 0.515 (0.513~0.517) | ||
| Median follow-up (Q1-Q3, d) | 100 (28–447) | 69 (19–351) | 118 (30–509) | 89 (25–391) | 103 (28–458) | 85 (22–393) | 118 (30–509) | 91 (27–412) | ||
| *Usage per day, mean (SD) | 1.04 (0.19) | 1.03 (0.16) | 1.03 (0.18) | 1.01 (0.10) | 0.086 | 1.04 (0.19) | 1.03 (0.16) | 1.03 (0.18) | 1.02 (0.10) | 0.076 |
| Sex, n (%) | 0.031 | 0.005 | ||||||||
| Female | 182,628 (48.6) | 286,603 (50.7) | 72,795 (49.1) | 158,936 (47.9) | 68206 (49.6) | 68,098 (49.5) | 67,885 (49.4) | 68,586 (49.9) | ||
| Male | 192,837 (51.4) | 278,438 (49.3) | 75,434 (50.9) | 172,860 (52.1) | 69,240 (50.4) | 69,348 (50.5) | 69,561 (50.6) | 68,860 (50.1) | ||
| Age at index, mean (SD) | 63.23 (12.51) | 63.80 (12.55) | 62.74 (12.32) | 62.41 (12.51) | 0.061 | 62.94 (12.37) | 62.81 (12.37) | 62.74 (12.32) | 63.06 (12.41) | 0.015 |
| 40–59, n (%) | 155,030 (41.3) | 222,186 (39.3) | 62,571 (42.2) | 145,863 (44.0) | 61,842 (41.7) | 62,415 (42.1) | 62,571 (42.2) | 61,507 (41.5) | ||
| 60–79, n (%) | 180,413 (48.1) | 277,970 (49.2) | 71,852 (48.5) | 153,730 (46.3) | 71,934 (48.5) | 71,684 (48.4) | 71,852 (48.5) | 71,838 (48.5) | ||
| 80+, n (%) | 40,022 (10.7) | 64,885 (11.5) | 13,806 (9.3) | 32,203 (9.7) | 14,453 (9.8) | 14,130 (9.5) | 13,806 (9.3) | 14,884 (10.0) | ||
| Charlson comorbidity index, mean (SD) | 0.080 | 0.030 | ||||||||
| 0 | 97,218 (25.9) | 146,103 (25.9) | 32,825 (22.1) | 92,613 (27.9) | 34,532 (23.3) | 34,659 (23.4) | 32,825 (22.1) | 34,297 (23.1) | ||
| 1 | 99,302 (26.4) | 147,943 (26.2) | 38,588 (26.0) | 88,786 (26.8) | 39,322 (26.5) | 40,337 (27.2) | 38,588 (26.0) | 39,690 (26.8) | ||
| 2 | 70,667 (18.8) | 105,622 (18.7) | 28,440 (19.2) | 60,360 (18.2) | 27,302 (18.4) | 27,270 (18.4) | 28,440 (19.2) | 27,372 (18.5) | ||
| 3 | 46,246 (12.3) | 70,284 (12.4) | 20,423 (13.8) | 38,116 (11.5) | 19,251 (13.0) | 19,109 (12.9) | 20,423 (13.8) | 18,818 (12.7) | ||
| 4+ | 62,032 (16.5) | 95,089 (16.8) | 27,953 (18.9) | 51,921 (15.6) | 27,822 (18.8) | 26,854 (18.1) | 27,953 (18.9) | 28,052 (18.9) | ||
| Type of insurance, n (%) | 0.083 | 0.005 | ||||||||
| Public health care | 344,316 (91.7) | 510,390 (90.3) | 136,784 (92.3) | 301,802 (91.0) | 136,957 (92.4) | 137,138 (92.5) | 136,784 (92.3) | 136,872 (92.3) | ||
| Medical aid | 30,332 (8.1) | 51,848 (9.2) | 11,026 (7.4) | 25,755 (7.8) | 10,868 (7.3) | 10,673 (7.2) | 11,026 (7.4) | 10,939 (7.4) | ||
| Patriots & Veterans | 817 (0.2) | 2803 (0.5) | 419 (0.3) | 4239 (1.3) | 404 (0.3) | 418 (0.3) | 419 (0.3) | 418 (0.3) | ||
| Comorbidities, n (%) | ||||||||||
| Hyperlipidemia | 21,647 (5.8) | 28,732 (5.1) | 7543 (5.1) | 19,479 (5.9) | 0.022 | 8515 (5.7) | 8073 (5.4) | 7543 (5.1) | 8467 (5.7) | 0.016 |
| Myocardial infarction | 7711 (2.1) | 8262 (1.5) | 2898 (2.0) | 4681 (1.4) | 0.031 | 2828 (1.9) | 2658 (1.8) | 2898 (2.0) | 2697 (1.8) | 0.007 |
| Congestive heart failure | 52,732 (14.0) | 54,807 (9.7) | 13,573 (9.2) | 28,615 (8.6) | 0.089 | 13,775 (9.3) | 13,330 (9.0) | 13,573 (9.2) | 13,468 (9.1) | 0.006 |
| Cerebrovascular disease | 69,734 (18.6) | 104,851 (18.6) | 38,320 (25.9) | 68,433 (20.6) | 0.097 | 39,153 (26.4) | 38,597 (26.0) | 38,320 (25.9) | 39,672 (26.8) | 0.012 |
| Peripheral vascular disease | 36,566 (9.7) | 56,517 (10.0) | 13,253 (8.9) | 27,444 (8.3) | 0.035 | 12,878 (8.7) | 12,346 (8.3) | 13,253 (8.9) | 13,006 (8.8) | 0.011 |
| Renal failure | 19,193 (5.1) | 25,025 (4.4) | 10,092 (6.8) | 14,434 (4.4) | 0.059 | 10,049 (6.8) | 9529 (6.4) | 10,092 (6.8) | 9353 (6.3) | 0.012 |
| Diabetes mellitus w/o complication | 132,863 (35.4) | 199,315 (35.3) | 56,003 (37.8) | 115,028 (34.7) | 0.033 | 55,195 (37.2) | 55,068 (37.2) | 56,003 (37.8) | 55,998 (37.8) | 0.008 |
| Diabetes mellitus w/ complication | 46,882 (12.5) | 76,364 (13.5) | 25,384 (17.1) | 41,885 (12.6) | 0.070 | 24,754 (16.7) | 24,382 (16.4) | 25,384 (17.1) | 24,589 (16.6) | 0.010 |
| Dementia | 2675 (0.7) | 4343 (0.8) | 1035 (0.7) | 2457 (0.7) | 0.005 | 985 (0.7) | 961 (0.6) | 1035 (0.7) | 992 (0.7) | 0.003 |
| Chronic Pulmonary disease | 73,813 (19.7) | 113,184 (20.0) | 26,090 (17.6) | 61,256 (18.5) | 0.036 | 25,807 (17.4) | 25,326 (17.1) | 26,090 (17.6) | 25,823 (17.4) | 0.007 |
| Mild liver disease | 78,046 (20.8) | 123,142 (21.8) | 28,971 (19.5) | 70,338 (21.2) | 0.029 | 28,611 (19.3) | 28,238 (19.1) | 28,971 (19.5) | 28,573 (19.3) | 0.006 |
| Moderate/severe liver disease | 2134 (0.6) | 4634 (0.8) | 881 (0.6) | 1951 (0.6) | 0.015 | 857 (0.6) | 857 (0.6) | 881 (0.6) | 865 (0.6) | 0.001 |
| Rheumatologic disease | 10,047 (2.7) | 16,694 (3.0) | 4166 (2.8) | 8991 (2.7) | 0.009 | 4034 (2.7) | 3967 (2.7) | 4166 (2.8) | 4097 (2.8) | 0.005 |
| Hemiplegia paraplegia | 12,266 (3.3) | 17,953 (3.2) | 7577 (5.1) | 12,815 (3.9) | 0.054 | 7335 (4.9) | 6987 (4.7) | 7577 (5.1) | 7581 (5.1) | 0.011 |
| †Any malignancy | 27,456 (7.3) | 47,772 (8.5) | 10,088 (6.8) | 22,566 (6.8) | 0.034 | 9829 (6.6) | 9984 (6.7) | 10,088 (6.8) | 10,073 (6.8) | 0.004 |
| Metastatic solid tumor | 4460 (1.2) | 8937 (1.6) | 1575 (1.1) | 3710 (1.1) | 0.024 | 1511 (1.0) | 1544 (1.0) | 1575 (1.1) | 1497 (1.0) | 0.003 |
| Co-medications, n (%) | ||||||||||
| Insulin | 50,918 (13.6) | 79,307 (14.0) | 23,974 (16.2) | 41,575 (12.5) | 0.054 | 23,167 (15.6) | 22,468 (15.2) | 23,974 (16.2) | 23,088 (15.6) | 0.014 |
| Metformin | 77,107 (20.5) | 122,363 (21.7) | 34,551 (23.3) | 71,225 (21.5) | 0.034 | 34,362 (23.2) | 33,847 (22.8) | 34,551 (23.3) | 34,823 (23.5) | 0.008 |
| Sulfonylurea | 55,963 (14.9) | 95,885 (17.0) | 25,465 (17.2) | 50,168 (15.1) | 0.039 | 25,263 (17.0) | 24,663 (16.6) | 25,465 (17.2) | 25,405 (17.1) | 0.008 |
| DPP4 inhibitors | 34,241 (9.1) | 40,456 (7.2) | 12,468 (8.4) | 28,608 (8.6) | 0.037 | 12,386 (8.4) | 12,167 (8.2) | 12,468 (8.4) | 12,332 (8.3) | 0.004 |
| SGLT2 inhibitors | 657 (0.2) | 539 (0.1) | 200 (0.1) | 432 (0.1) | 0.011 | 194 (0.1) | 186 (0.1) | 200 (0.1) | 210 (0.1) | 0.002 |
| GLP1 agonist | 39 (0.006) | 39 (0.007) | 25 (0.013) | 38 (0.009) | 0.005 | 21 (0.011) | 21 (0.011) | 25 (0.013) | 18 (0.008) | 0.002 |
| Other antidiabetic drugs | 17,452 (4.6) | 33,193 (5.9) | 8867 (6.0) | 16,577 (5.0) | 0.036 | 8738 (5.9) | 8517 (5.7) | 8867 (6.0) | 8693 (5.9) | 0.005 |
| TCA/SSRI/SNRI/Gabapentinoids | 59,320 (15.8) | 93,768 (16.6) | 24,230 (16.3) | 53,885 (16.2) | 0.011 | 24,019 (16.2) | 23,424 (15.8) | 24,230 (16.3) | 23,953 (16.2) | 0.008 |
| Antipsychotics | 83,111 (22.1) | 12,4858 (22.1) | 32,137 (21.7) | 70,809 (21.3) | 0.011 | 32,152 (21.7) | 31,708 (21.4) | 32,137 (21.7) | 32,431 (21.9) | 0.006 |
| Anticoagulants | 132,690 (35.3) | 179,305 (31.7) | 45,187 (30.5) | 101,317 (30.5) | 0.056 | 45,156 (30.5) | 44,226 (29.8) | 45,187 (30.5) | 44,749 (30.2) | 0.008 |
| ß-blockers | 126,935 (33.8) | 150,664 (26.7) | 44,069 (29.7) | 87,112 (26.3) | 0.094 | 44,677 (30.1) | 43,545 (29.4) | 44,069 (29.7) | 44,497 (30.0) | 0.009 |
| CCB | 185,714 (49.5) | 268,209 (47.5) | 66,572 (44.9) | 150,523 (45.4) | 0.053 | 66,248 (44.7) | 65,086 (43.9) | 66,572 (44.9) | 66,320 (44.7) | 0.010 |
| ACE inhibitors | 37,527 (10.0) | 40,871 (7.2) | 12,665 (8.5) | 22,724 (6.8) | 0.065 | 12,298 (8.3) | 12,112 (8.2) | 12,665 (8.5) | 12,291 (8.3) | 0.007 |
| Diuretics | 135,104 (36.0) | 206,376 (36.5) | 51,449 (34.7) | 110,038 (33.2) | 0.040 | 52,096 (35.1) | 50,943 (34.4) | 51,449 (34.7) | 51,264 (34.6) | 0.009 |
| Statins | 148,302 (39.5) | 185,641 (32.9) | 59,988 (40.5) | 118,175 (35.6) | 0.093 | 60,385 (40.7) | 59,718 (40.3) | 59,988 (40.5) | 60,952 (41.1) | 0.009 |
| NSAIDs | 152,377 (40.6) | 250,820 (44.4) | 57,267 (38.6) | 136,622 (41.2) | 0.060 | 57,283 (38.6) | 56,280 (38.0) | 57,267 (38.6) | 57,165 (38.6) | 0.007 |
| Digoxin | 13,936 (3.7) | 17,428 (3.1) | 3871 (2.6) | 7227 (2.2) | 0.050 | 3817 (2.6) | 3783 (2.6) | 3871 (2.6) | 3822 (2.6) | 0.002 |
Gabapentinoids: gabapentin/pregabalin.
ACE = angiotensin-converting enzyme inhibitors, AUC = area under the curve, CCB = calcium channel blockers, CCI = Charlson comorbidity index, CI = confidence interval, DDP = dipeptidyl Peptidase-4 inhibitors, GLP = glucagon-like peptide-1 agonist NSAIDs = non-steroidal anti-inflammatory drugs, SD = standard deviation, SGLT = sodium-Glucose Co-transporter-2 inhibitors, SMD = standardized mean difference, SNRI = serotonin-norepinephrine reuptake inhibitors, SSRI = selective serotonin reuptake inhibitor, Q = quartile, TCA = tricyclic antidepressant, w/o = without, w/ = with.
Mean number of times the drug was administered per day.
Including lymphomas and leukemias; excluding malignant neoplasms of the skin.
3.2. Analyzing the risk of cardio-cerebrovascular disease among valsartan, losartan, irbesartan, and telmisartan with different half-lives
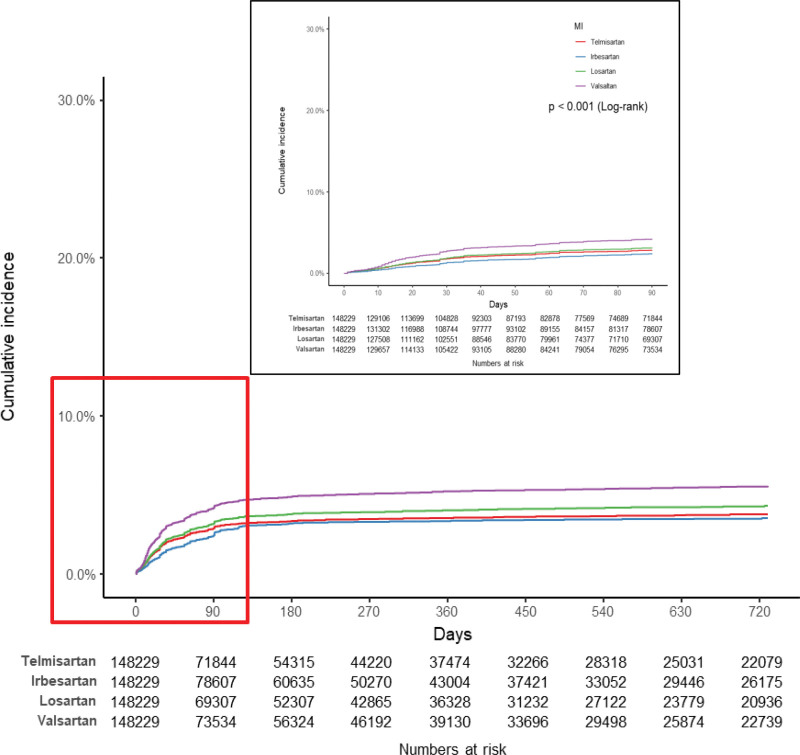
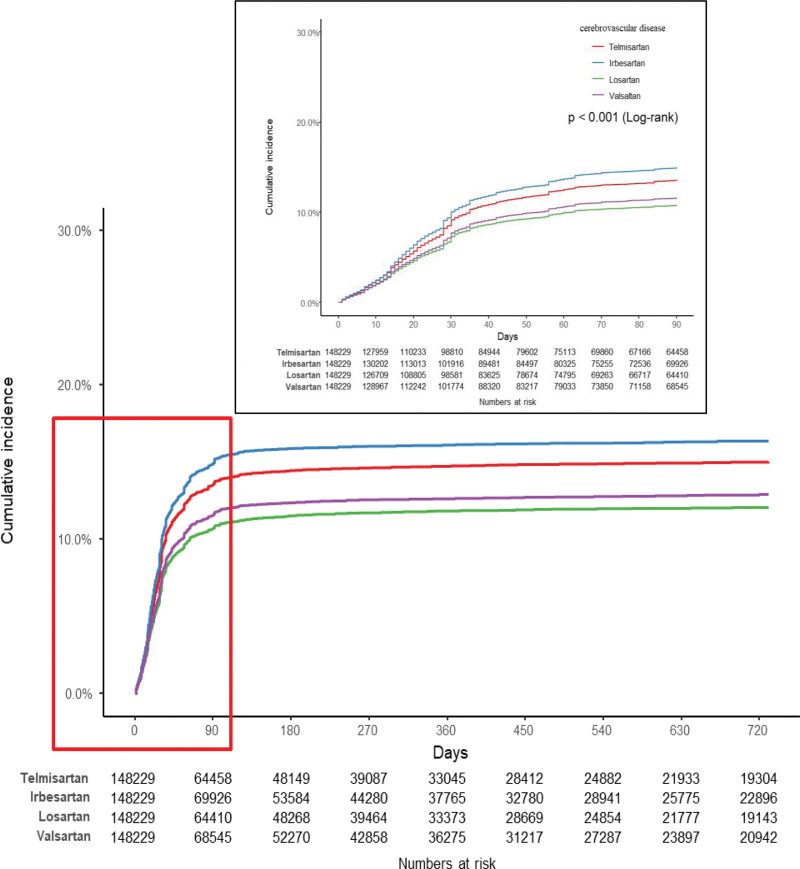
There were some differences in the incidence rates of MI per 1000 person-years among the matched cohorts with the use of the study drugs, with valsartan and losartan use being associated with relatively high incidence rates. However, compared to the other study drugs listed in Table 2, valsartan and losartan use was associated with relatively low incidence rates of cerebrovascular disease and cerebral infarction. In addition, the results of constructing Kaplan–Meier plots and performing log-rank tests on the matched cohort (Fig. 2) showed that the cumulative incidence of MI was highest with valsartan use, followed by losartan, telmisartan, and irbesartan use. Conversely, the same analysis revealed that the cumulative incidence of cerebrovascular disease was lowest with losartan use, followed by valsartan, telmisartan, and irbesartan use (Fig. 3). Compared to telmisartan use as the reference standard, valsartan (adjusted hazard ratio [aHR] 1.39, 95% CI 1.33–1.45) and losartan use (aHR 1.10, 95% CI 1.05–1.15) were associated with higher aHRs for MI. However, there was no significant difference in the aHRs for HF among the use of the drugs. The aHRs for cerebrovascular disease and cerebral infarction with losartan and valsartan use were lower than those with telmisartan use (Table 2).
Table 2.
Hazard ratios (HRs) for valsartan, losartan, and irbesartan, with telmisartan as the reference drug, in both the overall and matched cohorts.
| Outcome | Exposure | Overall cohort | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of patients | No. of event | Person yr | Incidence rate per 1000 Person-Yr | Crude HR (95% CI) | Adjusted* HR (95% CI) | Note | ||
| Myocardial infarction | Valsartan | 375,465 | 18,799 | 324,746.85 | 57.89 | 2.30 (2.24–2.36) | 1.50 (1.46–1.54) | †Short |
| Losartan | 565,041 | 12,794 | 447,815.50 | 28.57 | 1.09 (1.06–1.12) | 1.11 (1.08–1.15) | Short | |
| Irbesartan | 148,229 | 3456 | 151,743.84 | 22.78 | 1.01 (0.97–1.05)$ | 0.90 (0.87–0.94) | †Long | |
| Telmisartan | 331,796 | 7194 | 283,463.70 | 25.38 | Ref. | Ref. | Long | |
| Heart failure | Valsartan | 375,465 | 11,139 | 334,938.81 | 33.26 | 1.66 (1.61–1.71) | 1.32 (1.27–1.36) | ‡Approval |
| Losartan | 565,041 | 10,732 | 448,113.15 | 23.95 | 1.12 (1.08–1.15) | 0.95 (0.92–0.98) | ||
| Irbesartan | 148,229 | 2584 | 153,205.98 | 16.87 | 0.93 (0.89–0.98)$$ | 0.87 (0.83–0.91) | ||
| Telmisartan | 331,796 | 5867 | 284,367.16 | 20.63 | Ref. | Ref. | ||
| Cerebrovascular disease | Valsartan | 375,465 | 23,177 | 322,996.95 | 71.76 | 0.74 (0.73–0.75) | 0.70 (0.69–0.72) | |
| Losartan | 565,041 | 30,869 | 427,467.68 | 72.21 | 0.69 (0.68–0.70) | 0.67 (0.66–0.68) | ‡‡Approval | |
| Irbesartan | 148,229 | 18,425 | 134,559.93 | 136.93 | 1.49 (1.47–1.52) | 1.40 (1.37–1.42) | ||
| Telmisartan | 331,796 | 26,822 | 260,134.63 | 103.11 | Ref. | Ref. | ||
| Cerebral infarction | Valsartan | 375,465 | 17,431 | 330,745.40 | 52.70 | 0.72 (0.71–0.74) | 0.69 (0.68–0.71) | |
| Losartan | 565,041 | 22,349 | 437,374.15 | 51.10 | 0.65 (0.64–0.66) | 0.64 (0.63–0.65) | ||
| Irbesartan | 148,229 | 13,728 | 140,027.64 | 98.04 | 1.43 (1.40–1.47) | 1.31 (1.29–1.34) | ||
| Telmisartan | 331,796 | 20,692 | 267,242.72 | 77.43 | Ref. | Ref. | ||
| Outcome | Exposure | Matched cohort by PS | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of patients | No. of event | Person yr | Incidence rate per 1000 Person-Yr | Crude HR (95% CI) | Adjusted* HR (95% CI) | Note | ||
| Myocardial infarction | Valsartan | 148,229 | 5618 | 133,779.61 | 41.99 | 1.48 (1.42–1.54) | 1.39 (1.33–1.45) | †Short |
| Losartan | 148,229 | 4067 | 125,129.67 | 32.50 | 1.11 (1.06–1.16) | 1.10 (1.05–1.15) | Short | |
| Irbesartan | 148,229 | 3456 | 151,743.84 | 22.78 | 0.87 (0.84–0.92) | 0.90 (0.86–0.94) | †Long | |
| Telmisartan | 148,229 | 3756 | 130,417.75 | 28.80 | Ref. | Ref. | Long | |
| Heart failure | Valsartan | 148,229 | 2904 | 137,043.84 | 21.19 | 1.07 (1.02–1.13)! | 1.05 (0.99–1.10)!! | ‡Approval |
| Losartan | 148,229 | 2493 | 126,756.92 | 19.67 | 0.96 (0.90–1.01)!! | 0.95 (0.90–1.00)# | ||
| Irbesartan | 148,229 | 2584 | 153,205.98 | 16.87 | 0.92 (0.88–0.98)!!! | 0.94 (0.89–1.00)## | ||
| Telmisartan | 148,229 | 2672 | 131,754.08 | 20.28 | Ref. | Ref. | ||
| Cerebrovascular disease | Valsartan | 148,229 | 14,046 | 124,475.36 | 112.84 | 0.85 (0.83–0.87) | 0.85 (0.83–0.87) | |
| Losartan | 148,229 | 12,662 | 115,586.37 | 109.55 | 0.79 (0.77–0.81) | 0.80 (0.78–0.82) | ‡‡Approval | |
| Irbesartan | 148,229 | 18,425 | 134,559.93 | 136.93 | 1.10 (1.08–1.12) | 1.11 (1.09–1.13) | ||
| Telmisartan | 148,229 | 16,175 | 115,935.76 | 139.52 | Ref. | Ref. | ||
| Cerebral infarction | Valsartan | 148,229 | 10,720 | 128,790.07 | 83.24 | 0.83 (0.81–0.85) | 0.84 (0.82–0.86) | |
| Losartan | 148,229 | 9342 | 119,221.71 | 78.36 | 0.75 (0.73–0.77) | 0.76 (0.74–0.78) | ||
| Irbesartan | 148,229 | 13,728 | 140,027.64 | 98.04 | 1.05 (0.81–0.85) | 1.07 (1.04–1.09) | ||
| Telmisartan | 148,229 | 12,596 | 119,851.86 | 105.1 | Ref. | Ref. | ||
95% CI = confidence interval, HR = hazard ratio, PS = propensity score, Ref = reference.
$P=0.535,
$
$P=0.003.
!P=0.011,
!!P=0.099,
!!!P=0.005.
A multivariate Cox proportional hazard model.
half-life classification.
US & EU (Heart Failure and Post-Myocardial Infarction indicated to reduce the risk of cardiovascular mortality),
US & EU (Reduction of the risk of stroke in patients with hypertension).
#P = .067,
##P = .039.
Figure 2.
Kaplan–Meier plot showing the cumulative incidence of myocardial infarction in the matched cohorts for valsartan, losartan, irbesartan, and telmisartan.
Figure 3.
Kaplan–Meier plot showing the cumulative incidence of cerebrovascular disease in the matched cohorts for valsartan, losartan, irbesartan, and telmisartan.
3.3. Subgroup and sensitivity analyses
Subgroup analyses of the PS-matched cohort showed that the HR trends of the outcomes in the subgroups with losartan, valsartan, and irbesartan use were similar to those of the entire group. For all outcomes except HF, there was a correlation with age in the matched cohort (Supplemental table 2, http://links.lww.com/MD/K682 and 3, http://links.lww.com/MD/K683). The results of the sensitivity analysis indicated that the trends in hazard ratios (HRs) for the outcomes associated with the use of losartan, valsartan, and irbesartan compared to telmisartan remained consistent, whether the grace period was adjusted to 7 days, 15 days, or 30 days using the as-treated approach. (Supplemental table 4, http://links.lww.com/MD/K684 and 5, http://links.lww.com/MD/K685). E-value was conducted to assess the potential impact of unmeasured confounding factors (Supplemental table 6, http://links.lww.com/MD/K686). The E-value for hazard ratios (HRs) of irbesartan ranged from 1.50 to 1.78 for each outcome. Regarding losartan, the E-values for hazard ratios (HRs) were 1.58 for MI and heart failure, 2.09 for cerebrovascular disease, and 2.25 for cerebral infarction, respectively. The E-values for the hazard ratios (HRs) related to valsartan varied from 1.44 to 2.28 for each of the outcomes.
4. Discussion
Our study showed that the risk of MI was lower with irbesartan use and higher with valsartan and losartan use when compared to telmisartan use as the reference standard. Conversely, the risk of cerebrovascular disease was higher with irbesartan use and lower with valsartan and losartan use, compared to telmisartan use as the reference standard.
A previous meta-analysis of randomized clinical trials has shown no difference in the risk of MI and stroke among the use of the studied ARBs.[7] A recently published observational study has shown no difference in the risk of non-fatal MI and stroke among the use of 7 ARBs in patients with hypertension and without cardiovascular disease.[19] However, in our study, differences in the risks of MI and cerebrovascular disease among the use of ARBs were observed using telmisartan use as the reference standard. Valsartan and losartan use were associated with a higher risk of MI but a lower risk of cerebrovascular disease than telmisartan use. Conversely, irbesartan use was associated with a lower risk of MI but a higher risk of cerebrovascular disease compared to telmisartan use.
Telmisartan has a regulatory-approved indication for cardiovascular risk reduction because it showed an effect equivalent to that of ramipril in the Ongoing Telmisartan Alone and in Combination With Ramipril Global End Point Trial (ONTARGET).[20,21] Meanwhile, losartan has a regulatory-approved indication for stroke risk reduction because it showed an effect comparable to that of atenolol in the Losartan Intervention For Endpoint reduction in hypertension (LIFE) study.[22,23] Our findings showing the association of telmisartan use with a relatively low risk of MI and losartan use with a relatively low risk of cerebrovascular disease, support the licensed indications for these drugs.
The half-lives of ARBs can be classified as long or short. Telmisartan and irbesartan have long half-lives of 24 hours and 11 hours, respectively, whereas losartan and valsartan have short half-lives of 2 hours and 6 hours, respectively. The active metabolite of losartan, which is responsible for its pharmacologic effects, also has a short half-life of 6 to 9 hours.[24,25]
Our findings indicated that ARBs having long half-lives showed lower aHRs for MI but higher aHRs for cerebrovascular disease compared with ARBs having short half-lives. Therefore, we investigated how the difference in half-lives could affect aHRs for MI or cerebrovascular disease.
Telmisartan and irbesartan, which have long half-lives, are known to have longer-lasting and greater blood pressure-lowering effects than losartan or valsartan, which have short half-lives.[8] These properties may explain the greater cardiovascular risk reduction associated with telmisartan and irbesartan, but do not explain the greater cerebrovascular risk reduction associated with losartan and valsartan.
Cardiovascular diseases are affected not only by blood pressure but also by various other factors. In studies that evaluated the differences in cardiovascular risk reduction with the use of various antihypertensive medications, although blood pressure was controlled to a similar extent, the degree of cardiovascular risk reduction varied, suggesting that cardiovascular disease risk is determined by other factors in addition to blood pressure control.[23,26]
Aside from having blood pressure-lowering effects, ARBs are pleiotropic and have anti-inflammatory, antioxidant, antiproliferative, antifibrotic, antiplatelet, and neuroprotective effects.[24,27] These may have potential applications in the prevention of cardiovascular and cerebrovascular diseases. However, there is limited data from clinical and non-clinical trials on how these effects may differentially affect cardiovascular and cerebrovascular diseases. Variations in physiology, such as the dependence of coronary circulation on diastolic blood pressure and cerebral circulation on systolic blood pressure, may contribute to these differences.[28] Accordingly, different ARBs may have varying effects on cardiovascular or cerebrovascular risk. Liu et al have shown in a meta-analysis that low-dose aspirin is effective in preventing cardiovascular disease but not cerebrovascular disease, suggesting that understanding the unique properties of the cerebrovasculature is necessary for the prevention of cerebrovascular disease.[29]
The strength of our study lies in the utilization of the HIRA database, which contains comprehensive national health insurance claims data. As claims data are collected with minimal missing information and the majority of Koreans have national health insurance coverage, this database is highly representative of the entire Korean population.[30] However, there are some limitations in our study. First, during the follow-up period, censoring was performed at the earliest occurrence of the outcome of interest or when a patient was no longer prescribed the initial ARB. Several cases were censored at the beginning of the study, mainly because prescription records for the initial ARB were no longer found. To determine whether censoring affected the results, we evaluated different grace periods of 30 days, 15 days, and 7 days for medication discontinuation and found no significant differences. Second, blood pressure control could not be evaluated because of the lack of blood pressure measurements in the study. Because high blood pressure is a major risk factor for cardiovascular and cerebrovascular diseases, the confounding effect of the differences in the degree of blood pressure control cannot be excluded. Third, because this was an observational study, the unmeasured confounding effect of underlying diseases and concomitant medications also cannot be excluded. We matched the sex, age, comorbidities, and concomitant medications of patients at a 1:1 ratio for each group to ensure comparability between groups. To evaluate the potential impact of unmeasured confounding factors, we also calculated the E-value; however, the aforementioned limitations should be considered in the interpretation of the research results.
As established in several studies, cardiovascular and cerebrovascular diseases differ considerably. Our study results showed that the ARBs having long half-lives indicate lower aHRs for MI and higher aHRs for cerebrovascular events compared with the ARBs having short half-lives. We considered whether half-life could be one of the factors that led to these differences, but it is difficult to explain the differences in risks by half-life alone, as cardiovascular and cerebrovascular diseases are influenced by many factors other than blood pressure-lowering effects, including disease mechanisms and patients’ lifestyles. Further studies are required to expound on these differences.
5. Conclusions
We found differences in the risk of MI and cerebrovascular disease with the use of different ARBs compared to telmisartan use. Compared with telmisartan use, valsartan and losartan use, which showed a higher risk of MI, had a lower risk of cerebrovascular disease, and conversely, irbesartan use, which showed a lower risk of MI, had a higher risk of cerebrovascular disease. Further studies are required to expound on these differences.
Acknowledgments
We would like to acknowledge the Health Insurance and Review Assessment Service (HIRA) for providing a customized national database for national health insurance claim data (M20220615001). We would also like to thank Editage (www.editage.co.kr) for their assistance with English editing.
Author contributions
Conceptualization: Yung-Geun Yoo, Ju-Young Shin.
Data curation: Yung-Geun Yoo.
Formal analysis: Yung-Geun Yoo, Min-Jung Lim, Jin-Seob Kim, HeeJoo Ko.
Methodology: Yung-Geun Yoo, Jin-Seob Kim, HeeJoo Ko.
Resources: Yung-Geun Yoo, Jin-Seob Kim, Han-Eol Jeong, Ju-Young Shin.
Supervision: Ju-Young Shin.
Validation: Yung-Geun Yoo, Jin-Seob Kim, Han-Eol Jeong, HeeJoo Ko, Ju-Young Shin.
Writing – original draft: Yung-Geun Yoo, Min-Jung Lim.
Writing – review & editing: Yung-Geun Yoo, Min-Jung Lim, Han-Eol Jeong, Ju-Young Shin.
Supplementary Material
Abbreviations:
- aHR
- adjusted hazard ratio
- ARBs
- angiotensin receptor blockers
- CCI
- Charlson comorbidity index
- HF
- heart failure
- HIRA
- health insurance review and assessment service
- ICD
- international classification of diseases
- MI
- myocardial infarction
- PS
- propensity score
Posted history: This manuscript was previously posted to Research Square: doi: https://doi.org/10.21203/rs.3.rs-2925283/v1
Supplemental Digital Content is available for this article.
The authors have no funding and conflicts of interest to disclose.
The data that support the findings of this study are available from a third party, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available..
How to cite this article: Yoo Y-G, Lim M-J, Kim J-S, Jeong H-E, Ko H, Shin J-Y. Risk of myocardial infarction, heart failure, and cerebrovascular disease with the use of valsartan, losartan, irbesartan, and telmisartan in patients. Medicine 2023;102:46(e36098).
Contributor Information
Yung-Geun Yoo, Email: yunggeun@g.skku.edu.
Min-Jung Lim, Email: min-jung.lim@medisafe.kr.
Jin-Seob Kim, Email: jinseob2kim@gmail.com.
Han-Eol Jeong, Email: haneoljeong@hotmail.com.
HeeJoo Ko, Email: kohjoo7@catholic.ac.kr.
References
- [1].GBD. 2019 Diseases and Injuries Collaborators Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hajar R. Risk factors for coronary artery disease: historical perspectives. Heart Views. 2017;18:109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wu Y, Xiong Y, Wang P, et al. Risk factors of cardiovascular and cerebrovascular diseases in young and middle-aged adults: a meta-analysis. Medicine (Baltim). 2022;101:e32082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rapsomaniki E, Timmis A, George J, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1·25 million people. Lancet. 2014;383:1899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–67. [DOI] [PubMed] [Google Scholar]
- [6].Kim HC, Lee H, Lee HH, et al. Korea hypertension fact sheet 2021: analysis of nationwide population-based data with special focus on hypertension in women. Clin Hypertens. 2022;28:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tsoi B, Akioyamen LE, Bonner A, et al. Comparative efficacy of angiotensin II antagonists in essential hypertension: systematic review and network meta-analysis of randomised controlled trials. Heart Lung Circ. 2018;27:666–82. [DOI] [PubMed] [Google Scholar]
- [8].Nishimura T, Hashimoto J, Ohkubo T, et al. Efficacy and duration of action of the four selective angiotensin II subtype 1 receptor blockers, losartan, candesartan, valsartan and telmisartan, in patients with essential hypertension determined by home blood pressure measurements. Clin Exp Hypertens. 2005;27:477–89. [DOI] [PubMed] [Google Scholar]
- [9].Oparil S, Williams D, Chrysant SG, et al. Comparative efficacy of olmesartan, losartan, valsartan, and irbesartan in the control of essential hypertension. J Clin Hypertens (Greenwich). 2001;3:283–91, 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kim JA, Yoon SJ, Kim LY, et al. Towards actualizing the value potential of Korea Health Insurance Review and Assessment (HIRA) data as a resource for health research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. 2017;32:718–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Park B, Sung J, Park K, et al. Report of the evaluation for validity of discharged diagnoses in Korean Health Insurance Database. Seoul, Republic of Korea: Seoul National University; 2003:19–52. [Google Scholar]
- [12].Jeong HS, Lim HS, Park HJ, et al. Clinical outcomes between calcium channel blockers and angiotensin receptor blockers in hypertensive patients without established cardiovascular diseases during a 3-year follow-up. Sci Rep. 2021;11:1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chin HJ, Nam JH, Lee EK, et al. Comparative safety for cardiovascular outcomes of DPP-4 inhibitors versus glimepiride in patients with type 2 diabetes: a retrospective cohort study. Medicine (Baltim). 2017;96:e7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Park J, Kwon S, Choi EK, et al. Validation of diagnostic codes of major clinical outcomes in a National Health Insurance database. Int J Arrhythm. 2019;20:5. [Google Scholar]
- [15].Park JJ, Lee CJ, Park SJ, et al. Heart Failure Statistics in Korea, 2020: a report from the Korean Society of Heart Failure. Int J Heart Fail. 2021;3:224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Park JK, Kim KS, Kim CB, et al. The accuracy of ICD codes for cerebrovascular diseases in medical insurance claims. Korean J Prev Med. 2000;33:76–82. [Google Scholar]
- [17].Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 Administrative data. Med Care. 2005;43:1130–9. [DOI] [PubMed] [Google Scholar]
- [18].VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value 2017. Ann Intern Med. 2017;167:268–74. [DOI] [PubMed] [Google Scholar]
- [19].Lee W, Kang J, Park JB, et al. Long-term mortality and cardiovascular events of seven angiotensin receptor blockers in hypertensive patients: analysis of a national real-world database: a retrospective cohort study. Health Sci Rep. 2023;6:e1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].US FDA Prescribing Information of Telmisartan. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/020850s045lbl.pdf [access date Aug 6, 2023].
- [21].Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547–53. [DOI] [PubMed] [Google Scholar]
- [22].US FDA Prescribing Information of Losartan. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/020386s064lbl.pdf [access date Aug 6, 2023].
- [23].Dahlöf B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. [DOI] [PubMed] [Google Scholar]
- [24].Munger MA. Use of Angiotensin receptor blockers in cardiovascular protection: current evidence and future directions. P T. 2011;36:22–40. [PMC free article] [PubMed] [Google Scholar]
- [25].Abraham HM, White CM, White WB. The comparative efficacy and safety of the angiotensin receptor blockers in the management of hypertension and other cardiovascular diseases. Drug Saf. 2015;38:33–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shiraishi J, Sawada T, Koide M, et al. Cardio-cerebrovascular protective effects of valsartan in high-risk hypertensive patients with coronary artery disease (from the Kyoto Heart Study). Am J Cardiol. 2012;109:1308–14. [DOI] [PubMed] [Google Scholar]
- [27].Koh KK, Ahn JY, Han SH, et al. Pleiotropic effects of angiotensin II receptor blocker in hypertensive patients. J Am Coll Cardiol. 2003;42:905–10. [DOI] [PubMed] [Google Scholar]
- [28].Angeli F, Reboldi G, Verdecchia P. Hypertension and the J-curve phenomenon: implications for tight blood pressure control. Hypertens Res. 2013;36:109–11. [DOI] [PubMed] [Google Scholar]
- [29].Liu C, Du L, Wang S, et al. Differences in the prevention and control of cardiovascular and cerebrovascular diseases. Pharmacol Res. 2021;170:105737. [DOI] [PubMed] [Google Scholar]
- [30].Kim S, Kim MS, You SH, et al. Conducting and reporting a Clinical Research using Korean Healthcare Claims Database. Korean J Fam Med. 2020;41:146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.