Abstract
As an important member of Wnt/β-catenin signaling pathway, the aberrant expression of β-catenin has been implicated in many cancers. Chibby, a β-catenin binding partner, is an antagonist involved in this pathway. In contrast, thyroid cancer 1 (TC1) as an activator of this pathway can relieve the antagonistic activity of Chibby on the β-catenin-mediated transcription and is high expressed in human tumors. The objectives of this study were to examine the expression of TC1, Chibby, and β-catenin and investigate the association among them in laryngeal squamous cell carcinoma (LSCC). The expression of TC1, Chibby, β-catenin, c-Myc, Cyclin D1, and matrix metalloproteinase-7 (MMP-7) were examined by immunohistochemistry in samples from 53 LSCC patients. Compared with normal laryngeal squamous epithelium (NLSE), there were upregulated expression of TC1, downregulated expression of Chibby, and aberrant cytoplasmic expression of β-catenin in the LSCC tissues (P < .001). The high expression of TC1 was correlated with the tumor site, advanced TNM and T stage, lymphovascular invasion, and poor differentiation in LSCC tissues (P < .050). There were correlations between the aberrant expression of β-catenin and the tumor site, advanced TNM and T stage, lymphovascular invasion, perineurial invasion, and poor differentiation in LSCC tissues (P < .050). Upregulated TC1 and downregulated Chibby were correlated with aberrant expression of β-catenin (P < .001), but no correlation between them (P = .076). The percent of abnormal expression of β-catenin in LSCC was 96.00% in TC1+/Chibby−, 73.68% in TC1+/Chibby+, 0.00% in TC1-/Chibby−, and 0.00% in TC1-/Chibby + group (P < .001). High expression of c-Myc, Cyclin D1, and MMP-7 was observed in LSCC tissues (P < .001). There was statistically significant about the expression of Cyclin D1 and MMP-7 among the groups of TC1+/Chibby−, TC1+/Chibby+, TC1-/Chibby−, and TC1-/Chibby + (P < .001), but was not significance about the expression of c-Myc among them (P = .339). No association was found between overall survival and the expression of TC1, Chibby, and β-catenin (P > .05). The upregulated expression of TC1 and downregulated expression of Chibby were correlated with the aberrant expression of β-catenin and the high expression of Cyclin D1 and MMP-7 in LSCC tissues.
Keywords: Chibby, laryngeal squamous cell carcinoma, signaling pathway, thyroid cancer 1, Wnt, β-catenin
1. Introduction
Laryngeal squamous cell carcinoma (LSCC) is accounting for about 95% of all the larynx malignant tumors and is one of the most common malignances in the head and neck.[1,2] There are 184,615 new cases and 99,840 deaths of laryngeal cancer according to the global statistics in 2020 from the International Agency for Research on Cancer.[3] Despite treatment of LSCC has changed from surgery to the combined multidisciplinary treatments associated with concomitant or sequential chemotherapy and radiotherapy, the overall 5-year survival rate in patients with LSCC is slightly decreased from 66% to 63% over the last 40 years.[2] The biological therapy of LSCC has become a research hotspot in recent years. Therefore, seeking novel and effective therapeutic targets is crucial to patients with LSCC.
Dysregulaton of Wnt/β-catenin signaling pathway has been linked to the occurrence and development of a variety of tumors, promoting progression, metastasis, and poor prognosis.[4,5] Moreover, aberrant regulatory β-catenin as an essential element of Wnt/β-catenin pathway is the key to initiating this pathway, which is responsible for triggering transcription of a subset of downstream genes.[6] In fact, several reports have found that β-catenin, which reduced from cytomembrane and accumulated in cytoplasm, closely relate to differentiation, aggressive biological behavior and prognosis of laryngeal carcinoma.[7–9] According to these results, the Wnt/β-catenin signaling pathway may pay an important role in the development and progression of laryngeal cancer. Therefore, the determination of other positive or negative regulators of this pathway provides an important method to identify novel therapeutic targets for treatment of LSCC.
As we know, once the Wnt/β-catenin pathway is activated, it can result in the stability of cytosolic β-catenin. Stable β-catenin translocates into the nucleus and interacts with T-cell factor/lymphocyte enhancer factor transcription factors to induce the expression of Wnt target genes.[6,10] Chibby is a highly conserved protein that was originally identified as a β-catenin antagonist in a protein-protein interaction screen using the bait of C-terminal region of β-catenin in 2003.[11] Chibby represses β-catenin-mediated transcriptional activation by competing with T-cell factor/lymphocyte enhancer factor factors for β-catenin binding in nucleus and cooperating with 14-3-3 adaptor proteins to promote the export of β-catenin from the nucleus to the cytoplasm.[11–13] The regulatory effect of Chibby in the Wnt/β-catenin pathway suggests the biological importance of Chibby as a potential tumor suppressor gene.[11] However, thyroid cancer 1 (TC1) is first described in thyroid cancers[14] and functions as a positive regulator in the Wnt/β-catenin pathway.[15] It can release β-catenin from Chibby via its transient helical structure and relieve the antagonistic activity of Chibby on the β-catenin-mediated transcriptions, lending to the upregulation of many β-catenin target genes.[15,16] Therefore, TC1 is an upstream positive regulator of the Wnt/β-catenin pathway and could potentially be a therapeutic target of cancers.
The interactions and correlations among the expressions of TC1, Chibby, and β-catenin have been reports in many malignant tumors. On one hand, the upregulated TC1 is positively correlated with the abnormal expression of β-catenin in oral tongue squamous cell carcinoma,[17] lung cancer,[18] and cervical squamous cell carcinoma.[19] On the other hand, the downregulated Chibby is negatively correlated with the expression of β-catenin in lung cancers.[20] Moreover, the combination of Chibby and β-catenin may potentially to a biomarker for disease progression in patients with cervical cancer and hepatocellular carcinoma.[21,22] However, the interactions and correlations among them in LSCC have not been reported. In the present study, we examined the expression of TC1, Chibby, β-catenin, c-Myc, Cyclin D1, and matrix metalloproteinase-7 (MMP-7) in LSCC and corresponding normal laryngeal squamous epithelium (NLSE), investigated the correlations among them and the associations between their expressions and clinicopathological factors or prognosis of patients with LSCC.
2. Materials and methods
2.1. Study population
A total of 53 patients with LSCC who underwent surgery at the Department of Otolaryngology of the First People Hospital of Huzhou (Huzhou, Zhejiang, China) were recruited into this study. 19 patients of them were treated with plasma or carbon dioxide laser surgery under supportive laryngoscope. The other 34 patients received partial or total laryngectomy and neck lymph node dissection. Tumor tissues and paired adjacent normal laryngeal mucosa tissues were obtained from all of the 53 patients and embedded in paraffin for later experiments. Inclusion criteria were adults aged more than 18 years both male and female and were diagnosed and treated for the first time. Exclusion criteria included patients received radiotherapy or chemotherapy prior to surgery, were transferred to other hospitals during treatment, and with other types of cancer or other severe diseases.
The recruited patients in this study comprised 51 males and 2 females, and were aged between 51 and 93 years, with a mean age of 66.15 ± 8.21 years and a median age of 66 years. There were 38 patients smoking and 33 patients drinking. Tumor tissues of 29 patients were located at the glottis. According to tumor, node, metastases (TNM) staging of laryngeal cancer (2018 AJCC 8rd edition), samples were classified as T1 in 17 cases, T2 in 10 cases, T3 in 17 cases, and T4 in 9 cases or stage I in 17 cases, stage II in 8 cases, stage III in 14 cases, and stage IV in 14 cases. The lymphatic metastasis, lymphovascular invasion, and perineurial invasion were been observed in 8, 15, and 9 cases of them, respectively. The differentiation grade evaluated by 2 pathologists at the First People Hospital of Huzhou, was high in 20, moderate in 25, and poor in 8 samples. In addition, follow-up of these patients was only completed form 9 to 60 months, with a mean time of 27.04 ± 15.23 months and a median time of 23 months. 7 patients of them died during the follow-up period. They all agreed to take part in the study and signed an informed consent form. The Ethical Committee of the First People Hospital of Huzhou approved the study protocol (approval no. 2018027).
2.2. Immunohistochemistry (IHC)
The paraffin-embedded tissue blocks were cut into 4-μm sections. These continuous sections were dewaxed in xylene and rehydrated in graded ethanols. The antigen retrieval was performed by pressure cooking in an acitrate buffer (10mmol/L, pH 6) for 10 minutes. All sections were subsequently incubated with primary rabbit anti-TC1 polyclonal antibody (Dilution 1:200; 26279-1-AP), rabbit anti-Chibby polyclonal antibody (Dilution 1:50; 12239-1-AP), mouse anti-β-catenin monoclonal antibody (Dilution 1:5000; 66379-1-Ig), mouse anti-c-Myc monoclonal antibody (Dilution 1:1000; 67447-1-Ig), rabbit anti-Cyclin D1 polyclonal antibody (Dilution 1:500; 26939-1-AP) or rabbit anti-MMP-7 polyclonal antibody (Dilution 1:200; 10374-2-AP) overnight at 4 °C, and all of these antibodies were purchased from Proteintech, Wuhan, China. The detection of antibodies was accomplished using the streptavidin-peroxidase method. Slides were counterstained with hematoxylin, dehydrated and mounted. PBS was added instead of the primary antibodies as negative controls.
2.3. Evaluation of immunostaining
All the immunostained sections were evaluated by 2 independent investigators who were blinded to the clinical data. The intensity of immunostaining was scored from 0 to 3 to represent negative, weak, moderate, or strong, respectively. Five views per slide were randomly examined, and 100 tumor cells or normal squamous epithelial cells were observed per view at × 400 magnification. The positive rate of every case was obtained by calculating the average percentage of positively stained cells in each section. Percentage scores for TC1, Chibby, c-Myc, Cyclin D1, and MMP-7 were divided into 5 grades: 0 (0–5%), 1 (6–25%), 2 (26–50%), 3 (51–75%), and 4 (76–100%). The final score from each sample was obtained by multiplying the intensity score and percentage score and ranged from 0 to 12. According to the final score, the results of staining were finally estimated as negative expression (0), weak positive expression (1–4), moderate positive expression (5–8), and strong positive expression (9–12). It was worth noting that β-catenin was abnormally expressed when the percentage of positive cells stained in the cytomembrane was <70% in NLSE or that stained in the cytoplasm and/or nucleus was more than 10% in LSCC, which was scored 1. Otherwise, its expression was normal and scored 0. Therefore, the results of staining for β-catenin were finally determined as negative expression, weak positive expression, moderate positive expression, and strong positive expression according to a final score of 0, 1, 2, and 3, respectively.
2.4. Statistical analysis
Statistical analyses were performed using the software package SPSS version 23.0 (SPSS Inc., Chicago, IL, USA). The categorical variables were compared by the Chi-square (x 2) test or Fisher exact test when the theoretical frequency was smaller than 5. Data were presented as count and percentage. Spearman rank correlation test was used for analyzed correlations among TC1, Chibby, β-catenin, c-Myc, Cyclin D1, and MMP-7 and between them and the clinicopathologic characteristics of LSCC. Curves for survival rates were determined by the Kaplan–Meier analysis and compared using the log-rank test. Univariate Cox proportional hazards model was used to determine the factors of survival. All P values were 2-tailed. A difference was considered to be statistically significant when the P value was <.05.
3. Results
3.1. Upregulated TC1 was correlated with the some clinicopathologic characteristics in LSCC
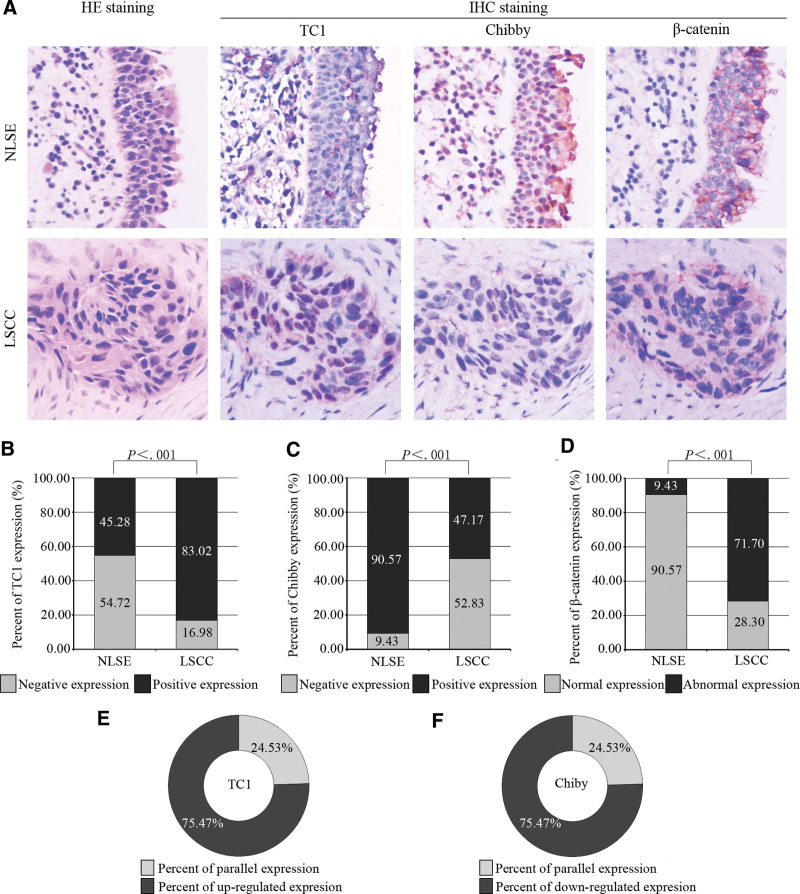
The subcellular localizations of TC1 in NLSE and LSCC tissues were mainly observed in the cytoplasm and some in the nucleus (Fig. 1A). The expression of TC1 was negative or weak positive in most NLSE tissues, but was positive in most LSCC tissues (Fig. 1A). Moreover, the percent of TC1 positive expression was 45.28% (24/53) in NLSE tissues and 83.02% (44/53) in LSCC tissues (P < .001, Fig. 1B). When we compared LSCC tissue with adjacent NLSE tissue from the same patient, upregulated expression of TC1 was detected in 75.47% (40/53) of them (Fig. 1E). As shown in Table 1, the high expression of TC1 in LSCC was correlated with tumor site (P = .031), advanced TNM stage (P = .009) and T stage (P = .024), lymphovascular invasion (P = .047), and poor differentiation (P = .019), but did not correlated with the sex (P = 1.000), age (P = 1.000), smoking (P = 1.000), drinking (P = .456), lymphatic metastasis (P = .324), and perineurial invasion (P = .329).
Figure 1.
Expressions of TC1, Chibby, and β-catenin in NLSE and LSCC tissues. (A) Results of immunostaining for TC1, Chibby, and β-catenin in NLSE and LSCC tissues were showed by continuous sections. The subcellular localizations of TC1 and Chibby were mainly in cytoplasm and nucleus of both NLSE and LSCC tissues, respectively. The intensity of immunostaining for β-catenin was strongest in cytomembrane of NLSE tissues and in cytoplasm of LSCC tissues. (B, C) Percent of positive expression for TC1 and Chibby; (D) Percent of abnormal expression for β-catenin; (E) Percent of upregulated expression for TC1 in LSCC tissues compare with NLSE tissues from the same patient; (F) Percent of downregulated expression for Chibby in LSCC tissues compare with NLSE tissues from the same patient. The dates were calculated by the x2 test and presented by number (%) in the figure B, C, and D. P value of <.05 was considered statistically significant. HE = hematoxylin-eosin, IHC = immunohistochemistry, LSCC = laryngeal squamous cell carcinoma, NLSE = normal laryngeal squamous epithelium, TC1 = thyroid cancer 1.
Table 1.
Relationships between the expression of TC1, Chibby, β-catenin and clinicopathological features in LSCC.
| Clinicopathological features | n | TC1 expression | P value | Chibby expression | P value | β-catenin expression | P value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Negative (%) | Positive (%) | Negative (%) | Positive (%) | Normal (%) | Abnormal (%) | |||||
| Sex | ||||||||||
| Male | 51 | 9 (17.65) | 42 (82.35) | 1.000* | 28 (54.90) | 23 (45.10) | .218* | 15 (29.41) | 36 (70.59) | 1.000* |
| Female | 2 | 0 (0.00) | 2 (100.00) | 0 (0.00) | 2 (100.00) | 0 (0.00) | 2 (100.00) | |||
| Agea | ||||||||||
| <66 | 25 | 4 (16.00) | 21 (84.00) | 1.000* | 14 (56.00) | 11 (44.00) | .662# | 7 (28.00) | 18 (72.00) | .963# |
| ≥66 yr | 28 | 5 (17.86) | 23 (82.14) | 14 (50.00) | 14 (50.00) | 8 (28.57) | 20 (71.43) | |||
| Smoking | ||||||||||
| No | 15 | 2 (13.33) | 13 (86.67) | 1.000* | 7 (46.67) | 8 (53.33) | .572# | 3 (20.00) | 12 (80.00) | .510* |
| Yes | 38 | 7 (18.42) | 31 (81.58) | 21 (55.26) | 17 (44.74) | 12 (31.58) | 26 (68.42) | |||
| Drinking | ||||||||||
| No | 20 | 2 (10.00) | 18 (90.00) | .456* | 10 (50.00) | 10 (50.00) | .748# | 7 (35.00) | 13 (65.00) | .399# |
| Yes | 33 | 7 (21.21) | 26 (78.79) | 18 (54.55) | 15 (45.45) | 8 (24.24) | 25 (75.76) | |||
| Tumor site | ||||||||||
| Glottic | 29 | 8 (27.59) | 21 (72.41) | .031* | 14 (48.28) | 15 (51.72) | .465# | 13 (44.83) | 16 (55.17) | .003# |
| Non-glottic | 24 | 1 (4.17) | 23 (95.83) | 14 (58.33) | 10 (41.67) | 2 (8.33) | 22 (91.67) | |||
| TNM stage | ||||||||||
| I + II | 25 | 8 (32.00) | 17 (68.00) | .009* | 12 (48.00) | 13 (52.00) | .506# | 12 (48.00) | 13 (52.00) | .003# |
| III + IV | 28 | 1 (3.57) | 27 (96.43) | 16 (57.14) | 12 (42.86) | 3 (10.71) | 25 (89.29) | |||
| T stage | ||||||||||
| 1 + 2 | 27 | 8 (29.63) | 19 (70.37) | .024* | 14 (51.85) | 13 (48.15) | .884# | 12 (44.44) | 15 (55.56) | .008# |
| 3 + 4 | 26 | 1 (3.85) | 25 (96.15) | 14 (53.85) | 12 (46.15) | 3 (11.54) | 23 (88.46) | |||
| Lymphatic metastasis | ||||||||||
| No | 45 | 9 (20.00) | 36 (80.00) | .324* | 22 (48.89) | 23 (51.11) | .256* | 13 (28.89) | 32 (71.11) | 1.000* |
| Yes | 8 | 0 (0.00) | 8 (100.00) | 6 (75.00) | 2 (25.00) | 2 (25.00) | 6 (75.00) | |||
| Lymphovascular invasion | ||||||||||
| No | 38 | 9 (23.68) | 29 (76.32) | .047* | 19 (50.00) | 19 (50.00) | .511# | 14 (36.84) | 24 (63.16) | .041* |
| Yes | 15 | 0 (0.00) | 15 (100.00) | 9 (60.00) | 6 (40.00) | 1 (6.67) | 14 (93.33) | |||
| Perineurial invasion | ||||||||||
| No | 44 | 9 (20.45) | 35 (79.55) | .329* | 23 (52.27) | 21 (47.73) | 1.000* | 15 (34.09) | 29 (65.91) | .047* |
| Yes | 9 | 0 (0.00) | 9 (100.00) | 5 (55.56) | 4 (44.44) | 0 (0.00) | 9 (100.00) | |||
| Differentiation | ||||||||||
| Highly | 20 | 7 (35.00) | 13 (65.00) | .019* | 9 (45.00) | 11 (55.00) | .374# | 11 (55.00) | 9 (45.00) | .001# |
| Moderately + Poorly | 33 | 2 (6.06) | 31 (93.94) | 19 (57.58) | 14 (42.42) | 4 (12.12) | 29 (87.88) | |||
LSCC = laryngeal squamous cell carcinoma, TC1 = thyroid cancer 1, TNM = tumor node metastases.
The patients were grouped according to median age (66 yr).
# Statistical significance was determined using the Χ2 test.
Statistical significance was determined using the Fisher exact test in 2 × 2 table because the theoretical frequency was smaller than 5. The results were showed by number (%). P value of <.05 was considered statistically significant.
3.2. Downregulated Chibby was not correlated with the clinicopathologic characteristics of LSCC
The expression of Chibby in NLSE and LSCC tissues was mainly discovered in the nucleus and a few in the cytoplasm (Fig. 1A). Chibby was highly expressed in NLSE tissues. In contrast, the level of its expression was remarkably reduced in LSCC tissues (Fig. 1A). The percent of Chibby positive expression was 90.57% (48/53) in NLSE tissues and 47.17% (25/53) in LSCC tissues (P < .001, Fig. 1C). There was 75.47% (40/53) patient showed the downregulated of Chibby expression according to the staining of tumor and normal tissues from the same case (Fig. 1F). There was no correlation between the low expression of Chibby and sex, age, smoking, drinking, tumor site, TNM stage, T stage, lymphatic metastatic status, lymphovascular invasion, perineurial invasion, and tumor differentiation in tumor samples (P > .05, Table 1).
3.3. Aberrant β-catenin expression was correlated with the some clinicopathologic characteristics in LSCC
The membranous expression of β-catenin was clear in the NLSE tissues, but was very weak or negative in most LSCC tissues. However, its expression in cytoplasm was obvious in most LSCC tissues and was little in the NLSE tissues. In addition, there was a few of its expression in the nucleus of LSCC tissues, but it was absence in the nucleus of NLSE tissues. In a word, we found that expression of β-catenin was reduced in cytomembrane and increased in cytoplasm and nucleus in tumor tissue (Fig. 1A). The level of its expression was separately evaluated in normal or cancer tissues according to the method described above. The percent of abnormal expression was 9.43% (5/53) in NLSE tissues and 71.70% (38/53) in LSCC tissues (P < .001, Fig. 1D). The relationships were discovered between aberrant expression of β-catenin and tumor site (P = .003), advanced TNM stage (P = .003) and T stage (P = .008), lymphovascular invasion (P = .041), and perineurial invasion (P = .047), and poor differentiation (P = .001), but not with the sex (P = 1.000), age (P = .963), smoking (P = .510), drinking (P = .399), lymphatic metastasis (P = 1.000), (Table 1).
3.4. Upregulated TC1 and Downregulated Chibby were correlated with aberrant expression of β-catenin, but no correlation between them
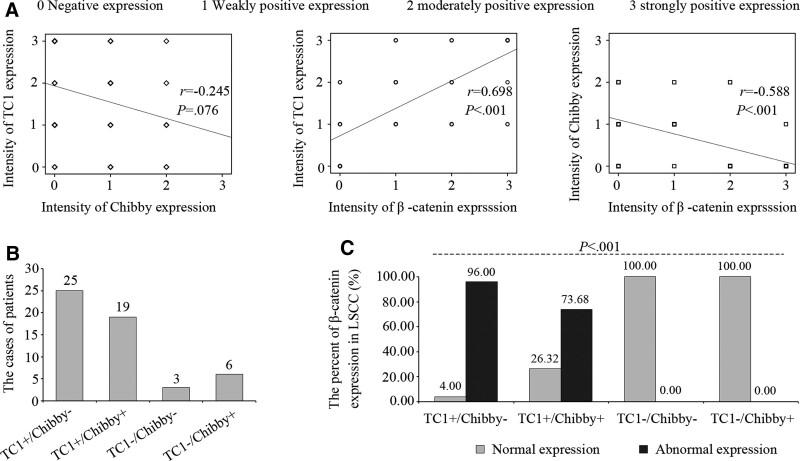
Spearman rank correlation test was used to determine the relationship among TC1, Chibby, and β-catenin (Fig. 2A). The expression of TC1 was positively correlated with the expression of β–catenin (R = 0.698, P < .001), but the expression of Chibby was negatively correlated with that (r = −0.588, P < .001). In addition, there was no correlation between TC1 and Chibby (r = −0.245, P = .076).
Figure 2.
Correlations among TC1, Chibby, and β-catenin in LSCC tissues. (A) Correlations among TC1, Chibby, and β-catenin in LSCC tissues. The dates represented the intensity of expression for TC1, Chibby, and β-catenin were calculated by Spearman rank correlation test. (B) Numbers of patients in groups of TC1+/Chibby−, TC1+/Chibby+, TC1-/Chibby−, and TC1-/Chibby+, which were divided according to the expression level of TC1 and Chibby. (C) Percent of abnormal expression of β-catenin in LSCC tissues among the 4 groups. Statistical significance was determined using the Fisher exact test in R × C table because of some theoretical frequency was smaller than 5. The results were showed by number (%). P value of <.05 was considered statistically significant. LSCC = laryngeal squamous cell carcinoma, TC1 = thyroid cancer 1, TC1+/Chibby− = positive expression of TC1 and negative expression of Chibby, TC1+/Chibby+ = positive expression of TC1 and Chibby, TC1-/Chibby− = negative expression of TC1 and Chibby, TC1-/Chibby+ = negative expression of TC1 and positive expression of Chibby.
To further clarify the common effect of TC1 and Chibby on β-catenin, we divided the 53 patients into 4 groups based on the expression level of TC1 and Chibby (Fig. 2B). The symbol “+” or “−” were used to represent the positive or negative expression, respectively. They were successively named as TC1+/Chibby− group including 25 cases, TC1+/Chibby + group including 19 cases, TC1-/Chibby− group including 3 cases, and TC1-/Chibby + group including 6 cases. The percent of abnormal expression of β-catenin in LSCC was 96.00% (24/25) in TC1+/Chibby− group, 73.68% (14/19) in TC1+/Chibby + group, 0.00% (0/3) in TC1-/Chibby− group, and 0.00% (0/6) in TC1-/Chibby + group (P < .001, Fig. 2C).
3.5. High expression of c-Myc, Cyclin D1, and MMP-7 was observed in LSCC
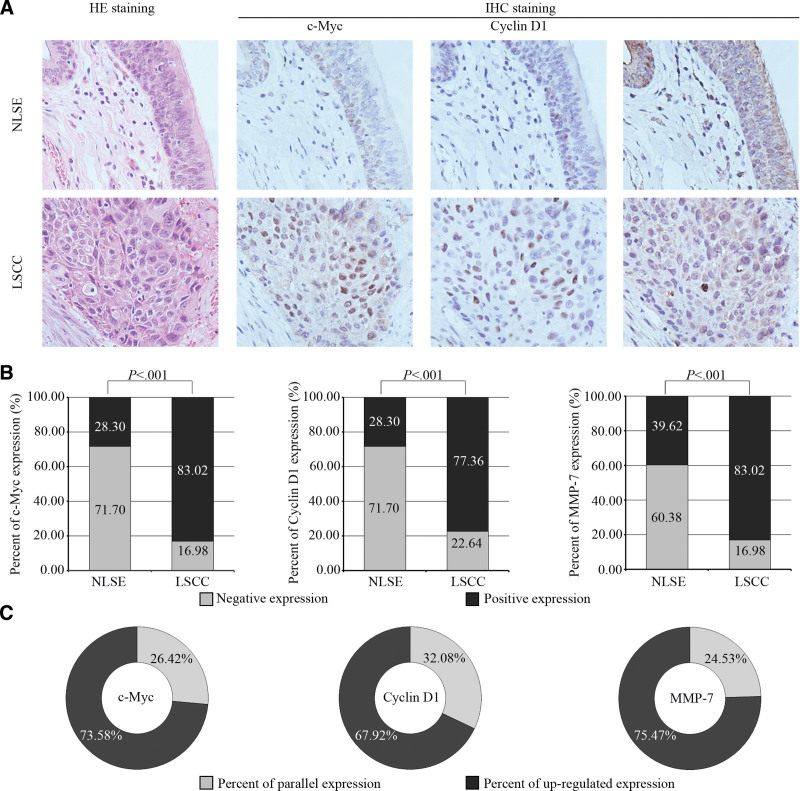
To investigate the changes of β-catenin target genes, we analyzed the expression of c-Myc, Cyclin D1, and MMP-7. The expressions of c-Myc and Cyclin D1 were mainly discovered in the nucleus, but that of MMP-7 was more in the cytoplasm (Fig. 3A). All of them were highly expressed in LSCC tissues and lowly expressed in NLSE tissues. The percents of c-Myc, Cyclin D1, and MMP-7 positive expression were 28.30%, 28.30%, and 39.62% in NLSE tissues and 83.02%, 77.36%, and 83.02% in LSCC tissues, respectively (P < .001, Fig. 3B). The expressions of c-Myc, Cyclin D1, and MMP-7 were upregulated in 73.58%, 67.92%, and 75.47% patients between tumor and normal tissues from the same case, respectively (Fig. 3C).
Figure 3.
Expressions of c-Myc, Cyclin D1, and MMP-7 in NLSE and LSCC tissues. (A) Results of immunostaining for c-Myc, Cyclin D1, and MMP-7 in NLSE and LSCC tissues were showed by continuous sections. The expressions of c-Myc and Cyclin D1 were mainly discovered in the nucleus, but that of MMP-7 was more in the cytoplasm. (B) Percent of positive expression for c-Myc, Cyclin D1, and MMP-7; (C) Percent of upregulated expression for c-Myc, Cyclin D1, and MMP-7 in LSCC tissues compare with NLSE tissues from the same patient. The dates were calculated by the x2 test and presented by number (%) in the figure B. P value of <.05 was considered statistically significant. HE = hematoxylin-eosin, IHC = immunohistochemistry, LSCC = laryngeal squamous cell carcinoma, MMP-7 = matrix metalloproteinase-7, NLSE = normal laryngeal squamous epithelium, TC1 = thyroid cancer 1.
3.6. Relationship between TC1, Chibby, β-catenin and β-catenin target genes in LSCC
Spearman rank correlation test confirmed the positive correlation between expression of TC1 and c-Myc (R = 0.279, P = .043), Cyclin D1 (R = 0.442, P = .001), and MMP-7 (R = 0.430, P = .001), (Table 2). The negative correlations were found between the expressions of Chibby and MMP-7 (r = −0.385, P = .004), but failed to observe any significance with the expression of c-Myc (r = −0.159, P = .257) and Cyclin D1 (r = −0.270 P = .050) (Table 2). The abnormal expression of β–catenin was also positively correlated with high expression of c-Myc (R = 0.289, P = .036), Cyclin D1 (R = 0.533, P < .001), and MMP-7 (R = 0.580 P < .001), (Table 2). In addition, there was statistically significant about the expression of Cyclin D1 and MMP-7 among the groups of TC1+/Chibby−, TC1+/Chibby+, TC1-/Chibby−, and TC1-/Chibby + (P < .001), but was not significance about the expression of c-Myc among them (P = .339), (Table 3).
Table 2.
Correlations between TC1, Chibby, β-catenin and c-Myc, Cyclin D1, MMP-7 in LSCC*.
| Variables | c-Myc | Cyclin D1 | MMP-7 | |||
|---|---|---|---|---|---|---|
| r | P value | r | P value | r | P value | |
| TC1 | 0.279 | .043 | 0.442 | .001 | 0.430 | .001 |
| Chibby | −0.159 | .257 | −0.270 | .050 | −0.385 | .004 |
| β-catenin | 0.289 | .036 | 0.533 | <.001 | 0.580 | <.001 |
LSCC = laryngeal squamous cell carcinoma, MMP-7 = matrix metalloproteinase-7, TC1 = thyroid cancer 1.
Analyzed by Spearman rank correlations. P value of <.05 was considered statistically significant.
Table 3.
Common effects of TC1 and Chibby on the expression β-catenin target gens in LSCC*.
| Groups | n | c-Myc expression | P value | Cyclin D1 expression | P value | MMP-7 expression | P value | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Negative (%) | Positive (%) | Negative (%) | Positive (%) | Negative (%) | Positive (%) | |||||
| TC1+/Chibby− | 25 | 2 (8.00) | 23 (92.00) | 1 (4.00) | 24 (96.00) | 0 (0.00) | 25 (100.00) | |||
| TC1+/Chibby+ | 19 | 4 (21.05) | 15 (78.95) | .339 | 4 (21.05) | 15 (78.95) | <.001 | 2 (10.53) | 17 (89.47) | <.001 |
| TC1-/Chibby− | 3 | 1 (33.33) | 2 (66.67) | 3 (100.00) | 0 (0.00) | 1 (33.33) | 2 (66.67) | |||
| TC1-/Chibby+ | 6 | 2 (33.33) | 4 (66.67) | 4 (66.67) | 2 (33.33) | 6 (100.00) | 0 (0.00) | |||
LSCC = laryngeal squamous cell carcinoma, MMP-7 = matrix metalloproteinase-7, TC1 = thyroid cancer 1.
All statistical significance was determined using the Fisher exact test in R × C table because of some theoretical frequency was smaller than 5. The results were showed by number (%). P value of <.05 was considered statistically significant.
3.7. Relationship between TC1, Chibby, β-catenin and overall survival rate in LSCC patients
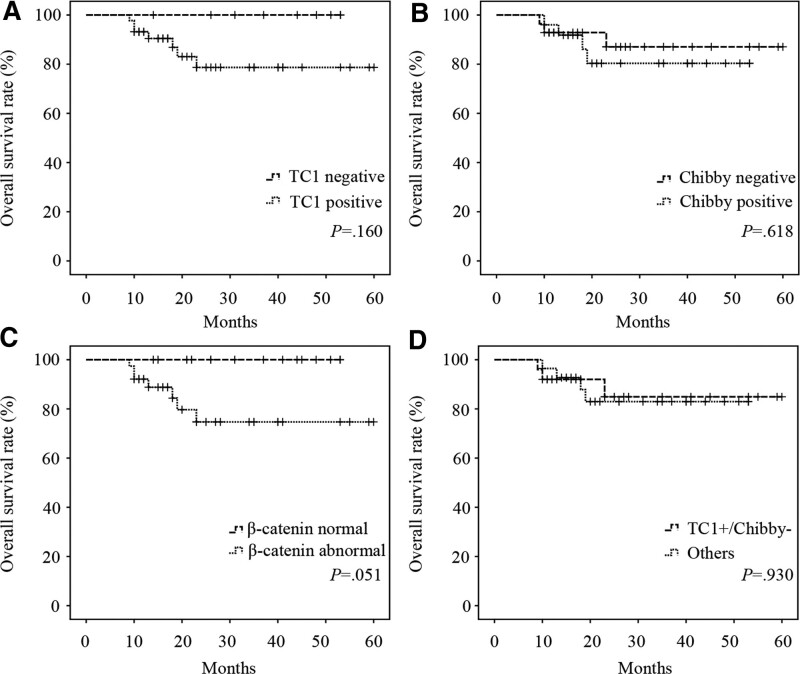
All specimens were divided into negative and positive expression groups about TC1 or Chibby, or normal and abnormal expression groups about β-catenin, or TC1+/Chibby− and others groups which included the groups of TC1+/Chibby+, TC1-/Chibby−, and TC1-/Chibby+. However, there was no significant difference in overall survival between these groups (P > .05, Fig. 4A, B, C, and D). At the same time, we found no association between overall survival and the sex, age, smoking, drinking, tumor site, TNM stage, T stage, lymphatic metastasis, lymphovascular invasion, perineurial invasion, differentiation, and the expression of TC1, Chibby, and β-catenin through univariate Cox proportional hazards model analysis (P > .05, Table 4).
Figure 4.
Correlations between TC1, Chibby, β-catenin and overall survival rate in LSCC patients. Kaplan–Meier overall survival curves of “TC1 negative” and “TC1 positive” group (A), “Chibby negative” and “Chibby positive” group (B), “β-catenin normal” and “β-catenin abnormal” group (C), and “TC1+/Chibby−” and “Others” group (D) from LSCC patients. “Others” represented the groups of TC1+/Chibby+, TC1-/Chibby−, and TC1-/Chibby+. Curves for survival rates were determined by the Kaplan–Meier analysis and compared using the log-rank test. P value of <.05 was considered statistically significant. LSCC = laryngeal squamous cell carcinoma, TC1 = thyroid cancer 1, TC1+/Chibby− = positive expression of TC1 and negative expression of Chibby.
Table 4.
Univariate analysis of factors associated with overall survival of patients with LSCC*.
| Variable | Comparison | HR (95%CI) | P value |
|---|---|---|---|
| Sex | Female vs Male | 0.05 (0.00–1699063.23) | .731 |
| Age | ≥66 vs <66 yr | 2.34 (0.45–12.05) | .310 |
| Smoking | Yes vs No | 0.55 (0.12–2.47) | .428 |
| Drinking | Yes vs No | 0.45 (0.10–2.00) | .293 |
| Tumor site | Non-glottic vs Glottic | 3.32 (0.64–17.14) | .151 |
| TNM stage | III + IV vs I + II | 6.81 (0.82–56.85) | .076 |
| T stage | 3 + 4 vs 1 + 2 | 7.82 (0.94–65.25) | .058 |
| Lymphatic metastasis | Yes vs No | 0.88 (0.11–7.28) | .902 |
| Lymphovascular invasion | Yes vs No | 1.93 (0.43–8.62) | .390 |
| Perineurial invasion | Yes vs No | 2.88 (0.54–15.43) | .217 |
| Differentiation | Moderately + Poorly vs Highly | 3.75 (0.45–31.18) | .221 |
| TC1 | Positive vs Negative | 30.10 (0.01–68114.11) | .388 |
| Chibby | Positive vs Negative | 1.46 (0.33–6.55) | .621 |
| β-catenin | Positive vs Negative | 40.98 (0.06–27977.50) | .265 |
| TC1/Chibby | TC1+/Chibby− vs othersa | 0.68 (0.28–1.66) | .392 |
CI = confidence interval, LSCC = laryngeal squamous cell carcinoma, TC1 = thyroid cancer 1, TNM = tumor node metastases.
“Others” represented the groups of TC1+/Chibby+, TC1-/Chibby−, and TC1-/Chibby+.
All statistical significance was determined using the univariate Cox proportional hazards model. P value of <.05 was considered statistically significant.
4. Discussion
The incidence of LSCC is one of the most common malignances in the head and neck, and the overall 5-year survival rate is still low.[1,2] Therefore, it is of great significance to change or modify some important genes or their expression products in the biological treatment of LSCC. For instance, the Wnt/β-catenin signaling pathway as an effective molecular target for cancer therapy has attracted widespread attention.[4,5] However, there is not enough knowledge regarding Wnt/β-catenin signaling cascade genes in LSCC. Hence, this study investigated the question of whether the expression of TC1 and Chibby were altered in LSCC tissues, and, if so, whether there was important influence of their abnormal expression on the Wnt/β-catenin signaling pathway.
TC1 is a novel activator of Wnt/β-catenin signaling pathway.[15,16] The over expression of TC1 has been reported in many tumors, such as thyroid cancer,[14] gastric cancer,[23] tongue cancer,[17] lung cancer,[18] and cervical squamous cell carcinoma.[19] In the present study, we found that the expression of TC1 was distinctly upregulated in LSCC relative to that in NLSE. Moreover, the upregulated expression of TC1 was correlated with non-glottic type tumors, advanced TNM and T stage, lymphovascular invasion, and poor differentiation, but did not correlated with the sex, age, smoking, drinking, lymphatic metastasis, and perineurial invasion in LSCC. Consistent with our study, most studies have presented that there were relationship between high expression of TC1 and TNM stage[17,18,23] and differentiation.[18,19,23] The correlation between TC1 expression and lymphatic metastasis was observed in gastric cancer[23] and lung cancer,[18] but not in tongue cancer.[17] The possible reason for the diversity was that the number of patients with lymphatic metastasis was too small to be conclusive.
Chibby is an antagonist involved in the Wnt/β-catenin pathway and is hypothesized to serve a function in tumorigenesis.[11] Several reports have shown that Chibby protein was downregulated in a number of types of cancer including lung cancers,[20] tongue cancer,[17] cervical cancer,[21] nasopharyngeal carcinoma,[24] gastric cancer,[25] and hepatocellular carcinoma.[22] At the same time, the expression of Chibby in LSCC was also decreased in the present and previous study.[26] However, its expression was not correlated with the clinicopathological features in tumor samples. In agreement with our study, there was no relationship between the expression of Chibby and TNM stage, lymphatic metastasis, and tumor differentiation in lung cancers and tongue cancer.[17,20] However, reduced expression of Chibby was associated with TNM stage in nasopharyngeal carcinoma[24] and with advanced TNM stage and histology grade in hepatocellular carcinoma.[22] These contentious views about the correlation of Chibby and clinicopathologic characteristics may be caused by different tissue types and severity of the disease.
As an important member of Wnt/β-catenin pathway, the aberrant expression of β-catenin has been reported in many tumors, such as lung cancers,[20] tongue cancer,[17] cervical squamous cell carcinoma,[19] and hepatocellular carcinoma.[22] The expression of β-catenin could be observed in cell membrane, cytoplasm and nucleus of these tumors.[17,20,22] It was worth noting that the abnormal expression of β-catenin means its expression was reduced in cell membrane, upregulated in cytoplasm or nucleus in tumor tissues compared with normal tissues.[17,20] In our study, we also found that expression of β-catenin was strong in membranous, but little in cytoplasm and absence in nucleus in NLSE tissues. However, its expression was clear in cytoplasm, few in nucleus, and very weak or negative in membranous in LSCC tissues. Therefore, we adopted different criteria to evaluate its abnormal expression in NLSE and LSCC tissues. That was to say that β-catenin was abnormally expressed when the percentage of positive cells stained in the cytomembrane was <70% in NLSE or that stained in the cytoplasm and/or nucleus was more than 10% in LSCC. According to this standard, our findings showed that the percent of its abnormal expression was higher in LSCC tissues than in NLSE tissues. A previous study has documented that aberrant expression of β-catenin in cytoplasm was detected significantly in laryngeal epithelial precursor lesions with progression to invasive laryngeal carcinoma and seemed to be related with more aggressive biological behavior.[9] At the same time, the abolishment of β-catenin from the membrane was correlated with supraglottic location of tumor, TNM stage, and dedifferentiation of LSCC.[7,8] The present results also illustrated there was relationship between aberrant expression of β-catenin and tumor site, advanced TNM and T stage, lymphovascular invasion, perineurial invasion, and poor differentiation, but not with the sex, age, smoking, drinking, and lymphatic metastasis in LSCC tissues.
The relationships among the expressions of TC1, Chibby, and β-catenin have reported in many type malignant tumors. First of all, the high cytoplasmic expression of TC1 was positively correlated with the abnormal expression of β-catenin in oral tongue squamous cell carcinoma,[17] lung cancer,[18] and cervical squamous cell carcinoma.[19] These studies were consistent with the present result, which documented that the positive relationship between TC1 and β-catenin in LSCC tissues. Secondly, there was negative correlation between the low expression of Chibby and aberrant β-catenin expression in lung cancers.[20] In addition, the Chibby/β-catenin ratio was identified to be downregulated and may potentially to a biomarker for disease progression in cervical cancer.[21] Our study also suggested that the down-regulation of Chibby in nucleus was associated with the abnormal cytoplasmic expression of β-catenin in LSCC tissues. Finally, there was no consensus about the association between TC1 and Chibby in carcinoma tissues. The correlation between high cytoplasmic expression of TC1 and low nuclear expression of Chibby was observed in lung cancer,[18] but not in oral tongue squamous cell carcinoma.[17] Although our data was presented in favor of the latter, further studies on the correlation between them should be conducted in the future. In order to discover whether there was important influence of the combination of TC1 and Chibby on β-catenin, all samples were divided into 4 groups according to the expression level of TC1 and Chibby. The result showed that the percent of abnormal expression of β-catenin was statistical differences among the 4 groups. In other words, the upregulated expression of TC1 and downregulated expression of Chibby were correlated with the aberrant expression of β-catenin in LSCC.
As we know, TC1 and Chibby as regulators of Wnt/β-catenin signaling pathway may impact the expression of β-catenin target genes. So, we further investigated the expression of some β-catenin target genes and the correlativity between TC1 or Chibby and them. These target genes have been implicated in cancer proliferation, such as c-Myc[27] and Cyclin D1,[28] and in cancer invasion, such as MMP-7.[29] Our results indicated that the expression of c-Myc, Cyclin D1, and MMP-7 were weak in NLSE tissues, but were distinctly increased in LSCC tissues. Meanwhile, there was positive correlation between expression of TC1 and c-Myc, Cyclin D1, and MMP-7. The similar relationship was discovered between abnormal expression of β-catenin and the 3 target genes. It has been demonstrated that the high expression of TC-1 in gastric cancer was related to the expression of Cyclin D1 and MMP-7, but not to the expression of c-Myc.[23] However, there was much controversy about the influence of Chibby on the 3 target genes. A previous report found that overexpression of Chibby did not affect the expression levels of the c-Myc protein and suggested that Chibby was not an effective inhibitor of β-catenin-mediated gene expression in gastric cancer cells.[25] In contrast to the finding, the other one demonstrated that Chibby knockdown induced the expression of c-Myc and Cyclin D1 protein, which promoted hepatocellular carcinoma cell proliferation and invasiveness.[22] In the current study, the negative association was discovered between Chibby and MMP-7, but not with c-Myc or Cyclin D1 in LSCC tissues. These findings suggested that the role of Chibby in Wnt/β-catenin signaling pathway may be tissue-specific and, perhaps, cell line-specific. At the same time, we found that there was statistically significant about the expression of these target genes excluding c-Myc among the groups of TC1+/Chibby−, TC1+/Chibby+, TC1-/Chibby−, and TC1-/Chibby+. These findings further supported the influence of upregulated expression of TC1 and downregulated expression of Chibby on the Wnt/β-catenin signaling pathway.
The Previous study has shown that there was a strong inverse correlation between TC1 expression and survival In accordance with the pathologic variable analysis.[23] At the same time, the low expression of Chibby and high expression of β-catenin in the nuclei was an independent risk factor for recurrence and overall survival of hepatocellular carcinoma.[22] We also analyzed the association between TC1, Chibby, β-catenin, clinicopathological factors and the overall survival of LSCC patients. However, there was no significant relationship between them. The possible reasons were that short follow-up of most patients after treatment.
5. Conclusions
The increased TC1 expression, decreased Chibby expression, and abnormal β-catenin expression were common in LSCC tissues compared with these in NLSE tissues. At the same time, the expression of β-catenin target genes, such as c-Myc, Cyclin D1, and MMP-7, were upregulated in the LSCC tissues. The upregulated expression of TC1 and downregulated expression of Chibby were correlated with the aberrant expression of β-catenin and the high expression of Cyclin D1 and MMP-7 in LSCC tissues. Our results suggested that TC1 and Chibby as regulators of Wnt/β-catenin signaling pathway might impact the activity of β-catenin and the expression of some β-catenin target genes in LSCC.
Acknowledgments
The authors are grateful to the Department of Pathology of the First People Hospital of Huzhou for providing technical support. We would like to thank Dr Hui Xia and Piwei Hu (Department of Pathology, the First People Hospital of Huzhou, Zhejiang, China) for evaluation of the immunostaining.
Author contributions
Conceptualization: Gang Ren, Chengyi Yin.
Data curation: Bingliang Ma.
Formal analysis: Xilin Zhang.
Funding acquisition: Gang Ren.
Investigation: Gang Ren, Bingliang Ma, Jianqiu Wang, Jue Xu, Xilin Zhang, Chengyi Yin.
Methodology: Gang Ren, Xilin Zhang, Chengyi Yin.
Software: Jianqiu Wang.
Supervision: Jue Xu.
Writing – original draft: Gang Ren.
Writing – review & editing: Chengyi Yin.
Abbreviations:
- CI
- confidence interval
- HE
- hematoxylin-eosin
- IHC
- Immunohistochemistry
- LSCC
- laryngeal squamous cell carcinoma
- MMP-7
- matrix metalloproteinase-7
- NLSE
- normal laryngeal squamous epithelium
- TC1
- thyroid cancer 1
- TCF/LEF
- T-cell factor/lymphocyte enhancer factor
- TNM
- tumor node metastases
This work was supported by grants from the Foundation of Huzhou Bureau of Science and Technology (grant number 2018GYB13 [to Gang Ren]).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical approval was obtained from the Ethics Committee of the First People Hospital of Huzhou (approval no. 2018027). Informed consent about participating in the study was obtained from all individual participants.
How to cite this article: Ren G, Ma B, Wang J, Xu J, Zhang X, Yin C. Upregulated TC1 and downregulated Chibby were correlated with the aberrant β-catenin expression in laryngeal squamous cell carcinoma. Medicine 2023;102:46(e36066).
Contributor Information
Gang Ren, Email: 176164137@qq.com.
Bingliang Ma, Email: 80703810@qq.com.
Jianqiu Wang, Email: 1700620861@qq.com.
Jue Xu, Email: 1098777082@qq.com.
Xilin Zhang, Email: 3490261481@qq.com.
References
- [1].Lefebvre JL. Laryngeal preservation in head and neck cancer: multidisciplinary approach. Lancet Oncol. 2006;7:747–55. [DOI] [PubMed] [Google Scholar]
- [2].Steuer CE, El-Deiry M, Parks JR, et al. An update on larynx cancer. CA Cancer J Clin. 2017;67:31–50. [DOI] [PubMed] [Google Scholar]
- [3].Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- [4].Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36:1461–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–99. [DOI] [PubMed] [Google Scholar]
- [6].Chatterjee A, Paul S, Bisht B, et al. Advances in targeting the Wnt/β-catenin signaling pathway in cancer. Drug Discov Today. 2022;27:82–101. [DOI] [PubMed] [Google Scholar]
- [7].Lopez-Gonzalez JS, Cristerna-Sanchez L, Vazquez-Manriquez ME, et al. Localization and level of expression of beta-catenin in human laryngeal squamous cell carcinoma. Otolaryngol Head Neck Surg. 2004;130:89–93. [DOI] [PubMed] [Google Scholar]
- [8].Goulioumis AK, Varakis J, Goumas P, et al. Differential beta-catenin expression between glottic and supraglottic laryngeal carcinoma. Eur Arch Otorhinolaryngol. 2010;267:1573–8. [DOI] [PubMed] [Google Scholar]
- [9].López F, Alvarez-Marcos C, Alonso-Guervós M, et al. From laryngeal epithelial precursor lesions to squamous carcinoma of the larynx: the role of cell cycle proteins and β-catenin. Eur Arch Otorhinolaryngol. 2013;270:3153–62. [DOI] [PubMed] [Google Scholar]
- [10].Xie J, Huang L, Lu YG, et al. Roles of the Wnt signaling pathway in head and neck squamous cell carcinoma. Front Mol Biosci. 2020;7:590912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Takemaru K, Yamaguchi S, Lee YS, et al. Chibby, a nuclear beta-catenin-associated antagonist of the Wnt/Wingless pathway. Nature. 2003;422:905–9. [DOI] [PubMed] [Google Scholar]
- [12].Li FQ, Mofunanya A, Harris K, et al. Chibby cooperates with 14-3-3 to regulate beta-catenin subcellular distribution and signaling activity. J Cell Biol. 2008;181:1141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Takemaru K, Fischer V, Li FQ. Fine-tuning of nuclear-catenin by Chibby and 14-3-3. Cell Cycle. 2009;8:210–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chua EL, Young L, Wu WM, et al. Cloning of TC-1 (C8orf4), a novel gene found to be overexpressed in thyroid cancer. Genomics. 2000;69:342–7. [DOI] [PubMed] [Google Scholar]
- [15].Jung Y, Bang S, Choi K, et al. TC1 (C8orf4) enhances the wnt/beta-catenin pathway by relieving antagonistic activity of Chibby. Cancer Res. 2006;66:723–8. [DOI] [PubMed] [Google Scholar]
- [16].Gall C, Xu H, Brickenden A, et al. The intrinsically disordered tc-1 interacts with Chibby via regions with high helical propensity. Protein Sci. 2007;16:2510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang P, Cao HY, Bai LL, et al. The high expression of TC1 (C8orf4) was correlated with the expression of β-catenin and cyclin D1 and the progression of squamous cell carcinomas of the tongue. Tumour Biol. 2015;36:7061–7. [DOI] [PubMed] [Google Scholar]
- [18].Zheng YW, Zhang L, Wang Y, et al. Thyroid cancer 1 (C8orf4) shows high expression, no mutation and reduced methylation level in lung cancers, and its expression correlates with β-catenin and dnmt1 expression and poor prognosis. Oncotarget. 2017;8:62880–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lan C, Huan DW, Nie XC, et al. Association of C8orf4 expression with its methylation status, aberrant β-catenin expression, and the development of cervical squamous cell carcinoma. Medicine (Baltim). 2019;98:e16715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Xu HT, Li QC, Dai SD, et al. The expression patterns and correlations of chibby, β-catenin, and DNA methyltransferase-1 and their clinicopathological significance in lung cancers. APMIS. 2011;119:750–8. [DOI] [PubMed] [Google Scholar]
- [21].Yang MC, Chien ST, Yang TF, et al. Downregulation of nuclear and cytoplasmic Chibby is associated with advanced cervical cancer. Oncol Lett. 2017;14:6632–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tsai MC, Huang CC, Wei YC, et al. Combined Chibby and β-catenin predicts clinical outcomes in patients with hepatocellular carcinoma. Int J Mol Sci. 2020;21:2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kim B, Koo H, Yang S, et al. TC1 (C8orf4) correlates with Wnt/beta-catenin target genes and aggressive biological behavior in gastric cancer. Clin Cancer Res. 2006;12:3541–8. [DOI] [PubMed] [Google Scholar]
- [24].Cai CF, Liu LM, Shangguan HJ, et al. Anti-oncogenic activity of Chibby in the development of human nasopharyngeal carcinoma. Oncol Lett. 2018;15:5849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li FQ, Chiriboga L, Black MA, et al. Chibby is a weak regulator of β-catenin activity in gastric epithelium. J Cell Physiol. 2019;234:1871–9. [DOI] [PubMed] [Google Scholar]
- [26].Ren G, Zhao DA, Xu J, et al. Expression of CBY and methylation of CBY at promoter region in human laryngeal squamous cell carcinoma. Tumori. 2015;101:215–22. [DOI] [PubMed] [Google Scholar]
- [27].He TC, Sparks AB, Rago C, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–12. [DOI] [PubMed] [Google Scholar]
- [28].Shtutman M, Zhurinsky J, Simcha I, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96:5522–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Crawford HC, Fingleton BM, Rudolph-Owen LA, et al. The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene. 1999;18:2883–91. [DOI] [PubMed] [Google Scholar]