Abstract
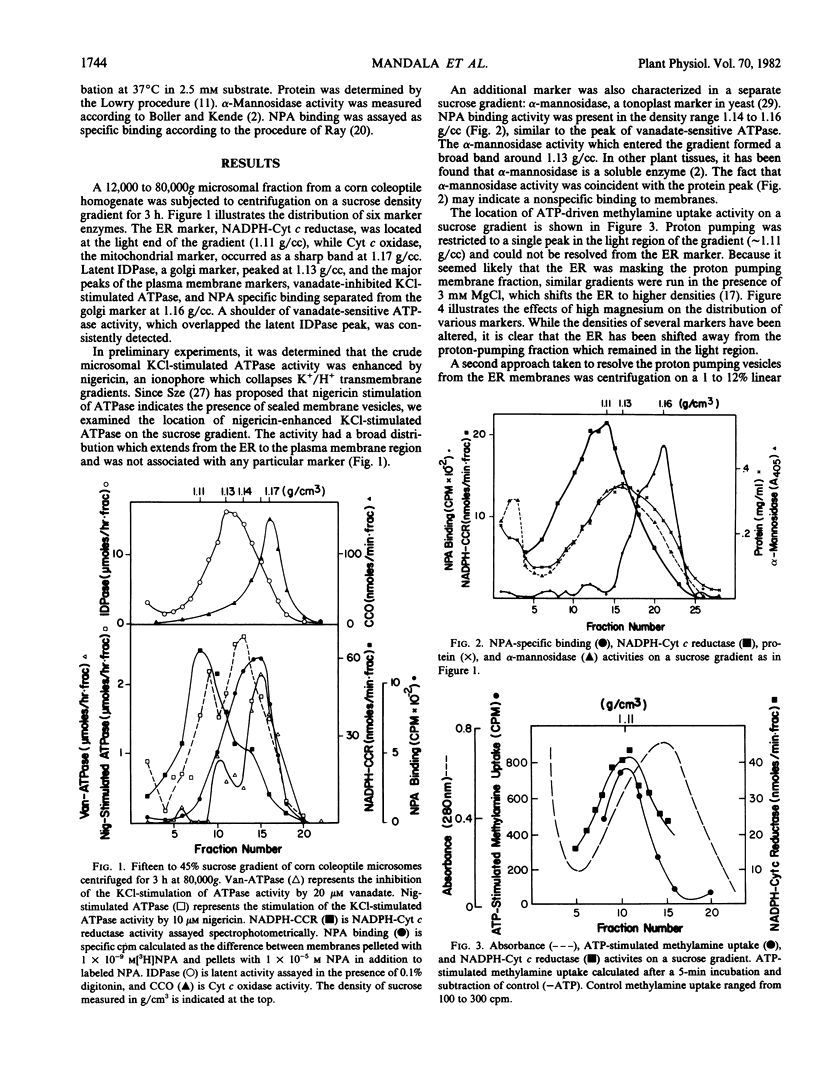
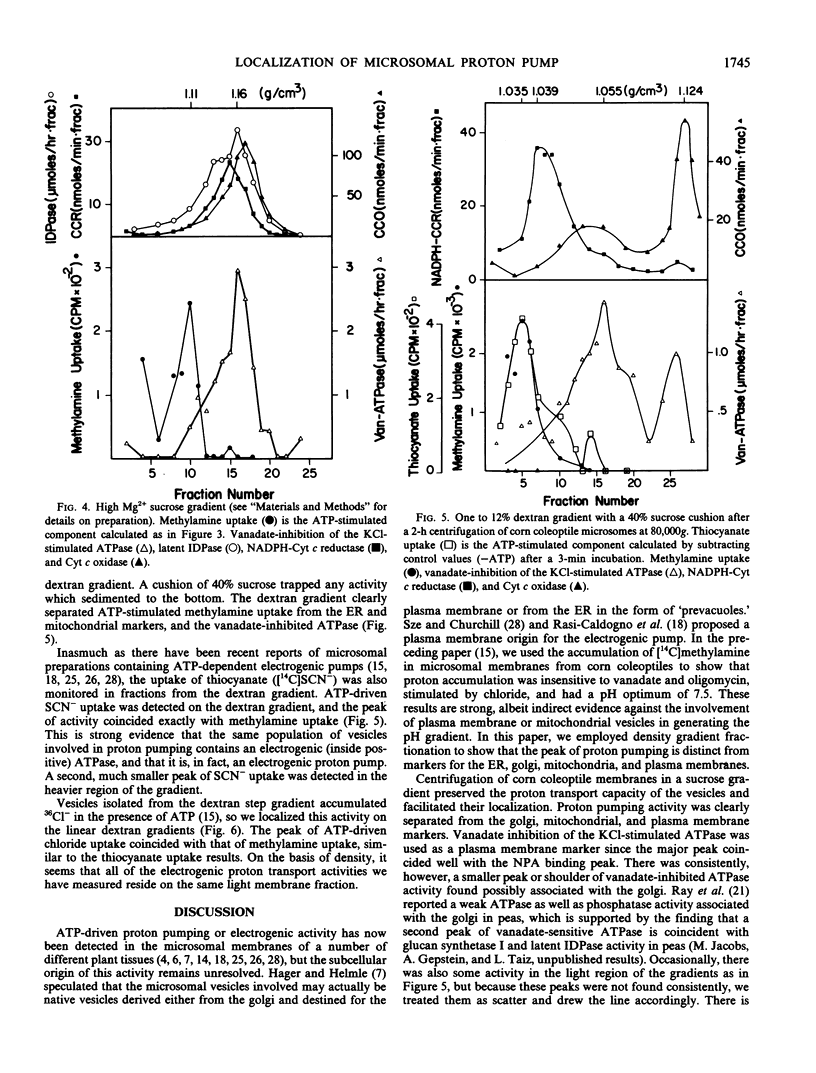
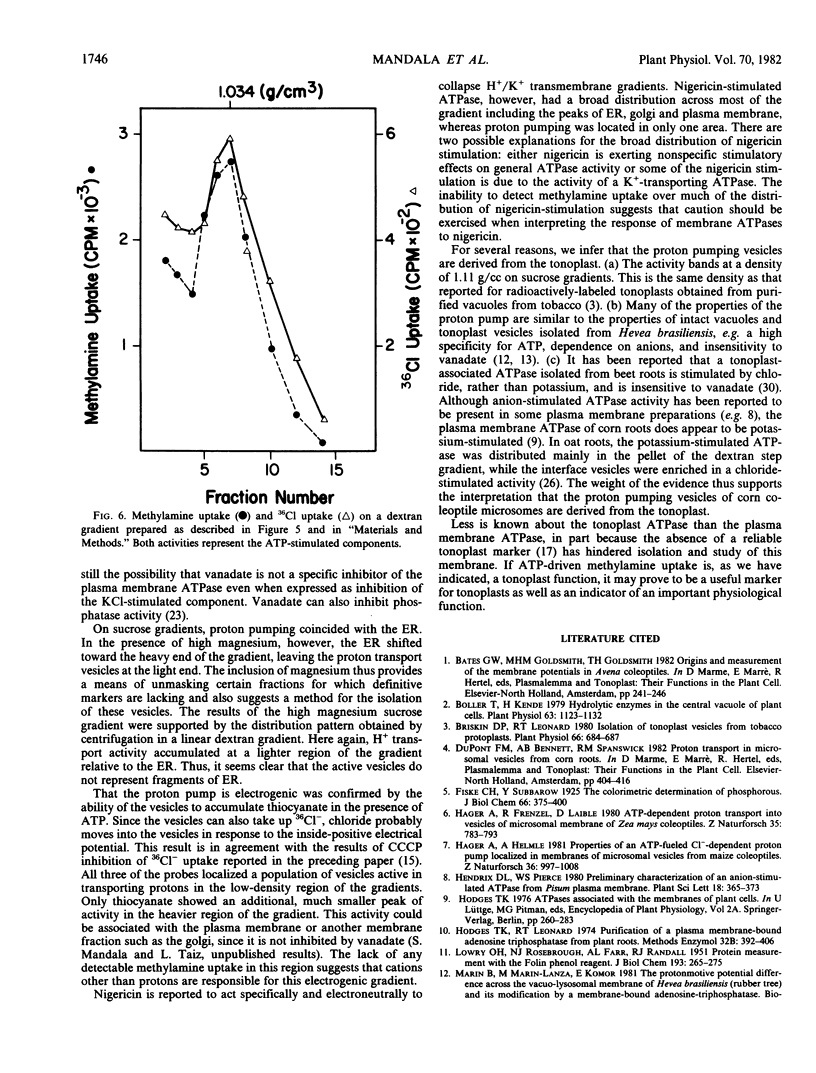
Previous studies characterizing an ATP-dependent proton pump in microsomal membrane vesicles of corn coleoptiles led to the conclusion that the proton pump was neither mitochondrial nor plasma membrane in origin (Mettler, Mandala, Taiz 1982 Plant Physiol 70: 1738-1742). To facilitate positive identification of the vesicles, corn coleoptile microsomal membranes were fractionated on linear sucrose and dextran gradients, with ATP-dependent [14C]methylamine uptake as a probe for proton pumping. On sucrose gradients, proton pumping activity exhibited a density of 1.11 grams/cubic centimeter and was coincident with the endoplasmic reticulum (ER). In the presence of high magnesium, the ER shifted to a heavier density, while proton pumping activity showed no density shift. On linear dextran gradients, proton pumping activity peaked at a lighter density than the ER. The proton pump appears to be electrogenic since both [14C]SCN− uptake and 36Cl− uptake activities coincided with [14C] methylamine uptake on dextran gradients. On the basis of density and transport properties, we conclude that the proton pumping vesicles are probably derived from the tonoplast. Nigericin-stimulated ATPase activity showed a broad distribution which did not coincide with any one membrane marker.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin D. P., Leonard R. T. Isolation of tonoplast vesicles from tobacco protoplasts. Plant Physiol. 1980 Oct;66(4):684–687. doi: 10.1104/pp.66.4.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager A., Frenzel R., Laible D. ATP-dependent proton transport into vesicles of microsomal membranes of Zea mays coleoptiles. Z Naturforsch C. 1980 Sep-Oct;35(9-10):783–793. doi: 10.1515/znc-1980-9-1021. [DOI] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mettler I. J., Mandala S., Taiz L. Characterization of in vitro proton pumping by microsomal vesicles isolated from corn coleoptiles. Plant Physiol. 1982 Dec;70(6):1738–1742. doi: 10.1104/pp.70.6.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasi-Caldogno F., de Michelis M. I., Pugliarello M. C. Evidence for an electrogenic ATPase in microsomal vesicles from pea internodes. Biochim Biophys Acta. 1981 Mar 20;642(1):37–45. doi: 10.1016/0005-2736(81)90135-8. [DOI] [PubMed] [Google Scholar]
- Ray P. M. Auxin-binding Sites of Maize Coleoptiles Are Localized on Membranes of the Endoplasmic Reticulum. Plant Physiol. 1977 Apr;59(4):594–599. doi: 10.1104/pp.59.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Shininger T. L., Ray M. M. ISOLATION OF beta-GLUCAN SYNTHETASE PARTICLES FROM PLANT CELLS AND IDENTIFICATION WITH GOLGI MEMBRANES. Proc Natl Acad Sci U S A. 1969 Oct;64(2):605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle D. L., Cleland R. Control of plant cell enlargement by hydrogen ions. Curr Top Dev Biol. 1977;11:187–214. doi: 10.1016/s0070-2153(08)60746-2. [DOI] [PubMed] [Google Scholar]
- Saxe H., Rajagopal R. Effect of vanadate on bean leaf movement, stomatal conductance, barley leaf unrolling, respiration, and phosphatase activity. Plant Physiol. 1981 Oct;68(4):880–884. doi: 10.1104/pp.68.4.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout R. G., Cleland R. E. Evidence for a Cl-Stimulated MgATPase Proton Pump in Oat Root Membranes. Plant Physiol. 1982 Apr;69(4):798–803. doi: 10.1104/pp.69.4.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H., Churchill K. A. Mg/KCl-ATPase of plant plasma membranes is an electrogenic pump. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5578–5582. doi: 10.1073/pnas.78.9.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H. Nigericin-stimulated ATPase activity in microsomal vesicles of tobacco callus. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5904–5908. doi: 10.1073/pnas.77.10.5904. [DOI] [PMC free article] [PubMed] [Google Scholar]


