Abstract
Various sperm preparation techniques have been developed to obtain functionally, genetically and morphologically high-quality competent spermatozoa for use in assisted reproductive technologies, which may affect treatment options and thus pregnancy outcomes and live birth rates. We aimed to compare swim-up washing procedure (SWP) and microfluidics sperm sorting (MSS) with regard to sperm separation, pregnancy outcomes and live birth rates in infertile couples receiving intrauterine insemination. A total of 326 couples with unexplained infertility who underwent intrauterine insemination were enrolled in this retrospective cohort study and were divided into 2 groups according to sperm preparation technique. The MSS and SWP methods were used to prepare sperm in 178 and 148 patients, respectively. The median sperm concentration reduced significantly from 51 (30–100) million/mL to 20 (10–40) million/mL in the MSS group, and from 45 (26–80) million/mL to 25 (11–48) million/mL in the SWP group (both P < .001). Median motility increased significantly from 30.43 ± 17.79 to 57.48 ± 20.24 in the MSS, and from 32.89 ± 13.92 to 43.91 ± 20.11 in SWP (both P < .001). There was a difference between groups after preparation regarding sperm concentration (better with SWP) and motility (better with MSS) (P = .018 and P < .001, respectively). A total of 86 (26.4%) pregnancies were observed in participants and the clinical pregnancy rate was 23% in the MSS group and 30.4% in the SWP group (P = .133). Fifty-one infants were born alive and a great majority (n = 47) were delivered at term. Multivariate logistic regression analysis showed that higher duration of infertility was independently associated with lower live birth success (odds ratio: 0.811, 95% confidence interval: 0.662–0.996; P = .045). Other variables, including female age, type and reason of infertility, number of cycles, and sperm motility and concentration, were found to be nonsignificant (P > .05). We observed nonsignificant worse reproductive results using microfluid sperm selection in comparison to the pellet swim-up technique (live birth rate = 12% vs 20%). Our evidence is of limited quality due to the retrospective design of this study and sufficiently powered RCTs are needed to evaluate whether sperm selection based using a microfluidic chip is better, similar, or worse than the pellet swim-up technique.
Keywords: intrauterine insemination, microchip, microfluidic sperm sorting, pregnancy rates, swim-up
1. Introduction
Infertility is defined as “the inability to conceive after at least 1 year of unprotected sexual intercourse” according to the World Health Organization (WHO).[1] Although the contribution of the male factor to global infertility is not well explained, it is considered to be present in up to 50% of couples applying for infertility.[2] Despite its low positive predictive value, semen analysis has long been used by as a primary tool to assess the health of sperm based on concentration, motility, volume, viability, morphology, and DNA integrity, among other parameters.[3] Achieving qualitatively- and quantitatively-viable sperm for fertilization is crucial for intrauterine insemination (IUI), in vitro fertilization/intracytoplasmic sperm injection, and cryopreservation of sperm. Various sperm preparation techniques (SPTs) have been developed to obtain functionally, genetically and morphologically high-quality competent spermatozoa for use in assisted reproductive technologies, which may affect treatment options and thus pregnancy outcomes and live birth rates.[4]
The swim-up washing method (SWP) and density-gradient centrifugation are among the traditionally preferred methods for sperm preparation. However, both methods have limitations in terms of sperm quality.[5] Most notably, both methods require the use of centrifugation in sperm separation, which is associated with sperm cell damage, reduced fertility potential and decreased pregnancy rate.[6] Furthermore, centrifugation-based preparation methods are time consuming and labor-intensive, and results may vary depending on operator experience.
Microfluidics sperm sorting (MSS) is a novel alternative SPT that uses a microfluidic system to select sperm and has recently gained widespread use in many infertility clinics to increase clinical pregnancy and live birth rates.[7] This method can provide precise selection of motile sperm in a shorter time while maintaining overall sperm quality with less DNA fragmentation and lower reactive oxygen species in semen.[8] However, there is a lack of data in the literature regarding the success of IUI with the use of MSS.
We aimed to evaluate the effectiveness of 2 different SPTs (conventional SWP and MSS chip) on pregnancy outcomes and live birth rates in subfertile couples receiving IUI.
2. Materials and Methods
2.1. Study design, patient selection and ethics
In this retrospective cohort study, we included data from couples who performed IUI between January 2014 and March 2022 in the assisted reproductive technologies centers of Acibadem Hospital, Kayseri, Turkey and Private Erciyes Hospital, Kayseri, Turkiye that met the following criteria:
(i) Women age between 20 to 43 years.
(ii) Basal follicle-stimulating hormone (FSH) level of < 15 IU/L.
(iii) Natural failure to conceive for at least 12 months.
(iv) Bilateral tubal patency evaluated by hysterosalpingography.
(v) Total motile sperm counts of > 5 million/mL and normal sperm analysis parameters according to the WHO 2010 criteria.[9]
(vi) No history of genetic disease or recurrent pregnancy loss.
Subjects with bilateral tubal pathology, history of genetic disease, recurrent abortion, and total motile sperm counts of < 5 million/mL were excluded from the study. A total of 326 couples with unexplained infertility who underwent IUI treatment were enrolled in the study. Subjects were divided into 2 groups according to their SPT (178 couples using MSS and 148 couples using SWT). All research procedures were evaluated and approved by the Research Ethics Committee of Acibadem University (date: 22.04.2022, decision no: 2022/07) and were conducted in agreement with the ethical standards specified in the Declaration of Helsinki.
Patients clinical characteristics, including age of female, type, duration and reasons of infertility, number of cycles, and pregnancy outcomes were obtained from patient files. Spermiogram parameters were recorded before and after SPT (MSS or SWP). The type of SPT was based on clinical data from the evaluation cycle and previous treatment outcomes and failure, as well as the couple’s choices and obstetrician’s recommendations. The process and equipment used were similar between the 2 groups, except for the SPT performed for each group.
2.2. Definitions
Clinical pregnancy was defined as the detection of 1 or 2 intrauterine gestational sac(s) at first ultrasonography observation along with an appropriately increasing beta-HCG level. Ongoing pregnancy was confirmed by ultrasound as the presence of a viable fetus after 12 weeks of gestation. Live birth outcome was accepted as the delivery of a live infant after at least 20 weeks of gestation. Ultrasonography measurements of all patients were performed by same researcher.
2.3. Female- and pregnancy-related evaluations
All women were assessed with a baseline transvaginal ultrasound examination on the day 3 of the menstrual cycle and received IUI protocol using standard controlled ovarian stimulation methods. Seventy-five IU of recombinant-FSH, GonalF, Merck, Serono, Germany) was administered to patients from cycle day to follicular maturation was completed. The duration and dosage of the recombinant-FSH was adjusted according to age, body mass index and number of antral follicles in transvaginal ultrasound. All women were regularly followed by ultrasound to monitor follicle development until 1 or 2 follicles with a maximum diameter > 17 mm and endometrial thickness of > 7 mm were detected. Cycles with more than 3 dominant follicles were canceled. Ovulation was triggered by an SC injection of 250 µg recombinant HCG (Ovitrelle, Merck Sereno, Italy) when at least 1 mature follicle measured > 17 mm. IUI procedure was performed 36 to 48 hours after the HCG injection. All women were checked with serum beta-HCG on the day 14 after insemination. Clinical pregnancy was established by detecting the gestational sac or fetal heartbeat on transvaginal ultrasound examination.
2.4. Semen acquisition and semen preparation
Semen samples were obtained by masturbation in a nearby special room after 2 to 5 days of sexual abstinence in a plastic, wide-mouthed sterile container and were allowed to liquefy at 37 ºC for 30 minutes. All semen samples were then prepared by either the SWP or MSS chip method and reassessed by the same technician who had analyzed the sperm samples before SPT, according to WHO standard technical guidelines and reference values.
In the SWP procedure, a liquefied semen sample was placed 15 mL conical centrifuge tube and diluted 1:1 with sperm preparation medium. The mixture was centrifuged for 10 minutes at 1200 rpm at room temperature. The supernatant was then removed and 1 mL of fresh sperm medium was gently added to the pellet. The tube was placed on a stand, inclined at an angle of 45º and then incubated at 37ºC for 40 minutes. Later, 0.5 mL of the supernatant from the top of the tube was placed into an empty tube to use for the IUI procedure.
The MSS chip method was performed using the Fertile Plus chip (Koek Biotechnology, Izmir, Turkey). This is a flow-free, single-use, dual chambered device with an inlet sample chamber linked with an outlet collection chamber through a narrow microfluidic channel (400 µL wide, 50 µL depth and 1.5 cm length) to select sperms passively without centrifugation steps. Furthermore, there is a porous membrane filter that allows the passage of morphologically normal-headed sperm. Following liquefaction, 850 µL of untreated semen samples was injected into the inlet chamber by a micro-pipette, and added 700 µL of sperm wash medium heated 37 ºC to the microporous membrane (outlet chamber). The chip was incubated for 30 minutes at 37 ºC, and then 650 µL of processed sperm sample was carefully collected from the outlet.
2.5. Statistical analysis
All analyses were performed on IBM SPSS Statistics for Windows, Version 25.0 (IBM Corp., Armonk, NY). Histogram and Q-Q plots were used to determine whether variables are normally distributed. Age of female was analyzed with the independent samples t test. Duration of infertility was analyzed with the Mann–Whitney U test. Repeated measurements of sperm concentrations were analyzed with the Wilcoxon signed ranks test, between groups comparisons of these were performed with the Mann–Whitney U test. Motility measurements were analyzed with the 2-way repeated measures analysis of variances. Categorical variables were analyzed with the chi-square test or Fisher exact test. Logistic regression analyses were performed to determine significant factors independently associated with the live birth. Two-tailed P values of < .05 were considered statistically significant.
3. Results
The MSS and SWP methods were used to prepare sperm in 178 and 148 patients, respectively. The median sperm concentration reduced significantly from 51 (30–100) million/mL to 20 (10–40) million/mL in the MSS group, and from 45 (26–80) million/mL to 25 (11–48) million/mL in the SWP group (both P < .001). Median motility increased significantly from 30.43 ± 17.79 to 57.48 ± 20.24 in the MSS, and from 32.89 ± 13.92 to 43.91 ± 20.11 in SWP (both P < .001). There was a difference between groups after preparation regarding sperm concentration (better with SWP) and motility (better with MSS) (P = .018 and P < .001, respectively) (Table 1).
Table 1.
Clinical characteristics of participants with regard to semen preparation technique.
| Overall (n = 326) | Semen preparation technique | P value | ||
|---|---|---|---|---|
| MSS microchip (n = 178) | Swim-up (n = 148) | |||
| Age of female | 28.88 ± 6.30 | 28.65 ± 6.36 | 29.15 ± 6.24 | .479 |
| Type of infertility | ||||
| Primary | 192 (58.9%) | 110 (61.8%) | 82 (55.4%) | .243 |
| Secondary | 134 (41.1%) | 68 (38.2%) | 66 (44.6%) | |
| Number of cycles | ||||
| 1 | 226 (69.3%) | 125 (70.2%) | 101 (68.2%) | .475 |
| 2 | 81 (24.8%) | 44 (24.7%) | 37 (25.0%) | |
| 3 | 14 (4.3%) | 8 (4.5%) | 6 (4.1%) | |
| 4 | 5 (1.5%) | 1 (0.6%) | 4 (2.7%) | |
| Duration of infertility, yr | 2 (1.5 to 3.5) | 2 (1.5 to4) | 2 (1.5 to3) | .557 |
| Reason of infertility | ||||
| Unexplained | 143 (43.9%) | 73 (41.0%) | 70 (47.3%) | .027 |
| Polycystic ovarian syndrome | 133 (40.8%) | 69 (38.8%) | 64 (43.2%) | |
| Other | 50 (15.3%) | 36 (20.2%) | 14 (9.5%) | |
| Sperm concentration (x106/mL) | ||||
| Before | 50 (30 to90) | 51 (30 to100) | 45 (26 to80) | .057 |
| After | 20 (10 to40) | 20 (10 to40) | 25 (11 to48) | .018 |
| P (within groups) | <.001 | <.001 | <.001 | |
| Change* | −23.5 (−50to −10) | −30 (−55to −15) | −15 (−34.5to −8.5) | <.001 |
| Motility (A + B) | ||||
| Before | 31.55 ± 16.17 | 30.43 ± 17.79 | 32.89 ± 13.92 | .171 |
| After | 51.32 ± 21.26 | 57.48 ± 20.24 | 43.91 ± 20.11 | <.001 |
| P (within groups) | <.001 | <.001 | <.001 | |
| Change* | 19.78 ± 18.56 | 27.06 ± 19.04 | 11.02 ± 13.55 | <.001 |
| Clinical pregnancy rate | 26.4% (86/326) | 23.0% (41/178) | 30.4% (45/148) | .133 |
| Biochemical pregnancy rate | 3.4% (11/326) | 3.9% (7/178) | 2.7% (4/148) | .760 |
| Ectopic pregnancy rate | 0.6% (2/326) | 0.6% (1/178) | 0.7% (1/148) | 1.000 |
| Miscarriage rate | 4.6% (15/326) | 3.4% (6/178) | 6.1% (9/148) | .369 |
| Ongoing pregnancy rate (>12 wk) | 17.8% (58/326) | 15.2% (27/178) | 20.9% (31/148) | .174 |
| Fetal anomaly | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | N/A |
| Multiple pregnancy rate | 2.1% (7/326) | 1.7% (3/178) | 2.7% (4/148) | .706 |
| Live birth rate | 15.6% (51/326) | 12.4% (22/178) | 19.6% (29/148) | .102 |
| Time of birth | ||||
| Preterm | 4 (7.8%) | 3 (13.6%) | 1 (3.4%) | .303 |
| Term | 47 (92.2%) | 19 (86.4%) | 28 (96.6%) | |
| Type of birth | ||||
| Normal | 16 (31.4%) | 11 (50.0%) | 5 (17.2%) | .028 |
| Cesarean | 35 (68.6%) | 11 (50.0%) | 24 (82.8%) | |
Data are given as mean ± standard deviation or median (1st quartile - 3rd quartile) for continuous variables according to normality of distribution and as frequency (percentage) for categorical variables.
MSS = microfluidics sperm sorting.
Negative values represent decrease and positive values represent increase.
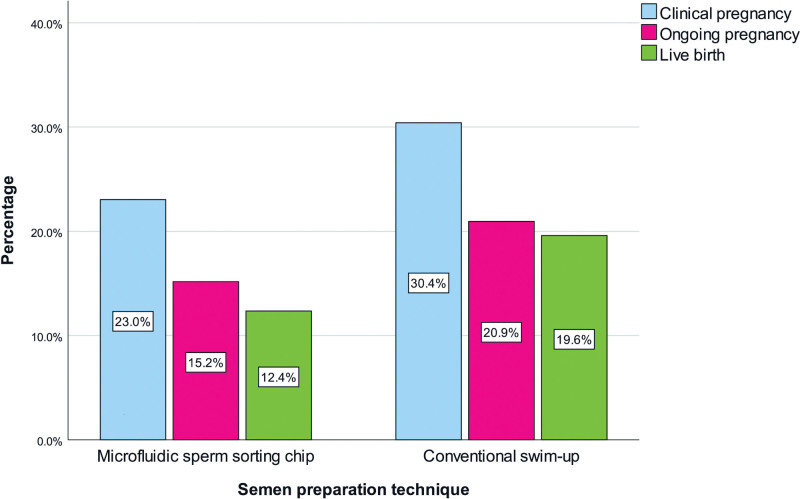
A total of 86 (26.4%) pregnancies were observed in participants and the clinical pregnancy rate was 23% in the MSS group and 30.4% in the SWP group (P = .133). Fifty-one infants were born alive and a great majority (n = 47) were delivered at term. Term delivery by cesarean section was higher in SWP recipients (P = .028). Pregnancy outcomes and live birth rates are visualized in Figure 1.
Figure 1.
Outcomes with regard to semen preparation technique.
Multivariate logistic regression analysis showed that higher duration of infertility was independently associated with lower live birth success (odds ratio: 0.811, 95% confidence interval: 0.662–0.996; P = .045). Other variables, including female age, type and reason of infertility, number of cycles, and sperm motility and concentration, were found to be nonsignificant (Table 2).
Table 2.
Association between clinical variables and live birth, logistic regression analysis results.
| Univariable | ||
|---|---|---|
| OR (95% CI) | P value | |
| Age of female | 0.964 (0.917–1.013) | .143 |
| Type of infertility, Secondary | 1.214 (0.665–2.216) | .528 |
| Number of cycles | 1.091 (0.696–1.710) | .704 |
| Duration of infertility, yr | 0.811 (0.661–0.996) | .045 |
| Reason of infertility* | ||
| Unexplained | 1.463 (0.518–4.131) | .472 |
| Polycystic ovarian syndrome | 2.187 (0.790–6.056) | .132 |
| Sperm concentration (x106/mL) | ||
| Before | 1.002 (0.996–1.007) | .570 |
| After | 1.004 (0.994–1.013) | .456 |
| Motility (A + B) | ||
| Before | 1.014 (0.996–1.032) | .125 |
| After | 1.003 (0.989–1.018) | .648 |
| Semen preparation technique, Swim-up | 1.728 (0.945–3.159) | .076 |
CI = confidence interval.
Reference category: Other, Multivariable analysis was not performed due to only one variable was significant. Nagelkerke R2 for duration of infertility = 0.026.
4. Discussion
This study compared the effectiveness of the SWP and MSS chip methods on sperm separation, pregnancy outcomes and live birth rates in infertile couples receiving IUI treatment. We found higher sperm motility but lower sperm concentration after SPT in patients who underwent MSS compared to SWP. Although not statistically significant, clinical pregnancy, ongoing pregnancy and live birth rates were lower in patients receiving MSS relative to those who underwent SWP. In addition, we demonstrated that higher duration of infertility was the only factor independently associated with lower live birth success.
There are several factors influencing the success rate of IUI, some of these are clinical parameters, including female age, duration of infertility, ovarian reserve, sperm parameters, and also, the sperm preparation method.[10–12] SPTs for assisted reproductive technologies aim to obtain as many motile, morphologically normal spermatozoa as possible without seminal plasma, debris, leukocytes, prostaglandins and immature germ cells, which may adversely affect sperm viability.[13] Traditional preparation methods, including density-gradient and SWP, separate sperm on the basis of migration or sedimentation, but sperm morphology and motility cannot be selected, nor can factors such as DNA integrity, membrane maturation and apoptotic properties be determined.[14] Microfluidic systems contain a micro-channel chip that mimics the intrauterine, cervical and vaginal canal microenvironment of sperm.[15] However, there are few studies evaluating the effect of MSS methods on sperm quality, and the results are inconsistent. Quinn et al[16], in a blinded, controlled study of 70 split samples from infertile men, reported that MSS yielded significantly reduced DNA fragmentation and increased motility of the sperm sample compared to those processed by density-gradient centrifugation. Yaylali and colleagues revealed in 112 couples with unexplained infertility that motility rate after sperm preparation, ratio of sperm with normal morphology and number of inseminated sperm were similar in density-gradient, SWP and MSS groups.[17] Gode et al[18] demonstrated in 265 patients using the density-gradient and microfluidic methods that higher sperm motility and lower sperm concentrations were found in the microfluidic group. In another study, Gode et al[19] showed in 57 split semen samples that lower total sperm concentration and higher progressive motility were found with use of SWP or MSS compared to preparation via the density-gradient approach. Recently, Mateizel et al[20] demonstrated in 52 semen samples that highly motile spermatozoa with uncompromised DNA integrity was achieved with MSS using Fertile plus. Similarly, our results confirm the majority of these prior findings. We found decreased sperm concentration and increased progressive sperm motility following sperm preparation via MSS compared to SWP. The count of progressive motile sperm obtained following preparation is one of the main determinants affecting the success of IUI. Due to the higher selectivity of the MSS sorting system, it was expected that the sperm concentration would be lower in the MSS method than in the SWP method. Considering the similar outcomes, the decreased sperm concentration appears to be balanced by the higher motility of the sperm sample following preparation with MSS. Obtaining sperm with higher motility in the MSS method may be associated with the method producing significantly less radical oxygen products and less DNA fragmentation. Our results support the hypothesis that the microfluidic sperm sorting approach may be more advantageous in motile sperm selection, prevent vulnerability to physical damage that may occur in other methods, and can reduce variability due to human error–with the added advantage of shorter processing time, which may be beneficial to preserve DNA quality and integrity. While microfluidics remains the latest development in the analysis and selection of high-quality sperm, with its small-scale size and microenvironment that mimics the mechanisms of natural selection, its full potential has not been fully realized. Further investigations of in vivo sperm behavioral characteristics and designing simple, user-friendly, innovative, and robust microfluidic designs in all species, especially humans, are needed, so that highly mobile, efficient sperm extraction with greater DNA integrity possible for use in assisted reproduction can be obtained.
Processing and selection of healthy sperm is essential for a successful pregnancy and healthy offspring. Sipahi et al[21], in 133 patients who had undergone IUI with microfluidic sorting, demonstrated that the clinical pregnancy rate was 19.5%, with the highest rate in younger patients. Although not statistically significant, Yaylali and colleagues found the highest clinical pregnancy rate with the use of MSS (22.73%) compared to SWP (15.91%) and the density-gradient (17.39%) methods.[17] Similarly, Gode et al[18] showed the pregnancy rate as 18.04% in the microfluidics group and 15.15% in the density-gradient group; however, again, comparisons revealed nonsignificant differences. Furthermore, Yetkinel et al[22] demonstrated in 122 couples with unexplained infertility treated with SWP or MSS that clinical pregnancy and live birth rates were similar in both groups. We found similar clinical pregnancy rates between 2 separation groups in our study. This may be related to the fact that patients treated with the MSS have more motile sperm but lower sperm concentration. Due to the retrospective design and sample size of the study, it may have been impossible to observe the potential drawback of the MSS approach in terms of pregnancy outcomes. Although larger scale, randomized, prospective studies are required to reach definitive answers regarding this matter, it must be noted that this study involved a reliable number of patients who had received SWP or MSS with respect to available recommendations. Therefore, notwithstanding the possible confounding effects of couples choice on the analysis, future studies should aim to build on these findings with strict inclusion criteria and prospective designs, thereby limiting the effects of bias.
The study had some limitations. First, as mentioned before, the effect of selection bias cannot be ignored since this was a retrospective study in which the 2 SPTs were not assigned in a randomized fashion. The effects of microfluidics methods could also be biased according to various confounding factors that may not be identified due to their relatively-recent introduction into the field. On the other hand, it must be noted that the baseline sperm concentration before preparation was somewhat higher in the MSS group, but analyses yielded a P value of .057.
5. Conclusion
We observed nonsignificant worse reproductive results using microfluid sperm selection in comparison to the pellet swim-up technique (live birth rate = 12% vs 20%). Our evidence is of limited quality due to the retrospective design of this study and sufficiently powered RCTs are needed to evaluate whether sperm selection based using a microfluidic chip is better, similar, or worse than the pellet swim-up technique.
Acknowledgements
The authors would like to thank all the investigators for their participation and continued commitment to the study.
Author contributions
Conceptualization: Bihter Senem Feyzioglu, Zerrin Avul.
Data curation: Bihter Senem Feyzioglu, Zerrin Avul.
Formal analysis: Bihter Senem Feyzioglu, Zerrin Avul.
Funding acquisition: Bihter Senem Feyzioglu, Zerrin Avul.
Investigation: Bihter Senem Feyzioglu, Zerrin Avul.
Methodology: Bihter Senem Feyzioglu, Zerrin Avul.
Project administration: Bihter Senem Feyzioglu, Zerrin Avul.
Resources: Bihter Senem Feyzioglu, Zerrin Avul.
Software: Bihter Senem Feyzioglu, Zerrin Avul.
Supervision: Bihter Senem Feyzioglu, Zerrin Avul.
Validation: Bihter Senem Feyzioglu, Zerrin Avul.
Visualization: Bihter Senem Feyzioglu, Zerrin Avul.
Writing – original draft: Bihter Senem Feyzioglu, Zerrin Avul.
Writing – review & editing: Bihter Senem Feyzioglu, Zerrin Avul.
Abbreviations:
- FSH
- follicle-stimulating hormone
- IUI
- intrauterine insemination
- MSS
- microfluidics sperm sorting
- SPTs
- sperm preparation techniques
- SWP
- swim-up washing procedure
- WHO
- World Health Organization
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors have no funding and conflicts of interest to disclose.
All research procedures were evaluated and approved by the Research Ethics Committee of Acibadem University (date: 22.04.2022, decisin no: 2022/07) and were conducted in agreement with the ethical standards specified in the Declaration of Helsinki.
How to cite this article: Feyzioglu BS, Avul Z. Effects of sperm separation methods before intrauterine insemination on pregnancy outcomes and live birth rates: Differences between the swim-up and microfluidic chip techniques. Medicine 2023;102:46(e36042).
References
- [1].Olatunji O, More A. A review of the impact of microfluidics technology on sperm selection technique. Cureus. 2022;14:e27369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Vaughan DA, Sakkas D. Sperm selection methods in the 21st century. Biol Reprod. 2019;101:1076–82. [DOI] [PubMed] [Google Scholar]
- [3].Baskaran S, Finelli R, Agarwal A, et al. Diagnostic value of routine semen analysis in clinical andrology. Andrologia. 2021;53:e13614. [DOI] [PubMed] [Google Scholar]
- [4].Jeyendran RS, Caroppo E, Rouen A, et al. Selecting the most competent sperm for assisted reproductive technologies. Fertil Steril. 2019;111:851–63. [DOI] [PubMed] [Google Scholar]
- [5].Yildiz K, Yuksel S. Use of microfluidic sperm extraction chips as an alternative method in patients with recurrent in vitro fertilisation failure. J Assist Reprod Genet. 2019;36:1423–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pinto S, Carrageta DF, Alves MG, et al. Sperm selection strategies and their impact on assisted reproductive technology outcomes. Andrologia. 2021;53:e13725. [DOI] [PubMed] [Google Scholar]
- [7].Guler C, Melil S, Ozekici U, et al. Sperm selection and embryo development: a comparison of the density gradient centrifugation and microfluidic chip sperm preparation methods in patients with astheno-teratozoospermia. Life (Basel). 2021;11:933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Anbari F, Khalili MA, Sultan Ahamed AM, et al. Microfluidic sperm selection yields higher sperm quality compared to conventional method in ICSI program: a pilot study. Syst Biol Reprod Med. 2021;67:137–43. [DOI] [PubMed] [Google Scholar]
- [9].WHO. Examination and processing of human semen. Switzerland: World Health Organization; 2010. [Google Scholar]
- [10].ESHRE Capri Workshop Group. Intrauterine insemination. Hum Reprod Update. 2009;15:265–77. [DOI] [PubMed] [Google Scholar]
- [11].Sinha P, Pandey K, Srivastava A. Factors determining successful intrauterine insemination. Int J Reprod Contracept Obstet Gynecol. 2017;6:3887–91. [Google Scholar]
- [12].Wang X, Zhang Y, Sun H-L, et al. Factors affecting artificial insemination pregnancy outcome. Int J Gen Med. 2021;14:3961–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dcunha R, Hussein RS, Ananda H, et al. Current insights and latest updates in sperm motility and associated applications in assisted reproduction. Reprod Sci. 2022;29:7–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Said TM, Land JA. Effects of advanced selection methods on sperm quality and ART outcome: a systematic review. Hum Reprod Update. 2011;17:719–33. [DOI] [PubMed] [Google Scholar]
- [15].Leung ET, Lee C-L, Tian X, et al. Simulating nature in sperm selection for assisted reproduction. Nat Rev Urol. 2022;19:16–36. [DOI] [PubMed] [Google Scholar]
- [16].Quinn MM, Jalalian L, Ribeiro S, et al. Microfluidic sorting selects sperm for clinical use with reduced DNA damage compared to density gradient centrifugation with swim-up in split semen samples. Hum Reprod. 2018;33:1388–93. [DOI] [PubMed] [Google Scholar]
- [17].Yaylali A. Retrospective comparison of the efficiency of sperm preparation methods in intrauterine insemination in unexplained infertility cases. Acta Med Alanya. 2020;4:132–6. [Google Scholar]
- [18].Gode F, Bodur T, Gunturkun F, et al. Comparison of microfluid sperm sorting chip and density gradient methods for use in intrauterine insemination cycles. Fertil Steril. 2019;112:842–848.e1. [DOI] [PubMed] [Google Scholar]
- [19].Gode F, Gürbüz AS, Tamer B, et al. The effects of microfluidic sperm sorting, density gradient and swim-up methods on semen oxidation reduction potential. Urol J. 2020;17:397–401. [DOI] [PubMed] [Google Scholar]
- [20].Mateizel I, Racca A, Aligianni E, et al. P-063 Microfluidic technology is highly effective in selecting a sperm population with high progressive motility and low DNA fragmentation index. Hum Reprod. 2023;38:i216–7. [Google Scholar]
- [21].Sipahi M, Tosun SA, Tutar SO. Experience of our clinic in intrauterine insemination cycles made with microfluidic sperm sorting chips. Aegean J Obstet Gynaecol. 2021;3:15–8. [Google Scholar]
- [22].Yetkinel S, Kilicdag EB, Aytac PC, et al. Effects of the microfluidic chip technique in sperm selection for intracytoplasmic sperm injection for unexplained infertility: a prospective, randomized controlled trial. J Assist Reprod Genet. 2019;36:403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]