Abstract
Dengue-associated complications, including dengue shock syndrome, severe respiratory distress, and pediatric acute liver failure (PALF), are associated with high mortality rates in patients with dengue. There is increasing prevalence of overweight and obesity among children worldwide. Obesity may activate inflammatory mediators, leading to increased capillary permeability and plasma leakage in patients with dengue. Several studies have shown a correlation between obesity and DSS, but did not include dengue fatality or PALF. Therefore, we hypothesized possible associations between obesity and critical dengue-associated clinical outcomes among PICU-admitted children with DSS, including dengue-related mortality, mechanical ventilation (MV) requirements, and dengue-associated PALF. The nutritional status of the participants was assessed using World Health Organization growth charts. A total of 858 participants with complete nutritional data were enrolled in this study. Obesity was significantly associated with risk of severe respiratory failure and MV support (odds ratio = 2.3, 95% CI: 1.31–4.06, P < .01); however, it was not associated with dengue-associated mortality or acute liver failure. Obese pediatric patients with DSS should be closely monitored for severe respiratory distress and the need for high-flow oxygenation support, particularly MV, soon after hospitalization.
Keywords: dengue, nutritional status, obesity, PICU, Vietnam
Key Points.
Obesity may activate inflammatory mediators, leading to increased capillary permeability and plasma leakage in dengue-infected patients.
Dengue shock syndrome, complicated by severe respiratory failure and dengue-associated acute liver failure (PALF) accounts for a high fatality rate.
Obesity was not statistically associated with both dengue-associated in-hospital mortality and PALF.
Obesity was significantly associated with the risk of developing severe respiratory failure and the need for mechanical ventilation support.
1. Introduction
Dengue infection is widespread in tropical and subtropical regions, and approximately 129 countries in Asia are at risk for dengue infection.[1,2] An estimated 390 million dengue cases occur annually worldwide, of which 96 million are clinically present.[3] Dengue infection was attributed to approximately 9221 deaths per year worldwide from 1990 to 2013 and a further 1.14 million disability-adjusted-life-years in 2013.[4] Dengue shock syndrome accounts for poor outcomes in hospitalized children.[5,6] In the absence of timely management, the in-hospital mortality rate of dengue-infected patients ranges from 20% to 25.6%.[5,6] Dengue continues to be a major global public health concern, requiring substantial joint efforts to reduce morbidity and mortality.
According to the 2021 Global Nutrition Report from the Nutrition Accountability Framework (NAF), approximately 234 million children under 5 years of age have nutritional abnormalities, including stunted status, wasted status, and overweight.[7] Notably, the global prevalence of overweight and obesity in children is on goingly increasing by 20 %.[7] The reported prevalence of overweight and obesity among Vietnamese children is 17.4 % and 8.6 %, respectively.[8] There is growing evidence that obesity can influence the host immune response owing to its impact on genomics and metabolisms.[9–11] Obesity may activate inflammatory mediators, leading to increased capillary permeability and plasma leakage in dengue-infected patients.[9–11] It has been shown a correlation between obesity and the development of progressive plasma leakage and dengue shock syndrome (DSS).[12–14] Severe respiratory distress requiring high-flow oxygen support may develop, resulting from severe plasma leakage in patients with severe dengue infection. Likewise, obese dengue-infected patients were reported to have a higher risk of developing severe hepatitis than a comparable nonobese cohort.[15] Notably, dengue-associated acute liver failure (ALF) is characterized by extensive liver necrosis and rapid deterioration of liver function, potentially resulting in death.[16] Despite its rarity, dengue-associated PALF is associated with a high mortality, ranging from 25% to roughly 60 %.[16–18]
Several previous studies have shown associations between patients’ nutritional status and dengue severity, including DSS, respiratory distress, and hepatitis.[19–21] However, to date, the impact of obesity on dengue-associated mortality, pediatric acute liver failure, and severe respiratory failure requiring mechanical ventilation (MV) support among pediatric intensive care unit (PICU)-admitted children has not been well studied and reported. Therefore, we aimed to conduct this study to fill this knowledge gap to improve the management and outcomes of dengue-infected pediatric patients. We hypothesized possible associations between obesity and clinical outcomes in DSS-experiencing children, including in-hospital mortality, acute liver failure, and MV.
2. Methods
2.1. Study design and participants
This retrospective, single-center study was conducted at Children Hospital No. 2, Ho Chi Minh City, Vietnam, which is one of the 3 largest tertiary referral pediatric hospitals in southern Vietnam, with a capacity of approximately 1400 in-hospital beds. We screened all critically ill Dengue-infected children admitted to the pediatric intensive care unit between 2013 and 2021. The eligibility criteria included age < 18 years, laboratory-confirmed dengue infection, DSS, and dengue-associated acute liver failure, irrespective of other causes of hepatic injury in patients with dengue infection.[22,23]
2.2. Study outcomes
The primary study outcomes were in-hospital dengue-associated mortality, severe respiratory distress requiring MV, and dengue-associated PALF.
2.3. Study definitions
Dengue infection was defined according to the World Health Organization (WHO) criteria (2009), with laboratory confirmation by the dengue-IgM antibody test or nonstructural 1 (NS1) antigen test.[22] Dengue shock syndrome was diagnosed according to the WHO dengue 2009 guidelines.[22]
Dengue-associated acute liver failure was defined as an acute episode of severe hepatic dysfunction with biochemical evidence of liver injury in children without preexisting chronic liver diseases, liver-induced coagulopathy not corrected by vitamin K supplementation, an international normalized ratio (INR) > 1.5, encephalopathy or INR > 2.0, with or without encephalopathy.[17,23]
The 2006 WHO growth chart for children aged 0–5 years and the 2007 WHO growth chart for those aged 5–19 years were used to categorize the nutritional status of the study participants.[24,25] For children aged ≥ 5 years, body mass index (BMI) was plotted in the 2007 WHO growth chart, and for children aged < 5 years, height for weight and/or weight was classified using the 2006 WHO growth chart. Nutritional categorization was performed based on the standard deviation (SD). According to the 2006 WHO growth chart, healthy or normal weight was plotted in the range from –2 SD to + 2 SD, obesity > +3 SD, overweight > +2 SD, and underweight <–2 SD.[24] Under the 2007 WHO growth chart, normal weight from –2 SD to + 1 SD, obesity was defined as > +2 SD, overweight > +1 SD, and underweight <–2 SD.[25]
2.4. Data collection
All patient data were de-identified to ensure compliance with the Good Clinical Practice. Case report forms were used to collect study data from medical records, which were then entered into an electronic database. The clinical outcomes of patients were reviewed during the PICU stay and at discharge. Nutritional data of the participants were assessed by collecting weight, height, and patient age at PICU admission.
2.5 . Ethics statement
The authors have no ethical issues to declare. This study was approved by the Institutional Review Board of Children’s Hospital No. 2, Ho Chi Minh City, Vietnam (approval number: 391, signed on 22 March, 2022). This study was performed in compliance with the principles of Good Clinical Practice and the Declaration of Helsinki.
2.6. Statistical analysis
Continuous variables were summarized as medians and interquartile ranges (IQRs). Categorical variables are presented as numbers (n) and percentages (%). Missing nutritional data from the retrospective data collection was considered the main study bias. Only participants with complete nutritional information were included in data analysis. We performed univariate and multivariable logistic regression models with appropriately adjusted covariates (age, sex, hematocrit levels, low platelet cell count, critical hepatic transaminases, severe bleeding, and standardized cummulative amount of infused fluid within 24 hours on PICU admission), which were chosen, based on the disease pathogenesis and our clinical experience. We performed the backward stepwise model selection, based on Akaike Information Criteria (or AIC) for all adjusted multivariable logistic analyses. No significant interactions were observed among the covariables of interest. Statistical significance was set at P values < .05 for all comparisons. The R statistical software (version 4.2.2, Boston, MA) was used for all analyses.
3. Results
3.1. Baseline characteristics of patients’ cohort on PICU admission
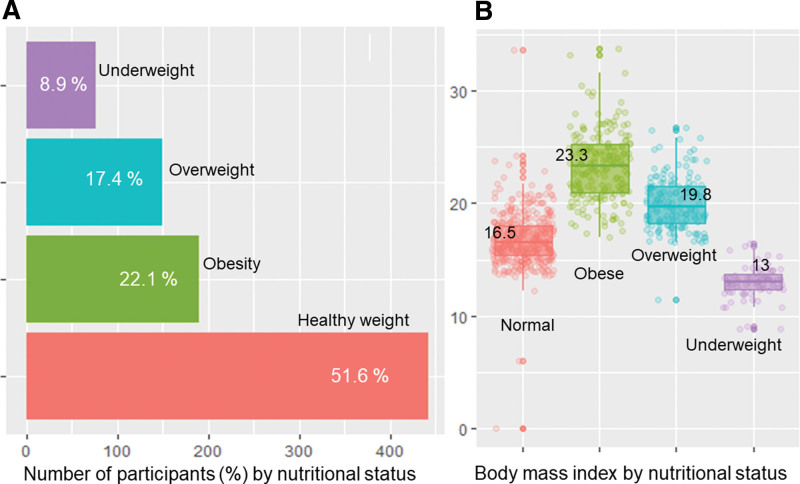
We identified 1045 dengue-infected children admitted to the pediatric intensive care unit, and of whom, a total of 858 patients with complete nutritional data retrieved from hospital paper-based medical records were enrolled and analyzed. The median patient age was 7.3 (IQR: 5–10) years, and males accounted for 53 % of study participants. The median BMI was 17.9 (IQR: 15.8–21) kg/m2. Proportions of participants with obesity, overweight, normal weight, and underweight were 22.1 %, 17.4 %, 51.6 %, and 8.9 %, respectively, as shown in Figure 1A. The BMI distribution by nutritional status is shown in Figure 1B.
Figure 1.
The percentages (%) of nutritional status of study participants (Fig. 1A) and the distributions of body mass index of obese cohort and other comparable groups (Fig. 1B).
3.2. Baseline clinical and laboratory characteristics of the cohort on PICU admission
Baseline clinical and laboratory data of the study participants are presented in Table 1. Notably, 793 (92 %) children were diagnosed with compensated DSS, and remaining 65 (8 %) patients with decompensated DSS. Forty-five (5.3%) participants had comorbidities. The median length of PICU stay was 2 (IQR, 1–3) days. Severe bleeding was observed in 68 (7.9 %) patients. The median respiratory rate was 26 breaths/min (IQR: 24–30) breaths per minutes. The median systolic shock index was 1.28 (IQR: 1.09–1.48) bpm/mm Hg and diastolic shock index was 1.71 (IQR: 1.44–2.0) bpm/mm Hg. Full blood counts revealed a significant increase in hematocrit and a decrease in platelet cell counts. Marked elevation of transaminase levels was also observed. The median blood lactate level was 2.2 (IQR: 1.6–3.2) mmol/L, and the median serum creatinine level was 52 (IQR: 44–61) µmol/L. Compared with the nonobese cohort, obese patients presented slightly higher levels of transaminases, serum lactate, and INR; however, these differences were not statistically significant.
Table 1.
Clinical characteristics of study participants on PICU admissions.
| Characteristics | All participants (N = 858) | Nonobese (n = 668) | Obese (n = 190) | P-value |
|---|---|---|---|---|
| Age, (years) | 7.3 (5–10) | 7.3 (4.7–10.2) | 7.3 (5.4–9.8) | .28 |
| Male, n (%) | 454 (53) | 322 (48) | 132 (69) | <.01 |
| Body mass index, kg/m2 | 17.9 (15.8–21) | 16.9 (15.1–18.9) | 23.3 (21–25.3) | <.001 |
| Underlying diseases, yes | 45 (5.3) | 30 (4.5) | 15 (7.9) | .09 |
| Grading of dengue severity* | .08 | |||
| Compensated DSS, n (%) | 793 (92) | 623 (93) | 170 (89) | |
| Decompensated DSS, n (%) | 65 (8) | 45 (7) | 20 (11) | |
| Length of PICU stay, days | 2 (1–3) | 2 (1–3) | 2 (2–4) | .13 |
| Severe bleeding, n (%) | 68 (7.9) | 52 (7.8) | 16 (8.4) | .87 |
| Respiratory rate, (breaths per min) | 26 (24–30) | 26 (24–30) | 26 (24–30) | .60 |
| Systolic shock index, (bpm/mm Hg) | 1.28 (1.09–1.48) | 1.3 (1.1–1.5) | 1.2 (1.03–1.42) | .06 |
| Diastolic shock index, (bpm/mm Hg) | 1.71 (1.44–2.0) | 1.71 (1.47–2.1) | 1.63 (1.38–1.88) | .07 |
| White blood cell count (x 109/L) | 4.9 (3.3–7.1) | 4.8 (3.3–7.0) | 5.2 (3.5–7.4) | .18 |
| Hemoglobin (g/dL) | 14.7 (13.2–16) | 14.6 (13.2–15.9) | 15.1 (13.7–16.3) | .36 |
| Peak hematocrit (%) | 48 (45–51) | 47 (44–50) | 49 (45–51) | <.01 |
| Nadir hematocrit (%) | 38 (35–42) | 38 (35–41) | 39 (35–42) | .03 |
| Platelet cell count (x 109/L) | 38 (24–58) | 40 (24–59) | 37 (23–56) | .49 |
| AST (IU/L) | 196 (106–609) | 179 (100–560) | 241 (123–660) | .37 |
| ALT (IU/L) | 87 (41–282) | 79 (38–266) | 123 (54–298) | .42 |
| International normalized ratio | 1.24 (1.1–1.53) | 1.24 (1.1–1.51) | 1.25 (1.1–1.55) | .15 |
| Serum lactate (mmol/L) | 2.2 (1.6–3.2) | 2.2 (1.6–3.0) | 2.6 (1.7–3.6) | .70 |
| Serum creatinin (µmol/L) | 52 (44–61) | 52 (44–60) | 54 (46–63) | .32 |
| Troponin I (ng/mL) | 0.03 (0.01–0.19) | 0.02 (0.01–0.15) | 0.1 (0.01–0.35) | .14 |
| Ascites on sonography, n (%) | 413 (59) | 320 (58) | 93 (63) | .30 |
| Respiratory support, n (%) | .58 | |||
| Normal breathing (room air) | 309 (36) | 247 (37) | 62 (33) | |
| Nasal cannula oxygenation | 403 (47) | 310 (46) | 93 (49) | |
| NCPAP | 34 (4) | 30 (5) | 4 (2) | |
| Mechanical ventilation | 112 (13) | 81 (12) | 31 (16) | |
| Severe hepatic transaminases | 108 (13) | 87 (13) | 21 (11) | .55 |
| Dengue-associated PALF, n (%) | 59 (6.9) | 49 (7.3) | 10 (5.3) | .41 |
| Fatal outcome, n (%) | 24 (2.8) | 19 (2.8) | 5 (2.6) | 1.0 |
Notes: Summary statistics are presented as median (interquartile range, IQR) for continuous variables and frequency (%) for categorical variables.
ALT = alanine aminotransferase; AST = aspartate aminotransferase; DSS = dengue shock syndrome; NCPAP = nasal continuous positive airway pressure; PALF = dengue-associated acute liver failure; PICU = pediatric intensive care unit.
DSS, classified according to WHO 2009 dengue guidelines.
3.3. Association between patient’s obese status and in-hospital dengue mortality rate
Overall, 24/858 (2.8 %) patients died during hospitalization, including 5/190 (2.6 %) patients in obese cohort and 19/668 (2.8 %) patients in the nonobese group. Both univariable and adjusted multivariable analyses showed no associations between obesity and in-hospital mortality rate (adjusted odds ratio [OR] = 0.82, 95% CI: 0.26–0.64, P = .71, as shown in Table 2).
Table 2.
Associations between obesity and in-hospital mortality among children with dengue shock syndrome.
| Groups | Fatal (n = 24) | Alive (n = 834) | Crude OR* (95% CI) | P * | Adjusted OR† (95% CI) | P † |
|---|---|---|---|---|---|---|
| Obese (n = 190) | 5 (2.6) | 185 (97.3) | 0.92 (0.34–2.51) | 0.87 | 0.82 (0.26–2.54) | .72 |
| Nonobese (n = 668) | 19 (2.8) | 649 (97.2) |
CI = confidence interval; OR = odds ratio.
Crude OR and P-values from univariate logistic analysis.
Adjusted OR and P-values from multivariable logistic analysis adjusted for age, sex, peak hematocrit level, platelet cell count, severe bleeding within 24 hours PICU admission.
3.4. Association between patient’s obesity and dengue-associated acute liver failure
A total of 36/858 (4.2 %) DSS children developed dengue-associated PALF. There was no statistical difference in proportion of dengue-associated acute liver failure in obese cohort (4.2 %) versus nonobese group (4.1 %), as shown in Table 3. Both univariate and adjusted multivariate analyses showed no significant association between obesity and dengue-associated PALF (adjusted OR = 1.32, 95% CI: 0.48–3.64, P = .6).
Table 3.
Associations between obesity and dengue-associated acute liver failure among children with dengue shock syndrome.
| Groups | PALF (n = 36) | Non-PALF (n = 822) | Crude OR* (95% CI) | P * | Adjusted OR† (95% CI) | P † |
|---|---|---|---|---|---|---|
| Obese, (n = 190) | 8 (4.2) | 182 (95.8) | 1.0 (0.45–2.24) | 0.99 | 1.32 (0.48–3.64) | 0.60 |
| nonobese, (n = 668) | 28 (4.1) | 640 (95.9) |
CI = confidence interval; OR = odds ratio; PALF = pediatric acute liver failure.
Crude OR and P-values from univariate logistic analysis.
The OR and P-values from multivariable logistic analysis adjusted for age, sex, and severe transaminases.
3.5. Association between obesity and severe respiratory distress requiring MV among children with DSS
A total of 112/858 (13 %) children with DSS experienced severe respiratory failure, indicated for MV support. More obese than nonobese patients required MV, as shown 31/112 (27.7 %) versus 159/746 (21.3 %), with a difference of 6.4 % (Table 4). Although univariate analysis showed that although obesity increased risk of MV support, this was not statistically significant (unadjusted OR = 1.41; 95% CI, 0.9–2.21 and P = .13). More importantly, the multivariable logistic analysis revealed that obese children with DSS had a significantly higher risk (over 2-fold) of developing severe respiratory failure and MV support, with adjusted OR = 2.3, 95% CI, 1.31–4.06, P < .01, as presented in Table 4. In addition, other significant determinants of MV support among DSS children included female patients, severe bleeding, severe hepatic transaminases, hematocrit level and excessive intravenous fluid administration.
Table 4.
Associations between obesity and mechanical ventilation requirement among children with dengue shock syndrome.
| Factors | MV (n = 112) | Non-MV (n = 746) | Crude OR (95% CI), P* | Adjusted OR (95% CI), P† |
|---|---|---|---|---|
| Obesity | ||||
| No Yes |
81 (72.3) 31 (27.7) |
587 (78.7) 159 (21.3) |
1.41 (0.90–2.21), P = .13 |
2.3 (1.31–4.06), P < .01 |
| Age (years), median (IQR) | 7 (5–10) | 7 (5–10) | 0.97 (0.92–1.02), P = .27 |
- |
| Gender | ||||
| Male Female |
52 (46.4) 60 (53.6) |
402 (53.9) 344 (46.1) |
1.35 (0.91–2.01), P = .14 |
1.75 (1.04–2.93), P = .03 |
| Severe bleeding‡ | ||||
| No Yes |
65 (58) 47 (42) |
725 (97) 21 (3) |
24.8 (14.0–44.0), P < .001 |
16.8 (8.61–32.7), P < .001 |
| Hematocrit (%), median (IQR) | 45 (40–50) | 48 (45–51) | 0.9 (0.87–0.93), P < .001 |
0.91 (0.87–0.95), P < .001 |
| Low platelet count (<20,000 cells × 109/L) | - | |||
| No Yes |
81 (72.3) 31 (27.7) |
635 (85.1) 111 (14.9) |
2.16 (1.36–3.42), P = .001 |
|
| Severe hepatic transaminases‡ | ||||
| No Yes |
76 (67.9) 36 (32.1) |
674 (90.3) 72 (9.7) |
4.43 (2.79–7.06), P < .001 |
2.99 (1.56–5.74), P = .001 |
| Square root of cumulative infused fluid from referral hospitals and 24 hours PICU admission (mL/kg)§ | 13.1 (10.3–15.3) | 9.4 (0–11.7) | 1.15 (1.1–1.20), P < .001 |
1.15 (1.1–1.21), P < .001 |
CI = confidence interval; IQR = interquartile range; MV = mechanical ventilation; OR = odds ratio; PICU = pediatric intensive care unit.
Crude OR and P-values from bivariate logistic analyses.
Adjusted OR and P-values from multivariable logistic analyses.
These factors were defined by the WHO dengue guidelines in 2009.
Standardized covariate of the cumulative amount of fluid from referral hospitals and within 24h PICU admission by square root.
4. Discussion
The impact of obesity on dengue-associated mortality, acute liver failure, and severe respiratory failure requiring MV in children with DSS has not been well studied. In this retrospective single-center study, we aimed to examine the effect of obesity on in-hospital fatality rate, dengue-associated PALF, and MV requirements among PICU-admitted pediatric patients with DSS. Our study revealed that a higher proportion of decompensated DSS was observed in the obese cohort than in the nonobese group. Notably, obese children had significantly higher levels of hepatic transaminases, coagulation disorders, hematocrit (peak and nadir levels), and cardiac troponin I on PICU admission than nonobese patients. These data are consistent with those of previous reports.[15,26] Additionally, dengue-related complications, including severe bleeding and ascites, were noticeably higher in the obese group than in the nonobese group. Pathophysiologically, published studies have shown associations between obesity and metabolism, the host immune response, overexpression of proinflammatory cytokines, and heightened vascular inflammation. These factors may compromise vascular endothelial cells, leading to increased capillary permeability and plasma leakage in obese dengue-infected patients.[9–11] These mechanisms explain the elevations in hematocrit and biomarkers among obese DSS patients in our study. Markedly, obesity has been shown to be associated with increased risks of developing DSS, dengue-induced hepatitis, severe plasma leakage and respiratory distress.[12–15] However, data on the effects of obesity on dengue-associated mortality and acute liver failure are lacking.
The in-hospital mortality rate of pediatric dengue-infected patients was reported approximately 25%.[6] Nonetheless, dengue fatality can be dramatically reduced to <1% with appropriate and timely treatments by intervening predictors of dengue severity identified on PICU admission.[5,27] Predictors of dengue-related death include significantly elevated transaminases, high blood lactate levels, positive fluid balance, DSS, and systolic shock index > 1, requiring multiple vasoactive drugs.[6,8,27] Therefore, we identified these prognostic factors on hospital admission for early and judicious interventions to reduce dengue-related mortality, particularly in obese children with DSS.
Our study clearly showed no association between obesity and dengue-associated mortality among hospitalized children with DSS, although obesity was significantly associated with severe respiratory distress and MV. There is growing evidence that obesity can injure vascular endothelial cells, leading to increased capillary permeability and plasma leakage.[9,11,28] Therefore, substantial plasma leakage causes excessive fluid accumulation in the pleural and abdominal cavities, which can further result in mild to severe respiratory failure. Obesity is a significant risk factor of intra-abdominal hypertension and abdominal compartment syndrome.[29] To the best of our knowledge, ascites in children with obesity may increase more rapidly than that in nonobese patients. Hence, obesity can potentially aggravate high intra-abdominal pressures. In addition, enlargement of internal organs resulting from substantial plasma leakage may lead to early manifestation of respiratory failure in obese patients with severe DSS.
In this regard, our study group recently reported case reports of patients with dengue obstructive shock syndrome, a fatal dengue complication occurring in the late critical stage of dengue infection.[30] A dramatic rise in thoracic pressure resulting from severe plasma leakage and MV can impede the heart from pumping effectively, reducing the adequacy of blood venous return to the cardiac chambers. Obesity, severe ascites, and pleural effusion combined with mechanical ventilatory support have been reported as significant risk factors for developing dengue obstructive shock syndrome.[30] Another point to note is that obese patients with DSS were shown to have longer duration of PICU stay in our study, although the difference was not statistically significant. Combining all points, obesity had a marginal effect on dengue in-hospital mortality, but it was significantly associated with respiratory failure. Thus, close monitoring of respiratory distress and early respiratory intervention for obese patients with DSS on hospital admission will yield clinical benefits, including shortened length of PICU stay and reduced PICU-related sepses.[15]
Dengue-associated acute liver failure is characterized by extensive liver necrosis and rapid deterioration of hepatic function, resulting in death.[16] Dengue-associated PALF is rare, but associated with a high fatality rate, ranging from 25 % to roughly 60 %.[17,18] Notably, dengue-induced liver injury encompasses direct viral cytopathic effect, hepatocyte apoptosis, cytokine storm, and immune-mediated dengue hepatitis.[31] The main pathogenesis of dengue-associated PALF involves a detrimentally dysregulated cytokine cascade resulting from sophisticated interactions between dengue viruses and host factors.[18] Excessive proinflammatory cytokine levels and liver-associated toxicities are significantly correlated with a high mortality rate in dengue-infected patients with PALF.[32] Previous studies have shown that obesity is significantly associated with an increased risk of elevated transaminase levels and severe hepatitis.[15,26] Most Notably, approximately 0.35 % of patients with dengue-induced hepatitis may develop acute liver failure.[33] To date, the association between obesity and dengue-associated PALF has not been studied and reported yet. Mouse experiments revealed that obesity could potentially affect host immune responses and increase cytokine expression against dengue infection.[34] Based on these findings, we hypothesized an association between obesity and dengue-associated PALF. Our study clearly showed no association between obesity and PALF among PICU-admitted children with severe dengue infection. Nevertheless, our study revealed that obese patients with DSS experienced elevated levels of biomarkers associated with hepatocyte damage, including hepatic transaminases and coagulation dysfunction. In this respect, our study findings confirmed the results from previously published reports[15,26] and further showed no correlation between obesity and dengue-associated PALF.
5. Limitations
Our study had several limitations inherent to the nature of a single-center, retrospective cohort study. Other limitations included unstandardized collections of clinical and laboratory data, particularly nutritional data of the participants.
6. Conclusions
This study demonstrated the prevalence of nutritional status among PICU-admitted children with DSS. Obesity was significantly associated with the risk of severe respiratory failure requiring MV support; however, it was not associated with dengue-associated mortality or acute liver failure. Obese children with DSS should be closely monitored for the manifestation of severe respiratory distress and the need for high-flow oxygenation, particularly MV support, soon after hospitalization.
Acknowledgements
We are grateful to the patients and administrative staff for their support with this study.
Author contributions
Conceptualization: Thanh Tat Nguyen, Dat Tat Nguyen, Luan Thanh Vo.
Data curation: Thanh Tat Nguyen, Dat Tat Nguyen, Tien Thi-Hong Vo, Oanh Tran-Hoang Dang, Bao Trung Nguyen, Dung Thi-Thuy Pham, Thuong Thi-Kim Nguyen, Yen Nguyen-Hoang Duong, Duong Hung Doan, Truc Huynh Nguyen, Lien Thi Ho, Phuc Hoang Nguyen, Dung Ngoc Phan, Tin Van Tran, Tuyet Kim Nguyen, Duc Cong Luong, Anh Thi-Mai Pham, Thuy Thi-Diem Dinh, Viet Chau Do, Luan Thanh Vo.
Formal analysis: Thanh Tat Nguyen, Dat Tat Nguyen.
Funding acquisition: Thuong Thi-Kim Nguyen, Luan Thanh Vo.
Investigation: Thanh Tat Nguyen, Dat Tat Nguyen, Yen Nguyen-Hoang Duong, Thuy Thi-Diem Dinh, Luan Thanh Vo.
Methodology: Thanh Tat Nguyen, Luan Thanh Vo.
Project administration: Tuyet Kim Nguyen, Anh Thi-Mai Pham, Thuy Thi-Diem Dinh.
Supervision: Thuy Thi-Diem Dinh, Viet Chau Do, Luan Thanh Vo.
Writing – original draft: Thanh Tat Nguyen, Dat Tat Nguyen.
Writing – review & editing: Thanh Tat Nguyen, Luan Thanh Vo.
Abbreviations:
- BMI
- body mass index
- DSS
- dengue shock syndrome
- INR
- international normalized ratio
- IQRs
- interquartile ranges
- MV
- mechanical ventilation
- PALF
- dengue-associated pediatric acute liver failure
- PICU
- pediatric intensive care unit
- SD
- standard deviation
- WHO
- World Health Organization
TTN and DTN contributed equally to this work.
This study was funded by Kim Oanh Corp. in Vietnam. The funder did not have any role in the study design, data analysis, or publication decisions.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Nguyen TT, Nguyen DT, Vo TT-H, Dang OT-H, Nguyen BT, Pham DT-T, Nguyen TT-K, Duong YN-H, Doan DH, Nguyen TH, Ho LT, Nguyen PH, Phan DN, Tran TV, Nguyen TK, Luong DC, Pham AT-M, Dinh TT-D, Do VC, Vo LT. Associations of obesity and dengue-associated mortality, acute liver failure and mechanical ventilation in children with dengue shock syndrome. Medicine 2023;102:46(e36054).
Contributor Information
Dat Tat Nguyen, Email: tuyet1706@gmail.com.
Tien Thi-Hong Vo, Email: vothanhluan@gmail.com.
Oanh Tran-Hoang Dang, Email: droanhdang@gmail.com.
Bao Trung Nguyen, Email: tuyet1706@gmail.com.
Dung Thi-Thuy Pham, Email: maianh2406@gmail.com.
Thuong Thi-Kim Nguyen, Email: tuyet1706@gmail.com.
Yen Nguyen-Hoang Duong, Email: Yenduong160691@gmail.com.
Duong Hung Doan, Email: hungduong365@gmail.com.
Truc Huynh Nguyen, Email: tuyet1706@gmail.com.
Lien Thi Ho, Email: Lienho1421@gmail.com.
Phuc Hoang Nguyen, Email: tuyet1706@gmail.com.
Dung Ngoc Phan, Email: phanngocdung01021993@gmail.com.
Tin Van Tran, Email: tranvantin035@gmail.com.
Tuyet Kim Nguyen, Email: tuyet1706@gmail.com.
Duc Cong Luong, Email: Luongcongduc53@gmail.com.
Anh Thi-Mai Pham, Email: maianh2406@gmail.com.
Thuy Thi-Diem Dinh, Email: dtdiemthuy@yahoo.com.vn.
Viet Chau Do, Email: dr.dochauviet@gmail.com.
Luan Thanh Vo, Email: vothanhluan@gmail.com.
References
- [1].Phanitchat T, Zhao B, Haque U, et al. Spatial and temporal patterns of dengue incidence in northeastern Thailand 2006–2016. BMC Infect Dis. 2019;19:743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Brady OJ, Gething PW, Bhatt S, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6:e1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Stanaway JD, Shepard DS, Undurraga EA, et al. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16:712–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Alejandria MM. Dengue haemorrhagic fever or dengue shock syndrome in children. BMJ Clin Evid. 2015;2015:0917. [PMC free article] [PubMed] [Google Scholar]
- [6].Sachdev A, Pathak D, Gupta N, et al. Early predictors of mortality in children with severe dengue fever: a prospective study. Pediatr Infect Dis J. 2021;40:797–801. [DOI] [PubMed] [Google Scholar]
- [7].The 2021 global nutrition report from the Nutrition Accountability Framework (NAF). Available from: https://globalnutritionreport.org/reports/2021-global-nutrition-report/. [accessed and cited on 21th June 2023]
- [8].Phan HD, Nguyen TNP, Bui PL, et al. Overweight and obesity among Vietnamese school-aged children: National prevalence estimates based on the World Health Organization and International Obesity Task Force definition. PLoS One. 2020;15:e0240459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Raqib R, Cravioto A. Nutrition, immunology, and genetics: future perspectives. Nutr Rev. 2009;67(Suppl 2):S227–236. [DOI] [PubMed] [Google Scholar]
- [10].Calabro P, Chang DW, Willerson JT, et al. Release of C-reactive protein in response to inflammatory cytokines by human adipocytes: linking obesity to vascular inflammation. J Am Coll Cardiol. 2005;46:1112–3. [DOI] [PubMed] [Google Scholar]
- [11].Gallagher P, Chan KR, Rivino L, et al. The association of obesity and severe dengue: possible pathophysiological mechanisms. J Infect. 2020;81:10–6. [DOI] [PubMed] [Google Scholar]
- [12].Kurnia B, Suryawan IWB. The association between obesity and severity of dengue hemorrhagic fever in children at Wangaya General Hospital. Open Access Maced J Med Sci. 2019;7:2444–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zulkipli MS, Dahlui M, Jamil N, et al. The association between obesity and dengue severity among pediatric patients: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12:e0006263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Te H, Sriburin P, Rattanamahaphoom J, et al. Association between nutritional status and dengue severity in Thai children and adolescents. PLoS Negl Trop Dis. 2022;16:e0010398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tan VPK, Ngim CF, Lee EZ, et al. The association between obesity and dengue virus (DENV) infection in hospitalised patients. PLoS One. 2018;13:e0200698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kye Mon K, Nontprasert A, Kittitrakul C, et al. Incidence and clinical outcome of acute liver failure caused by dengue in a Hospital for Tropical Diseases, Thailand. Am J Trop Med Hyg. 2016;95:1338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Thanh NT, Dat NT, Thinh TN, et al. Therapeutic plasma exchange and continuous renal replacement therapy in pediatric dengue-associated acute liver failure: a case series from Vietnam. Transfus Apher Sci. 2023;62:103617. [DOI] [PubMed] [Google Scholar]
- [18].Vo LT, Do VC, Trinh TH, et al. Combined therapeutic plasma exchange and continuous renal replacement therapy in children with dengue-associated acute liver failure and shock syndrome: single-center cohort from Vietnam. Pediatr Crit Care Med. 2023;24:818–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Marón GM, Clará AW, Diddle JW, et al. Association between nutritional status and severity of dengue infection in children in El Salvador. Am J Trop Med Hyg. 2010;82:324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Maneerattanasak S, Suwanbamrung C. Impact of nutritional status on the severity of dengue infection among pediatric patients in Southern Thailand. Pediatr Infect Dis J. 2020;39:e410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zulkipli MS, Rampal S, Bulgiba A, et al. Is there any association between body mass index and severity of dengue infection? Trans R Soc Trop Med Hyg. 2021;115:764–71. [DOI] [PubMed] [Google Scholar]
- [22].WHO. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva: World Health Organization, 2009. [PubMed] [Google Scholar]
- [23].Wendon J, Cordoba J, Dhawan A, et al. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047–81. [DOI] [PubMed] [Google Scholar]
- [24].World Health Organization (WHO). Child growth standards. 2020. Available from: https://www.who.int/tools/child-growth-standards/standards/weight-for-age. [Accessed and cited on 21th June 2023]
- [25].World Health Organization (WHO). Growth reference data from 5–19 years old. 2020. Available from: https://www.who.int/tools/growth-reference-data-for-5to19-years [Accessed and cited on 21st June 2023]
- [26].Chiu YY, Lin CY, Yu LS, et al. The association of obesity and dengue severity in hospitalized adult patients. J Microbiol Immunol Infect. 2023;56:267–73. [DOI] [PubMed] [Google Scholar]
- [27].Jain S, Mittal A, Sharma SK, et al. Predictors of dengue-related mortality and disease severity in a tertiary care center in North India. Open Forum Infect Dis. 2017;4:ofx056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Iantorno M, Campia U, Di Daniele N, et al. Obesity, inflammation and endothelial dysfunction. J Biol Regul Homeost Agents. 2014;28:169–76. [PubMed] [Google Scholar]
- [29].Holodinsky JK, Roberts DJ, Ball CG, et al. Risk factors for intra-abdominal hypertension and abdominal compartment syndrome among adult intensive care unit patients: a systematic review and meta-analysis. Crit Care. 2013;17:R249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nguyen TT, Le NT, Nguyen NM, et al. Clinical features and management of children with dengue-associated obstructive shock syndrome: a case report. Medicine (Baltim). 2022;101:e31322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Leowattana W, Leowattana T. Dengue hemorrhagic fever and the liver. World J Hepatol. 2021;13:1968–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Antoniades CG, Berry PA, Wendon JA, et al. The importance of immune dysfunction in determining outcome in acute liver failure. J Hepatol. 2008;49:845–61. [DOI] [PubMed] [Google Scholar]
- [33].Devarbhavi H, Ganga D, Menon M, et al. Dengue hepatitis with acute liver failure: Clinical, biochemical, histopathological characteristics and predictors of outcome. J Gastroenterol Hepatol. 2020;35:1223–8. [DOI] [PubMed] [Google Scholar]
- [34].Chuong C, Bates TA, Akter S, et al. Nutritional status impacts dengue virus infection in mice. BMC Biol. 2020;18:106. [DOI] [PMC free article] [PubMed] [Google Scholar]