Abstract
Background
The current absence of gold‐standard or all‐aspect favorable therapies for COVID‐19 renders a focus on multipotential drugs proposed to prevent or treat this infection or ameliorate its signs and symptoms vitally important. The present well‐designed randomized controlled trial (RCT) sought to evaluate the efficacy and safety of N‐acetylcysteine (NAC) as adjuvant therapy for 60 hospitalized Iranian patients with COVID‐19.
Methods
Two 30‐person diets, comprising 15 single diets of Kaletra (lopinavir/ritonavir) + hydroxychloroquine (HCQ) with/without NAC (600 mg TDS) and atazanavir/ritonavir + HCQ with/without NAC (600 mg TDS), were administered in the study.
Results
At the end of the study, a further decrease in C‐reactive protein was observed in the NAC group (P = 0.008), and no death occurred in the atazanavir/ritonavir + HCQ + NAC group, showing that the combination of these drugs may reduce mortality. The atazanavir/ritonavir + HCQ and atazanavir/ritonavir + NAC groups exhibited the highest O2 saturation at the end of the study and a significant rise in O2 saturation following intervention commencement, including NAC (P > 0.05). Accordingly, oral or intravenous NAC, if indicated, may enhance O2 saturation, blunt the inflammation trend (by reducing C‐reactive protein), and lower mortality in hospitalized patients with COVID‐19.
Conclusion
The NAC could be more effective as prophylactic or adjuvant therapy in stable non‐severe cases of COVID‐19 with a particularly positive role in the augmentation of O2 saturation and faster reduction of the CRP level and inflammation or could be effective for better controlling of COVID‐19 or its therapy‐related side effects.
Keywords: antiviral, atazanavir, COVID‐19, hydroxychloroquine, Kaletra, lopinavir, NAC, N‐acetylcysteine, ritonavir
The present well‐designed randomized controlled trial sought to evaluate the efficacy and safety of N‐acetylcysteine (NAC) as adjuvant therapy in hospitalized Iranian patients with coronavirus disease 2019 (COVID‐19). The NAC could be more effective as prophylactic or adjuvant therapy in stable and nonsevere cases of COVID‐19 with a particularly positive role in the augmentation of O2 saturation and faster reduction of the C‐reactive protein level and inflammation.

1. INTRODUCTION
On March 11, the World Health Organization (WHO) announced the outbreak of coronavirus disease 2019 (COVID‐19) and declared it to be an epidemic. 1 , 2 The virus is transmitted through respiratory droplets or aerosols. 3 One of the theories concerning the coronavirus pathogenesis is that the virus binds to host cells through angiotensin‐converting enzyme 2 (ACE2). ACE2 is expressed by the epithelial cells of the lung, intestine, kidney, and blood vessels. 4 Diabetes, ACE inhibitors, and angiotensin II receptor blockers, which are used for hypertension control, increase ACE2 expression and COVID‐19 risk. 4 The symptoms of COVID‐19 include dry coughs; malaise; fever; dyspnea; multiorgan failure; acute respiratory distress syndrome (ARDS) requiring mechanical ventilation and oxygen therapy in the intensive care unit (ICU); coagulopathy with thrombosis; systemic manifestations such as sepsis, septic shocks, and multiorgan dysfunction syndrome; and mucocutaneous involvement. 5 , 6 , 7 , 8 , 9 Inflammatory responses, cytokine storms, and chemokines are critical issues allied to the complications of COVID‐19. 10 , 11 About 33% of the patients with COVID‐19 require ICU admission, with a mortality rate of 20% reported in some investigations. 12 , 13 Additionally, a mortality rate of 49.0% has been reported among critical patients with comorbid cardiovascular diseases, hypertension, diabetes, chronic respiratory diseases, or cancer. 12 Polymerase chain reaction (PCR) tests of the upper respiratory tract samples, lung computed tomography (CT) scans, and blood tests are accepted by the WHO for the diagnosis of COVID‐19. 3 , 14 N‐acetylcysteine (NAC) is a multipotential drug suggested by the literature for the prevention and treatment of COVID‐19. 1 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 NAC is an antioxidant with a wide variety of use in different medical conditions such as pulmonary disorders, cystic fibrosis, bronchitis, chronic obstructive pulmonary disease, and pneumonia. 6 , 24 Evidence indicates the important roles of NAC in the prevention and treatment of COVID‐19. 24 , 25 , 26 COVID‐19 can manifest itself through neurological disorders such as Guillain–Barre syndrome, seizure, headache, and stroke. 27 NAC is capable of exerting protective effects on the nervous system and helps prevent or treat these manifestations. 5 Liver failure can develop in patients with COVID‐19 for several reasons, including metabolic acidosis and complications induced by certain drugs such as remdesivir, which is one of the most commonly used drugs in these patients. In this regard, one of the most well‐known effects of NAC is the prevention and treatment of hepatotoxicity. 28 , 29 , 30
1.1. A gap of knowledge
In spite of the multitude of research dedicated to COVID‐19, a definitive and universally accepted treatment for this ailment remains elusive. As a result, current strategies primarily revolve around providing supportive care. The most effective approach to tackling the illness continues to be prevention, with global vaccination efforts already in progress. However, it is important to note that instances of COVID‐19‐linked infections and fatalities persistently persist. Most COVID‐19 supportive drugs modulate the immune system to regulate inflammatory storms. 5 Many of the immune modulators have immunosuppressive properties that may not work properly in viral disorders. 5 NAC is one of the few immune modulators without immunosuppressive properties. 31 However, all the articles suggesting the use of NAC in the treatment of COVID‐19 recommend further well‐designed randomized controlled clinical trials (RCTs).
1.2. Aim
This RCT, conducted under double‐blind conditions (with both secondary assessors and analysts unaware of the details), aimed to assess the impact of oral NAC in the management of COVID‐19 patients admitted to the hospital.
While there's evidence suggesting notable advantages of NAC for individuals with mild COVID‐19 before hospitalization, this specific investigation concentrated exclusively on patients already in the hospital setting. This study stands out as one of the meticulously planned RCTs aimed at gauging the effectiveness and safety of NAC as supplementary treatment for hospitalized COVID‐19 patients.
2. METHODS
2.1. Design and settings
The present double‐blind RCT was performed in Rasool Akram Medical Complex, Tehran, Iran, on 60 patients with COVID‐19. The diagnosis of COVID‐19 was established according to the opinion of the treating physician, based on clinical signs and PCR or paraclinical or laboratory findings.
2.2. Sampling and allocation
The sampling convenience method was employed. Eligible participants were classified by stratified blocked randomization and based on diet therapy (four regimens). Thereafter, they were randomly assigned to either the intervention group or the routine treatment regimen group. Randomization was done separately within each group. The size of the blocks was four; in other words, two allocations to the intervention group and two allocations to the routine treatment group were considered.
2.3. Eligibility criteria
The indication for hospitalization according to the national protocol was as follows: fever above 39° or being toxic in the examination, respiratory distress, the use of respiratory muscle relaxants, the use of suprasternal or intercostal retraction, a respiratory rate greater than 30/min, a heart rate higher than 120 beats/min, a peripheral blood O2 saturation level less than 93%, having an underlying disease (e.g., diabetes, hypertension, heart failure, immune system disorders, renal or hepatic impairment, a history of asthma or chronic obstructive pulmonary disease, and smoking), age over 50 years in the case of being symptomatic, and the involvement of one‐third of 3–5 pulmonary lobes. The criteria for exclusion encompassed individuals who were minors, those with unstable vital signs, individuals either already intubated or requiring intubation, those with diminished levels of consciousness, a respiratory rate exceeding 24, blood pressure readings below 90/60 mm Hg, showing multilobular infiltration in CT scans or chest X‐rays, experiencing persistent hypoxia, pregnant or nursing individuals, and those with past hypersensitivity reactions to NAC or medications containing glutathione. The withdrawal criteria were comprised of drug intolerance, severe complications probably related to NAC during treatment, and unwillingness to continue collaboration with the study at any point and for any reason.
2.4. Interventions and follow‐up
Two 30‐person diets, comprising 15 single diets of Kaletra (lopinavir/ritonavir) (LOPINAVIR/RITONAVIR Aurobindo 200/50 MG Tablet) + hydroxychloroquine (HCQ) (HYDROXYCHLOROQUINE AMIN 200MG TAB) with/without NAC (ACETYLCYSTEINE‐HAKIM 600 MG EFF TAB) (600 mg total dissolved solids [TDS]) and atazanavir/ritonavir (atazanavir (300 mg) + ritonavir (100 mg), India) + HCQ with/without NAC (600 mg TDS), were administered in the study. Sixty patients completed the study (15 patient: Kaletra + HCQ/15 patient: Kaletra + HCQ + NAC/15 patient: atazanavir/ritonavir + HCQ/15 patient: atazanavir/ritonavir + HCQ + NAC). The control and intervention groups received the national protocol treatment, and NAC was added to the treatment of the intervention group. Since the eligible patients had no contraindications for NAC, the protocol was 600 mg orally every 8 h for 14 days.
2.5. Blinding
The secondary assessor and the data analyst were blinded to the treatment regimens.
2.6. Paraclinical data
Laboratory parameters were evaluated by using the peripheral blood samples of the patients. Additionally, lactate dehydrogenase (LDH), tumor necrosis factor‐α (TNF‐α), interleukin‐6 (IL‐6), complete blood count (CBC), erythrocyte sedimentation rate (ESR), and C‐reactive protein (CRP) tests were performed daily for the patients, and the course of laboratory changes was monitored. Radiological examinations of the lungs were performed by CT scanning twice: at admission and discharge, and differences in radiological findings were recorded and compared. In the patients with minor underlying problems or gastrointestinal intolerance, 600 mg of oral tablets every 12 or 24 h were given.
2.7. Response to treatment criteria
Time of improvement in symptoms such as coughs, shortness of breath, and lethargy.
Improvements in O2 saturation without changes in the treatment protocol and reductions in the need for O2.
Duration of hospitalization according to the course of symptom improvement.
Readmission after discharge.
Serial evaluations of laboratory parameters, consisting of LDH, TNF‐α, IL‐6, CBC, ESR, and CRP, and comparison of parameters at hospitalization, during hospitalization, and at discharge.
Investigation of changes in anti‐inflammatory parameters.
Examination of radiological changes at the beginning of hospitalization and during hospitalization.
Need for ICU admission during hospitalization.
2.8. Primary and secondary outcomes
The primary outcomes of the study were the efficacy and side effects of NAC, and the secondary outcomes were drug tolerance and treatment satisfaction. The effectiveness of treatment was evaluated according to the duration of hospitalization; improvement in O2 saturation, laboratory and paraclinical findings, and clinical symptoms; and the assessment of complications based on a questionnaire.
2.9. Ethical considerations
The research adhered to the tenets of the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of Iran University of Medical Sciences (ethical code #IR.IUMS.REC.1399.206 registered on August 16, 2020), and the study protocol was registered in the Iranian Registry of Clinical Trials (#IRCT20200623047897N1; https://en.irct.ir/trial/49277). Written informed consent was obtained from all the patients.
2.10. Statistical analysis
After entering the required data from the patient's records, the data were analyzed using SPSS version 21. Besides, descriptive data for continuous variables and qualitative statistics were used as bar charts and tables. One‐way Analysis of variance (ANOVA) repeated measure was used to compare the quantitative variables among groups as well as linear regression was applied to predict the factors affecting the length of stay at the ICU. p Value less than .05 was considered significant.
3. RESULTS
In this research study, the average age of the patients was 57.82 years with a standard deviation of 18.23 years, and the average duration of hospitalization was 10.13 days with a standard deviation of 6.07 days. In terms of gender distribution, 31 patients (51.7%) were female, and there were no statistically significant differences observed between the various groups. Detailed clinical and paraclinical characteristics of the study population at both admission and discharge are presented in Tables 1 and 2. Notably, the analysis revealed differences among the four groups in terms of certain parameters at discharge.
Table 1.
Clinical and paraclinical characteristics of the study population admission.
| Variable | Mean | SD | F a | p‐Valueb |
|---|---|---|---|---|
| Hospitalization days | ||||
| Kaletra + HCQ | 10.60 | 7.298 | 2.347 | .082 |
| Kaletra + HCQ + NAC | 11.87 | 4.941 | ||
| Atazanavir/ritonavir + HCQ | 6.73 | 3.058 | ||
| Atazanavir/ritonavir + HCQ + NAC | 11.33 | 7.158 | ||
| ICU days | ||||
| Kaletra + HCQ | 3.47 | 6.174 | 1.714 | .174 |
| Kaletra + HCQ + NAC | 2.47 | 4.704 | ||
| Atazanavir/ritonavir + HCQ | 0.00 | 0.000 | ||
| Atazanavir/ritonavir + HCQ + NAC | 1.80 | 3.783 | ||
| WBC | ||||
| Kaletra + HCQ | 8.5000 | 5.26661 | 0.963 | .417 |
| Kaletra + HCQ + NAC | 10.0200 | 6.00990 | ||
| Atazanavir/ritonavir + HCQ | 12.4533 | 17.83836 | ||
| Atazanavir/ritonavir + HCQ + NAC | 6.4667 | 3.88471 | ||
| diff_segment | ||||
| Kaletra + HCQ | 68.1467 | 28.47184 | 1.210 | .316 |
| Kaletra + HCQ + NAC | 80.7462 | 8.23120 | ||
| Atazanavir/ritonavir + HCQ | 74.7231 | 9.10386 | ||
| Atazanavir/ritonavir + HCQ + NAC | 73.5714 | 13.89363 | ||
| diff_lymphocyte | ||||
| Kaletra + HCQ | 16.3000 | 9.91396 | 1.679 | .184 |
| Kaletra + HCQ + NAC | 15.5231 | 8.01105 | ||
| Atazanavir/ritonavir + HCQ | 22.1154 | 9.19464 | ||
| Atazanavir/ritonavir + HCQ + NAC | 21.2071 | 10.23782 | ||
| seg_lymph_ratio | ||||
| Kaletra + HCQ | 7.8954 | 6.13541 | 3.007 | .039 |
| Kaletra + HCQ + NAC | 6.6869 | 3.82775 | ||
| Atazanavir/ritonavir + HCQ | 4.0519 | 1.88692 | ||
| Atazanavir/ritonavir + HCQ + NAC | 4.3714 | 2.22906 | ||
| RBC | ||||
| Kaletra + HCQ | 4.5567 | 0.62525 | 0.958 | .419 |
| Kaletra + HCQ + NAC | 4.7333 | 0.59904 | ||
| Atazanavir/ritonavir + HCQ | 4.4107 | 0.94044 | ||
| Atazanavir/ritonavir + HCQ + NAC | 4.3373 | 0.53022 | ||
| HGB | ||||
| Kaletra + HCQ | 12.9533 | 1.61946 | 0.212 | .888 |
| Kaletra + HCQ + NAC | 13.3475 | 1.80408 | ||
| Atazanavir/ritonavir + HCQ | 12.9467 | 2.96211 | ||
| Atazanavir/ritonavir + HCQ + NAC | 12.7800 | 1.32891 | ||
| PLT | ||||
| Kaletra + HCQ | 184.9333 | 81.10528 | 0.469 | .705 |
| Kaletra + HCQ + NAC | 209.1333 | 135.86436 | ||
| Atazanavir/ritonavir + HCQ | 169.2000 | 84.42934 | ||
| Atazanavir/ritonavir + HCQ + NAC | 202.6000 | 97.13378 | ||
| ESR | ||||
| Kaletra + HCQ | 50.7273 | 23.57580 | 1.492 | .233 |
| Kaletra + HCQ + NAC | 58.5000 | 25.39685 | ||
| Atazanavir/ritonavir + HCQ | 36.2222 | 23.28507 | ||
| Atazanavir/ritonavir + HCQ + NAC | 55.4444 | 28.08964 | ||
| CRP | ||||
| Kaletra + HCQ | 38.6711 | 14.42544 | 4.434 | .009 |
| Kaletra + HCQ + NAC | 48.0080 | 0.00422 | ||
| Atazanavir/ritonavir + HCQ | 26.4983 | 19.56495 | ||
| Atazanavir/ritonavir + HCQ + NAC | 28.8020 | 17.16058 | ||
| PT | ||||
| Kaletra + HCQ | 14.9071 | 2.37567 | 0.633 | .597 |
| Kaletra + HCQ + NAC | 14.4000 | 1.78282 | ||
| Atazanavir/ritonavir + HCQ | 14.1500 | 1.15719 | ||
| Atazanavir/ritonavir + HCQ + NAC | 14.0867 | 1.38248 | ||
| INR | ||||
| Kaletra + HCQ | 1.1936 | 0.27712 | 0.259 | .855 |
| Kaletra + HCQ + NAC | 1.1443 | 0.21429 | ||
| Atazanavir/ritonavir + HCQ | 1.1500 | 0.14460 | ||
| Atazanavir/ritonavir + HCQ + NAC | 1.1267 | 0.17915 | ||
| PTT | ||||
| Kaletra + HCQ | 34.2143 | 4.02260 | 3.853 | .015 |
| Kaletra + HCQ + NAC | 40.0714 | 9.16065 | ||
| Atazanavir/ritonavir + HCQ | 33.5500 | 4.31414 | ||
| Atazanavir/ritonavir + HCQ + NAC | 33.6600 | 4.70741 | ||
| BUN | ||||
| Kaletra + HCQ | 26.6000 | 24.81589 | 2.045 | .118 |
| Kaletra + HCQ + NAC | 17.1429 | 12.30295 | ||
| Atazanavir/ritonavir + HCQ | 17.2000 | 9.90815 | ||
| Atazanavir/ritonavir + HCQ + NAC | 13.4667 | 7.15009 | ||
| Cr | ||||
| Kaletra + HCQ | 1.5400 | 1.05343 | 1.932 | .135 |
| Kaletra + HCQ + NAC | 1.3357 | 0.44826 | ||
| Atazanavir/ritonavir + HCQ | 1.6467 | 0.75201 | ||
| Atazanavir/ritonavir + HCQ + NAC | 1.0733 | 0.28402 | ||
| AST | ||||
| Kaletra + HCQ | 42.6923 | 15.93416 | 0.634 | .597 |
| Kaletra + HCQ + NAC | 51.4167 | 45.73532 | ||
| Atazanavir/ritonavir + HCQ | 46.1667 | 28.94457 | ||
| Atazanavir/ritonavir + HCQ + NAC | 60.1333 | 42.96156 | ||
| ALT | ||||
| Kaletra + HCQ | 28.3077 | 13.44981 | 1.114 | .353 |
| Kaletra + HCQ + NAC | 46.2500 | 59.31599 | ||
| Atazanavir/ritonavir + HCQ | 35.8333 | 24.87362 | ||
| Atazanavir/ritonavir + HCQ + NAC | 52.3333 | 37.21303 | ||
| LDH | ||||
| Kaletra + HCQ | 901.7143 | 222.52170 | 5.981 | .003 |
| Kaletra + HCQ + NAC | 576.5000 | 170.14085 | ||
| Atazanavir/ritonavir + HCQ | 694.6000 | 206.46622 | ||
| Atazanavir/ritonavir + HCQ + NAC | 515.6250 | 188.30366 | ||
| CPK | ||||
| Kaletra + HCQ | 294.8571 | 186.19562 | 0.771 | .519 |
| Kaletra + HCQ + NAC | 272.4615 | 488.81380 | ||
| Atazanavir/ritonavir + HCQ | 302.8571 | 293.94412 | ||
| Atazanavir/ritonavir + HCQ + NAC | 639.1111 | 1006.70408 | ||
| CPK_M | ||||
| Kaletra + HCQ | 28.3333 | 8.14453 | 0.198 | .896 |
| Kaletra + HCQ + NAC | 20.6667 | 7.37111 | ||
| Atazanavir/ritonavir + HCQ | 24.4286 | 9.50188 | ||
| Atazanavir/ritonavir + HCQ + NAC | 24.4286 | 16.27736 | ||
| ALK_P | ||||
| Kaletra + HCQ | 253.5000 | 243.59130 | 0.989 | .407 |
| Kaletra + HCQ + NAC | 246.4000 | 108.58095 | ||
| Atazanavir/ritonavir + HCQ | 153.7273 | 62.06463 | ||
| Atazanavir/ritonavir + HCQ + NAC | 225.7143 | 137.50437 | ||
| bili_t | ||||
| Kaletra + HCQ | 1.0875 | 0.87413 | 2.322 | .093 |
| Kaletra + HCQ + NAC | 0.8750 | 0.32842 | ||
| Atazanavir/ritonavir + HCQ | 0.7556 | 0.27889 | ||
| Atazanavir/ritonavir + HCQ + NAC | 1.5154 | 0.97455 | ||
| bili_d | ||||
| Kaletra + HCQ | 0.2875 | 0.13562 | 1.377 | .266 |
| Kaletra + HCQ + NAC | 0.2875 | 0.13562 | ||
| Atazanavir/ritonavir + HCQ | 0.2333 | 0.12247 | ||
| Atazanavir/ritonavir + HCQ + NAC | 0.4538 | 0.42743 |
Abbreviations: ALK_P, alkaline phosphatase; ALT, alanine aminotransferase; ANOVA, analysis of variance; AST, aspartate aminotransferase; bili_d, direct bilirubin; bili_t, total bilirubin; BUN, blood urea nitrogen; CPK, creatine phosphokinase; Cr, creatinine; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; HCQ, hydroxychloroquine; HGB, hemoglobin; ICU, intensive care unit; INR, international normalized ratio; LDH, lactate dehydrogenase; NAC, N‐acetylcysteine; PLT, platelet; PT, prothrombin time; PTT, partial prothrombin time; RBC, red blood cell; WBC, white blood cell.
Statistics of one‐way ANOVA test.
All p‐values in this table are originated from ANOVA mean comparison.
Table 2.
Clinical and paraclinical characteristics (quantitative variables) of the study groups at discharge time.
| Variable | Mean | SD | F a | p‐Valueb |
|---|---|---|---|---|
| WBC_dis | ||||
| Kaletra + HCQ | 6.7167 | 3.38280 | 0.631 | .600 |
| Kaletra + HCQ + NAC | 7.9231 | 3.07738 | ||
| Atazanavir/ritonavir + HCQ | 7.9273 | 3.63045 | ||
| Atazanavir/ritonavir + HCQ + NAC | 9.0200 | 3.52449 | ||
| diff_segment_dis | ||||
| Kaletra + HCQ | 77.4500 | 9.16951 | 5.156 | .007 |
| Kaletra + HCQ + NAC | 62.9714 | 9.53078 | ||
| Atazanavir/ritonavir + HCQ | 78.4875 | 10.05861 | ||
| Atazanavir/ritonavir + HCQ + NAC | 79.9600 | 5.87435 | ||
| diff_lymphocyte_dis | ||||
| Kaletra + HCQ | 7.2667 | 8.98480 | 0.929 | .433 |
| Kaletra + HCQ + NAC | 9.8800 | 13.09238 | ||
| Atazanavir/ritonavir + HCQ | 7.3467 | 9.15883 | ||
| Atazanavir/ritonavir + HCQ + NAC | 4.0000 | 6.25996 | ||
| seg_lymph_ratio_discharge | ||||
| Kaletra + HCQ | 8.9700 | 7.69231 | 0.922 | .445 |
| Kaletra + HCQ + NAC | 3.9786 | 2.45806 | ||
| Atazanavir/ritonavir + HCQ | 12.4725 | 16.45986 | ||
| Atazanavir/ritonavir + HCQ + NAC | 7.5540 | 3.34222 | ||
| RBC_dis | ||||
| Kaletra + HCQ | 3.9717 | 0.66835 | 0.989 | .409 |
| Kaletra + HCQ + NAC | 4.3777 | 0.76260 | ||
| Atazanavir/ritonavir + HCQ | 4.3540 | 0.49552 | ||
| Atazanavir/ritonavir + HCQ + NAC | 4.3740 | 0.70734 | ||
| HGB_dis | ||||
| Kaletra + HCQ | 11.7500 | 1.85497 | 1.061 | .378 |
| Kaletra + HCQ + NAC | 12.3154 | 1.95356 | ||
| Atazanavir/ritonavir + HCQ | 13.1500 | 2.01674 | ||
| Atazanavir/ritonavir + HCQ + NAC | 12.8800 | 1.90316 | ||
| PLT_dis | ||||
| Kaletra + HCQ | 182.5833 | 92.96770 | 1.550 | .219 |
| Kaletra + HCQ + NAC | 265.0000 | 89.59302 | ||
| Atazanavir/ritonavir + HCQ | 256.0000 | 109.84231 | ||
| Atazanavir/ritonavir + HCQ + NAC | 220.4000 | 137.21990 | ||
| ESR_dis | ||||
| Kaletra + HCQ | 64.0000 | 0.00000 | 1.523 | .317 |
| Kaletra + HCQ + NAC | 65.5000 | 0.70711 | ||
| Atazanavir/ritonavir + HCQ | 30.2500 | 36.73668 | ||
| Atazanavir/ritonavir + HCQ + NAC | 9.0000 | – | ||
| CRP_dis | ||||
| Kaletra + HCQ | 40.0033 | 13.85929 | 9.102 | .008 |
| Kaletra + HCQ + NAC | 36.0000 | 16.97056 | ||
| Atazanavir/ritonavir + HCQ | 7.9967 | 3.46699 | ||
| Atazanavir/ritonavir + HCQ + NAC | 5.9900 | 0.00000 | ||
| PT_dis | ||||
| Kaletra + HCQ | 15.9000 | 2.73057 | 0.870 | .478 |
| Kaletra + HCQ + NAC | 14.1000 | 1.23982 | ||
| Atazanavir/ritonavir + HCQ | 14.8000 | 2.54558 | ||
| Atazanavir/ritonavir + HCQ + NAC | 14.3333 | 2.59294 | ||
| INR_dis | ||||
| Kaletra + HCQ | 1.2350 | 0.18738 | 1.985 | .163 |
| Kaletra + HCQ + NAC | 1.0663 | 0.07671 | ||
| Atazanavir/ritonavir + HCQ | 1.0000 | – | ||
| Atazanavir/ritonavir + HCQ + NAC | 1.1000 | 0.17321 | ||
| PTT_dis | ||||
| Kaletra + HCQ | 32.6667 | 5.57375 | 0.627 | .609 |
| Kaletra + HCQ + NAC | 31.7500 | 4.13176 | ||
| Atazanavir/ritonavir + HCQ | 32.3000 | 5.23259 | ||
| Atazanavir/ritonavir + HCQ + NAC | 28.4000 | 1.75784 | ||
| BUN_dis | ||||
| Kaletra + HCQ | 26.7500 | 16.55363 | 1.000 | .403 |
| Kaletra + HCQ + NAC | 18.3000 | 7.48406 | ||
| Atazanavir/ritonavir + HCQ | 20.0000 | 13.42318 | – | – |
| Atazanavir/ritonavir + HCQ + NAC | 21.5714 | 4.42934 | ||
| Cr_dis | ||||
| Kaletra + HCQ | 1.1083 | 0.46409 | 1.690 | .186 |
| Kaletra + HCQ + NAC | 1.2900 | 0.31429 | ||
| Atazanavir/ritonavir + HCQ | 1.2417 | 0.49627 | ||
| Atazanavir/ritonavir + HCQ + NAC | 0.8714 | 0.22887 | ||
| AST_dis | ||||
| Kaletra + HCQ | 57.0000 | 44.49157 | 0.143 | .868 |
| Kaletra + HCQ + NAC | 46.9000 | 34.40430 | ||
| Atazanavir/ritonavir + HCQ | 49.0000 | 15.72683 | ||
| Atazanavir/ritonavir + HCQ + NAC | – | – | ||
| ALT_dis | ||||
| Kaletra + HCQ | 46.0000 | 42.13668 | 0.413 | .668 |
| Kaletra + HCQ + NAC | 48.5000 | 44.10152 | ||
| Atazanavir/ritonavir + HCQ | 68.7500 | 31.45764 | ||
| Atazanavir/ritonavir + HCQ + NAC | – | – | ||
| LDH_dis | ||||
| Kaletra + HCQ | 837.3333 | 497.61464 | 2.275 | .115 |
| Kaletra + HCQ + NAC | 698.5000 | 235.20268 | ||
| Atazanavir/ritonavir + HCQ | 500.5714 | 189.82435 | ||
| Atazanavir/ritonavir + HCQ + NAC | 477.1667 | 78.03183 | ||
| CPK_dis | ||||
| Kaletra + HCQ | 107.6667 | 81.98984 | 0.246 | .862 |
| Kaletra + HCQ + NAC | 87.2500 | 52.42375 | ||
| Atazanavir/ritonavir + HCQ | 85.2500 | 51.21442 | ||
| Atazanavir/ritonavir + HCQ + NAC | 122.0000 | 35.35534 | ||
| CPK_mb_dis | ||||
| Kaletra + HCQ | 55.0000 | – | ||
| Kaletra + HCQ + NAC | – | – | ||
| Atazanavir/ritonavir + HCQ | 10.0000 | – | ||
| Atazanavir/ritonavir + HCQ + NAC | – | – | ||
| ALK_P_dis | ||||
| Kaletra + HCQ | 56.9333 | 84.17878 | 7.650 | .001 |
| Kaletra + HCQ + NAC | 137.2000 | 114.94048 | ||
| Atazanavir/ritonavir + HCQ | 31.1333 | 82.19738 | ||
| Atazanavir/ritonavir + HCQ + NAC | 0.0000 | 0.00000 | ||
| Bili_t_dis | ||||
| Kaletra + HCQ | 1.3800 | 1.02567 | 2.648 | .119 |
| Kaletra + HCQ + NAC | 0.8500 | 0.52820 | ||
| Atazanavir/ritonavir + HCQ | 2.5500 | 1.62635 | ||
| Atazanavir/ritonavir + HCQ + NAC | – | – | ||
| Bili_d_dis | ||||
| Kaletra + HCQ | 0.4400 | 0.21909 | 0.455 | .647 |
| Kaletra + HCQ + NAC | 0.3333 | 0.23381 | ||
| Atazanavir/ritonavir + HCQ | 0.3000 | 0.00000 | ||
| Atazanavir/ritonavir + HCQ + NAC | – | – |
Abbreviations: ALK_P, alkaline phosphatase; ALT, alanine aminotransferase; ANOVA, analysis of variance; AST, aspartate aminotransferase; bili_d, direct bilirubin; bili_t, total bilirubin; BUN, blood urea nitrogen; CPK_M, creatine phosphokinase; Cr, creatinine; CRP, C‐reactive protein; _dis, at discharge; ESR, erythrocyte sedimentation rate; HCQ, hydroxychloroquine; HGB, hemoglobin; ICU, intensive care unit; INR, international normalized ratio; LDH, lactate dehydrogenase; NAC, N‐acetylcysteine; PLT, platelet; PT, prothrombin time; PTT, partial prothrombin time; RBC, red blood cell; WBC, white blood cell.
Statistics of one‐way ANOVA test.
All p‐values in this table are originated from ANOVA mean comparison.
Specifically, there were significant variations in CRP levels (F = 9.102, p = .008), alkaline phosphatase (ALP) levels (F = 7.650, p = .001), and differential segment (diff_segment) values (F = 5.156, p = .007) across the groups. The atazanavir/ritonavir + HCQ + NAC group exhibited the lowest CRP value, indicating a favorable outcome. In contrast, the Kaletra + HCQ + NAC group displayed the highest ALP value at discharge. Furthermore, the highest diff_segment value was observed in the atazanavir/ritonavir + HCQ + NAC group, suggesting distinctive patterns among the treatment groups.
Based on the χ 2 test, there were noteworthy differences in the utilization of intravenous immune globulin (IVIG) between the Kaletra + HCQ group (six patients) and the atazanavir/ritonavir + HCQ + NAC group (where no IVIG was administered), with this distinction proving statistically significant (p = .015). The initial random assignment of patients into four distinct groups led to discernible variations in terms of fatigue, anorexia, cardiovascular diseases, and hypertension, as outlined in Table 3.
Table 3.
Clinical and paraclinical characteristics (qualitative variables) across the four study groups.
| Variable | Group | χ 2 a | p‐Valueb | |||
|---|---|---|---|---|---|---|
| Kaletra + HCQ | Kaletra + HCQ + NAC | Atazanavir/ritonavir + HCQ | Atazanavir/ritonavir + HCQ + NAC | |||
| Sex | ||||||
| Female | 9 | 5 | 8 | 9 | 2.870 | .412 |
| Male | 6 | 10 | 7 | 6 | ||
| IVIG | ||||||
| Negative | 9 | 10 | 14 | 15 | 10.833 | .015 |
| Positive | 6 | 5 | 1 | 0 | ||
| PCR | ||||||
| Negative | 11 | 11 | 9 | 10 | 3.487 | .942 |
| Positive | 4 | 4 | 4 | 4 | ||
| Fever | ||||||
| Negative | 4 | 6 | 5 | 3 | 1.587 | .662 |
| Positive | 11 | 9 | 10 | 12 | ||
| Cough | ||||||
| Negative | 4 | 4 | 4 | 1 | 2.369 | .542 |
| Positive | 11 | 11 | 11 | 13 | ||
| Dyspnea | ||||||
| Negative | 5 | 3 | 2 | 1 | 3.498 | .352 |
| Positive | 10 | 12 | 12 | 13 | ||
| Fatigue | ||||||
| Negative | 2 | 6 | 0 | 1 | 10.214 | .013 |
| Positive | 11 | 9 | 15 | 13 | ||
| Anorexia | ||||||
| Negative | 1 | 10 | 2 | 3 | 17.434 | .001 |
| Positive | 12 | 4 | 13 | 11 | ||
| Body pain | ||||||
| Negative | 5 | 5 | 3 | 3 | 1.200 | .773 |
| Positive | 10 | 10 | 12 | 11 | ||
| Diarrhea | ||||||
| Negative | 9 | 15 | 13 | 13 | 6.584 | .068 |
| Positive | 4 | 0 | 2 | 1 | ||
| Sore throat | ||||||
| Negative | 9 | 10 | 11 | 7 | 1.961 | .624 |
| Positive | 4 | 5 | 4 | 7 | ||
| Sputum | ||||||
| Negative | 7 | 10 | 7 | 10 | 2.660 | .485 |
| Positive | 7 | 5 | 8 | 4 | ||
| Chest discomfort | ||||||
| Negative | 6 | 13 | 9 | 9 | 5.287 | .160 |
| Positive | 7 | 2 | 6 | 5 | ||
| Headache | ||||||
| Negative | 7 | 9 | 8 | 8 | 0.172 | .982 |
| Positive | 6 | 6 | 7 | 6 | ||
| Vertigo | ||||||
| Negative | 10 | 14 | 8 | 11 | 6.638 | .091 |
| Positive | 3 | 1 | 7 | 3 | ||
| Illusion | ||||||
| Negative | 8 | 10 | 12 | 13 | 4.236 | .248 |
| Positive | 5 | 2 | 3 | 1 | ||
| Seizure | ||||||
| Negative | 13 | 12 | 15 | 14 | 2.716 | .999 |
| Positive | 0 | 0 | 0 | 1 | ||
| LOC | ||||||
| Negative | 9 | 10 | 13 | 12 | 1.754 | .690 |
| Positive | 4 | 2 | 2 | 2 | ||
| Smell loss | ||||||
| Negative | 11 | 7 | 12 | 12 | 4.930 | .196 |
| Positive | 2 | 6 | 3 | 2 | ||
| Taste disorders | ||||||
| Negative | 13 | 8 | 12 | 12 | 6.659 | .082 |
| Positive | 0 | 5 | 3 | 2 | ||
| Heart disease | ||||||
| Negative | 6 | 12 | 9 | 11 | 8.017 | .044 |
| Positive | 9 | 3 | 6 | 2 | ||
| Lung disease | ||||||
| Negative | 11 | 13 | 12 | 14 | 2.400 | .660 |
| Positive | 4 | 2 | 3 | 1 | ||
| Kidney disease | ||||||
| Negative | 10 | 14 | 11 | 13 | 5.576 | .145 |
| Positive | 3 | 1 | 4 | 0 | ||
| Dialysis | ||||||
| Negative | 13 | 15 | 12 | 13 | 6.078 | .166 |
| Positive | 0 | 0 | 2 | 0 | ||
| Immunodeficiency | ||||||
| Negative | 13 | 15 | 13 | 14 | 5.804 | .237 |
| Positive | 0 | 0 | 2 | 0 | ||
| DM | ||||||
| Negative | 10 | 11 | 10 | 9 | 0.176 | .999 |
| Positive | 4 | 4 | 5 | 4 | ||
| HTN | ||||||
| Negative | 5 | 13 | 9 | 11 | 8.547 | .035 |
| Positive | 8 | 2 | 6 | 3 | ||
| Malignancy | ||||||
| Negative | 12 | 15 | 13 | 13 | 2.058 | .681 |
| Positive | 1 | 0 | 2 | 1 | ||
| Dexamethasone | ||||||
| Negative | 15 | 10 | 6 | 3 | 21.991 | .001 |
| Positive | 0 | 5 | 9 | 12 | ||
| Acetaminophen | ||||||
| Negative | 9 | 5 | 7 | 3 | 5.556 | .152 |
| Positive | 6 | 10 | 8 | 12 | ||
| Azithromycin | ||||||
| Negative | 9 | 5 | 15 | 13 | 18.730 | .001 |
| Positive | 6 | 10 | 0 | 2 | ||
| Ceftriaxone | ||||||
| Negative | 8 | 7 | 13 | 14 | 11.746 | .007 |
| Positive | 7 | 8 | 2 | 1 | ||
| Heparin | ||||||
| Negative | 2 | 1 | 0 | 2 | 2.400 | .740 |
| Positive | 13 | 14 | 15 | 13 | ||
| DiphenHCQamine | ||||||
| Negative | 8 | 11 | 11 | 9 | 1.978 | .659 |
| Positive | 7 | 4 | 4 | 6 | ||
| Interferon‐β | ||||||
| Negative | 15 | 15 | 9 | 9 | 15.000 | .002 |
| Positive | 0 | 0 | 6 | 6 | ||
| Levofloxacin | ||||||
| Negative | 7 | 5 | 13 | 5 | 11.467 | .009 |
| Positive | 8 | 10 | 2 | 10 | ||
Abbreviations: DM, diabetes mellitus; HCQ, hydroxychloroquine; HTN, hypertension; IVIG, intravenous immune globulin; LOC, level of consciousness; NAC, N‐acetylcysteine; PCR, polymerase chain reaction.
Statistics of χ 2 test.
All p‐values in this table are originated from crosstab (χ 2) frequency comparison.
In the realm of binary variables, the atazanavir/ritonavir + HCQ + NAC group demonstrated the highest incidence of fever (n = 12), cough (n = 13), and dyspnea (n = 13). Notably, fatigue was most frequently reported in both the atazanavir/ritonavir + HCQ + NAC group (n = 13) and the atazanavir/ritonavir + HCQ group (n = 13). Furthermore, the Kaletra + HCQ group accounted for 12 instances of body aches, while the Kaletra + HCQ group reported four cases of diarrhea. In the context of sore throat, the atazanavir/ritonavir + HCQ + NAC group documented seven occurrences, whereas chest discomfort was observed seven times in the Kaletra + HCQ group. Instances of headache were prevalent in the atazanavir/ritonavir + HCQ group (seven cases) and dizziness was notable in the Kaletra + HCQ + NAC group (14 cases). Interestingly, only one case of seizure was recorded in the atazanavir/ritonavir + HCQ + NAC group. Olfactory dysfunction was reported among six patients in the Kaletra + HCQ + NAC group (with the fewest cases noted in the Kaletra + HCQ + NAC and atazanavir/ritonavir + HCQ + NAC groups), while five instances of taste disorders were identified in the Kaletra + HCQ + NAC group.
The utilization of dexamethasone, acetaminophen, azithromycin, ceftriaxone, interferon‐β, and levofloxacin differed across the four groups. For instance, within the atazanavir/ritonavir + HCQ and atazanavir/ritonavir + HCQ + NAC groups, six patients each were administered interferon‐β (p = .002), which contributed to the absence of mortality in the atazanavir/ritonavir + HCQ + NAC group. Although two patients in the Kaletra + HCQ group, one patient in the atazanavir/ritonavir + HCQ group, and one patient in the Kaletra + HCQ + NAC group did not survive, this distinction was not statistically significant (χ 2 = 2.134, p = .896). Notably, all patients within the atazanavir/ritonavir + HCQ + NAC group were discharged in favorable health conditions (refer to Table 4).
Table 4.
Comparison of the final condition between the four study groups receiving four types of medicine.
| Group | Final condition | χ 2 a | p‐Valueb | |
|---|---|---|---|---|
| Died n (%) | Discharged n (%) | |||
| Kaletra + HCQ | 2 (50%) | 13 (23.2%) | 2.134 | .896 |
| Kaletra + HCQ + NAC | 1 (25%) | 14 (25%) | ||
| Atazanavir/ritonavir + HCQ | 1 (25%) | 14 (25%) | ||
| Atazanavir/ritonavir + HCQ + NAC | – | 15 (26.8%) | ||
| Total | 4 (100%) | 56 (100%) | ||
Abbreviations: HCQ, hydroxychloroquine; NAC, N‐acetylcysteine.
Statistics of χ 2 test.
All p‐values in this table are originated from crosstab (χ 2) frequency comparison.
Applying a linear regression model to forecast ICU stay duration, variables including the usage of IVIG, elevation of creatine phosphokinase (CPK), and decrease in ESR were associated with prolonged ICU stays (see Table 5). No significant discrepancies in terms of mortality were observed among the various medications employed (see Table 6 and Figure 1).
Table 5.
Linear regression to predict ICU days as a dependent variable.
| Variable | Unstandardized coefficients | Standardized coefficients | p‐Valuea | |
|---|---|---|---|---|
| β | Standard error | β b | ||
| Gender | 2.245 | 1.762 | .327 | .212 |
| Age | .036 | 0.045 | .454 | .438 |
| O2_sat_before | −.045 | 0.070 | −.805 | .528 |
| NAC | −.440 | 1.658 | −.065 | .792 |
| Atazanavir/ritonavir | 21.483 | 17.421 | 3.186 | .227 |
| Lopinavir/ritonavir | 24.658 | 18.230 | 3.657 | .186 |
| IVIG | 5.910 | 2.864 | .554 | .048 |
| PCR | −.969 | 1.502 | −.115 | .524 |
| WBC | −.025 | 0.069 | −.071 | .719 |
| diff_segment | −.012 | 0.051 | −.194 | .812 |
| diff_lymphocyte | −.223 | 0.171 | −.973 | .204 |
| seg_lymph_ratio | −.842 | 0.447 | −1.214 | .070 |
| RBC | −3.689 | 2.004 | −3.529 | .076 |
| HGB | 1.144 | 0.750 | 3.155 | .138 |
| PLT | .007 | 0.009 | .303 | .472 |
| ESR | −.096 | 0.042 | −1.104 | .031 |
| CRP | −.012 | 0.057 | −.093 | .837 |
| PT | −.600 | 1.225 | −1.822 | .628 |
| INR | .361 | 9.449 | .089 | .970 |
| PTT | −.014 | 0.119 | −.107 | .906 |
| BUN | −.018 | 0.088 | −.092 | .838 |
| Cr | −1.516 | 1.733 | −.499 | .388 |
| AST | −.013 | 0.056 | −.160 | .821 |
| ALT | .011 | 0.056 | .122 | .847 |
| LDH | .003 | 0.005 | .465 | .542 |
| CPK | .006 | 0.003 | .713 | .034 |
| CPK_M | −.090 | 0.162 | −.479 | .582 |
| ALK_P | .013 | 0.008 | .688 | .108 |
| Bilirubin total | .595 | 2.600 | .158 | .821 |
| Bilirubin direct | 1.641 | 7.130 | .137 | .819 |
Abbreviations: ALK_P, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CPK_M, creatine phosphokinase; Cr, creatinine; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; HGB, hemoglobin; ICU, intensive care unit; INR, international normalization ratio; IVIG, intravenous immune globulin; LDH, lactate dehydrogenase; PCR, polymerase chain reaction; PLT, platelet; PT, prothrombin time; PTT, partial prothrombin time; RBC, red blood cell; _sat, saturation; WBC, white blood cell.
All p‐values in this table are originated from linear regression for prediction.
Standardized coefficients of linear regression.
Table 6.
Comparison of the study patients' final condition according to the drugs administered.
| Variable | Final condition | χ 2 a | p‐Valueb | |
|---|---|---|---|---|
| Died | Discharged | |||
| Dexamethasone | ||||
| Negative | 3 | 31 | 0.587 | .626 |
| Positive | 1 | 25 | ||
| Acetaminophen | ||||
| Negative | 2 | 22 | 0.179 | .999 |
| Positive | 2 | 34 | ||
| Azithromycin | ||||
| Negative | 4 | 38 | 1.837 | .306 |
| Positive | 0 | 18 | ||
| Ceftriaxone | ||||
| Negative | 2 | 40 | 0.816 | .576 |
| Positive | 2 | 16 | ||
| Heparin | ||||
| Negative | 0 | 5 | 0.390 | .999 |
| Positive | 4 | 51 | ||
| Diphenhidramine | ||||
| Negative | 2 | 37 | 0.424 | .606 |
| Positive | 2 | 19 | ||
| Interferon‐β | ||||
| Negative | 4 | 44 | 1.071 | .574 |
| Positive | 0 | 12 | ||
| Levofloxacin | ||||
| Negative | 1 | 29 | 1.071 | .612 |
| Positive | 3 | 27 | ||
Statistics of χ 2 test.
All p‐values in this table are originated from crosstab (χ 2) frequency comparison.
Figure 1.
The flow diagram shows the flow of patients through the trial.
Regarding the enhancement of oxygen saturation (O2 saturation) brought about by the four different treatment protocols, the findings indicated that both the atazanavir/ritonavir + HCQ + NAC group (p = .001) and the atazanavir/ritonavir + HCQ group (p = .008) demonstrated notable improvements. These groups exhibited a substantial increase in O2 saturation levels posttreatment, in comparison to the initial O2 saturation levels before intervention (as shown in Table 7). However, no statistically significant variations were noted in this aspect among the four groups (p > .05), as detailed in Table 8 and illustrated in Figure 2.
Table 7.
Comparison of O2 saturation levels between the four study groups receiving four types of medicine.
| Variable | Mean | SD | t a | p‐Valueb |
|---|---|---|---|---|
| Lopinavir/ritonavir + HCQ + NAC | ||||
| O2_sat_ before | 89.73 | 4.250 | 0.655 | .523 |
| O2_ sat_ after | 88.6667 | 7.73366 | ||
| Lopinavir/ritonavir + HCQ | ||||
| O2_sat_before | 89.80 | 6.689 | 1.817 | .091 |
| O2_ sat_ after | 79.7333 | 21.45582 | ||
| Atazanavir/ritonavir + HCQ | ||||
| O2_sat_before | 70.87 | 21.722 | −2.898 | .008 |
| O2_ sat_ after | 85.3478 | 9.62765 | ||
| Atazanavir/ritonavir + HCQ + NAC | ||||
| O2_sat_before | 74.56 | 13.395 | −4.138 | .001 |
| O2_ sat_ after | 85.9444 | 6.02419 |
Abbreviations: HCQ, hydroxychloroquine; NAC, N‐acetylcysteine; _sat, saturation.
Statistics of independent t‐test.
All p‐values in this table are originated from independent t‐test for two groups mean comparison.
Table 8.
Comparison of the opacification consolidation between the study groups according to the drugs administered.
| Variable | Kaletra + HCQ | Kaletra + HCQ + NAC | Atazanavir/ritonavir + HCQ | Atazanavir/ritonavir + HCQ + NAC | χ 2 a | p‐Valueb |
|---|---|---|---|---|---|---|
| Ground glass opacification consolidation | ||||||
| No | 9 | 9 | 4 | 6 | 4.821 | .225 |
| Yes | 6 | 6 | 11 | 9 | ||
| Bilateral opacification consolidation | ||||||
| No | 8 | 10 | 7 | 9 | 1.357 | .716 |
| Yes | 7 | 5 | 8 | 6 | ||
| Multifocal opacification zz | ||||||
| No | 11 | 10 | 8 | 10 | 1.392 | .778 |
| Yes | 4 | 5 | 7 | 5 | ||
Abbreviations: HCQ, hydroxychloroquine; NAC, N‐acetylcysteine.
Statistics of χ 2 test.
All p‐values in this table are originated from crosstab (χ 2) frequency comparison.
Figure 2.
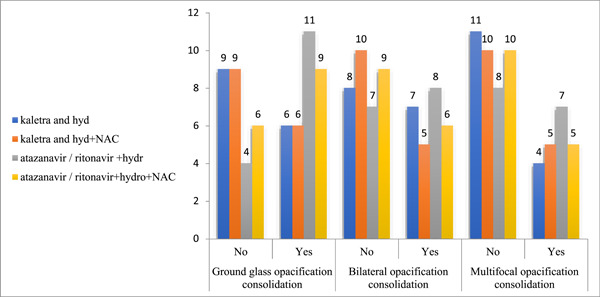
The image illustrates a comparison of opacification consolidation between the study groups according to the drugs administered.
4. DISCUSSION
There is accumulating evidence pointing toward the therapeutic potential of NAC in addressing COVID‐19 and its associated repercussions. For instance, NAC's impact on oxidative stress regulation, immune modulation, and apoptosis management, combined with its unique attributes such as enhancing oxygenation and circulation, can significantly contribute to improved respiratory outcomes and the prevention of end‐organ failure. Furthermore, well‐designed studies have highlighted NAC's roles as an antioxidant and immunomodulator in combatting viruses that target the respiratory system, such as influenza strains A and B, and the respiratory syncytial virus. This extends to addressing acute injuries like ARDS.
Notably, NAC's potential extends beyond its supportive role in ICU patients, those with sepsis, and individuals with nonpulmonary end‐organ complications. It may also offer positive contributions to patients with underlying health conditions. Moreover, NAC could serve as a promising supplementary therapeutic option for COVID‐19, taking into account patient conditions, indications, and contraindications. 5 , 32 , 33 , 34 Oral NAC could potentially serve as a preventive or treatment option for disease‐related outcomes in stable patients who are not experiencing sepsis and are not reliant on intubation. Intravenous (IV) administration of NAC has shown promise in moderate‐to‐severe cases of COVID‐19, particularly among individuals admitted to the ICU with complications like end‐organ failure. Numerous reports have highlighted the effectiveness of NAC in managing cytokine storms, alleviating dyspnea, and addressing ARDS associated with COVID‐19. 5 The utilization of NAC through different administration routes is contingent upon the specific context. At present, drawing from studies with robust evidence, it can be deduced that NAC's efficacy is particularly notable in stable patients when administered at the standard dose. Its most significant impact might lie in its preventive capacity—meaning its potential to improve the disease trajectory for noninfected individuals or those already infected. This multifaceted drug's primary role could thus be its ability to preemptively intervene. 35 To our current understanding, this study stands out as one of the most well‐designed RCTs conducted thus far to assess the effectiveness and safety of NAC in hospitalized patients afflicted with COVID‐19 infection. In brief, the results of this RCT revealed that the average duration of hospitalization (not confined to the ICU) was the shortest among individuals in the atazanavir/ritonavir + HCQ group (6.73 days) and the longest in those in the Kaletra + HCQ + NAC group (11.87 days). However, the discrepancies in hospitalization duration among the four treatment groups did not attain statistical significance (p = .082). Correspondingly, the mean duration of ICU stay was notably briefer for patients in the atazanavir/ritonavir + HCQ + NAC group (1.8 days) compared to the Kaletra + HCQ group (3.4 days), although these differences did not achieve statistical significance either (p = .172). Despite the absence of statistical significance, these outcomes suggest that patients administered with NAC exhibited more total hospitalization days and fewer ICU hospitalization days, implying a greater likelihood of maintaining a stable overall condition.
Upon hospitalization and before the initiation of the primary treatment, analysis of CT scans and severity scores indicated that ground glass opacification consolidation was most prevalent in the atazanavir/ritonavir + HCQ group (n = 11), and least common in the Kaletra + HCQ + NAC and Kaletra + HCQ groups (n = 6). Similarly, bilateral opacification consolidation was most frequent in the atazanavir/ritonavir + HCQ group (n = 8) and least frequent in the Kaletra + HCQ + NAC group (n = 5). The highest frequency of multifocal opacification consolidation was observed in the atazanavir/ritonavir + HCQ group (n = 7), and the lowest in the Kaletra + HCQ group (n = 4). Notably, these variations did not show any significant differences among the groups, indicating a relatively uniform distribution of lung involvement severity across the treatment groups within this RCT.
Upon hospitalization and preceding the initiation of the primary treatment, there were statistically significant differences among the four study groups in terms of seg_lymph_ratio, CRP, partial thromboplastin time, and LDH. However, at the conclusion of the study, only CRP exhibited sustained statistically significant disparities between the groups. Notably, the NAC group demonstrated a notable reduction in CRP levels by the study's end. Noteworthy was the observation that at discharge, the atazanavir/ritonavir + HCQ + NAC group displayed the lowest CRP value (5.99), while the Kaletra + HCQ group recorded the highest value (40.00). This disparity in CRP levels held statistical significance (p = .008). As for ESR at discharge, the atazanavir/ritonavir + HCQ + NAC group showed the least value (9.00), whereas the Kaletra + HCQ + NAC group exhibited the highest value (65.50). However, this discrepancy did not manifest as statistically significant, suggesting that CRP changes serve as a more sensitive measure of treatment response and inflammation reduction compared to ESR.
Outside of CRP, the statistically significant comparisons between laboratory findings upon hospitalization and at discharge are as follows:
For diff_segment at discharge, the Kaletra + HCQ + NAC group reported the lowest value (62.97%), while the atazanavir/ritonavir + HCQ + NAC group exhibited the highest value (79.96%).
In terms of ALP at discharge, the highest and lowest values were observed in the Kaletra + HCQ + NAC group and the atazanavir/ritonavir + HCQ + NAC group, respectively. This discrepancy held statistical significance (p = .001). This suggests that the atazanavir/ritonavir regimen exhibited a more pronounced effect on diff_segment and ALP, indicating a more substantial improvement trend compared to the Kaletra (lopinavir/ritonavir) regimen.
In the context of mortality, the absence of any deaths in the atazanavir/ritonavir + HCQ + NAC group hints at the potential for reduced mortality when these drugs are combined.
With regard to O2 saturation, the highest levels were noted in the atazanavir/ritonavir + HCQ group and the atazanavir/ritonavir + HCQ + NAC group at the study's conclusion. Furthermore, a significant increase in O2 saturation was observed post‐intervention in groups that received NAC (p < .05), marking a pivotal finding in this study.
The majority of COVID‐19 cases are characterized by a redox imbalance in alveolar epithelial cells, triggering apoptosis, heightened inflammation, and consequent impairment of gas exchange. Numerous studies have discussed the potential beneficial effects of NAC as a versatile drug in managing COVID‐19 and its associated complications. 5 Furthermore, several primary investigations encompassing case reports, case series, and clinical trials have directed their attention towards the utilization of NAC in the therapeutic approach and care of patients afflicted with COVID‐19, along with its accompanying complications. These complications encompass end‐organ failure, particularly instances of acute liver failure stemming from factors such as remdesivir‐induced liver dysfunction, heightened liver enzyme levels, and occurrences of intrahepatic hemorrhage. Additionally, NAC's potential has been explored in the management of ARDS and the mitigation of seizure occurrences. 36 , 37
A RCT was conducted in Brazil with the aim of assessing the effectiveness and safety of IV NAC in severe cases of COVID‐19, defined by oxyhemoglobin saturation falling below 94% or a respiratory rate surpassing 24 breaths/min. The results of this trial indicated that IV NAC did not yield a significant reduction in the requirement for mechanical ventilation when compared to the control group. Specifically, 20.6% of individuals in the NAC group necessitated mechanical ventilation, as opposed to 23.9% in the control group.
Furthermore, the trial outcomes revealed that various parameters, including the duration of mechanical ventilation, the frequency of ICU admission, the length of ICU stays, and the rate of mortality, did not demonstrate statistically significant differences between the group receiving NAC intervention and the control group. These results collectively suggest that the administration of high doses of NAC did not influence the progression of severe COVID‐19. 35 The current study exclusively enrolled stable COVID‐19 patients categorized as moderate‐to‐severe, who were not admitted to the ICU (N = 60). Among them, only 11 patients required ICU admission during the course of their illness, regardless of the treatment regimen they were on. It is worth noting that the patients' severity scores and the mode of NAC administration (IV vs. oral) differed between our present RCT and the one conducted in Brazil. Despite these differences, the interpretation of findings from both trials suggests that NAC might exhibit greater effectiveness as a preventive or supplementary therapy in stable, nonsevere cases of COVID‐19. It particularly seems to play a positive role in improving oxygen saturation levels and hastening the reduction of CRP levels and inflammation.
Beyond NAC's therapeutic applications, which include its use as an adjunct therapy, the potential prophylactic benefits of this versatile drug in the context of COVID‐19 infection are also of notable significance 38 , 39 , 40 and its related complications 41 have been discussed in many reviews 40 , 42 , 43 , 44 , 45 , 46 , 47 and original studies 48 , 49 all of which have focused mainly on the drug as an anti‐inflammatory and antiapoptotic agent.
In a meticulously designed study examining the use of NAC for treating COVID‐19 patients, high doses of the drug did not result in improved outcomes for cases classified as severe and requiring admission to the ICU. 35 , 50 An additional clinical trial showcased that the amalgamation of methylene blue, vitamin C, and NAC led to an elevated survival rate among patients with COVID‐19. 16 Findings from a series of cases suggested that both oral and IV administration of glutathione, along with glutathione precursors like N‐acetyl‐cysteine and α‐lipoic acid, could potentially offer a new therapeutic avenue for inhibiting nuclear factor kappa B (NF‐κB), managing cytokine storms, and addressing ARDS in individuals diagnosed with COVID‐19 pneumonia. 51 The outcomes of a case series propose that a fresh therapeutic approach might be viable through the oral and IV application of glutathione, in addition to its precursors like N‐acetyl‐cysteine and α‐lipoic acid. This approach shows promise in potentially curtailing NF‐κB activity, regulating cytokine storms, and addressing the onset of ARDS among individuals diagnosed with COVID‐19 pneumonia. 17 The findings of a case series suggest the potential for a novel therapeutic strategy involving the oral and IV administration of glutathione, along with its precursors like N‐acetyl‐cysteine and α‐lipoic acid. This approach holds promise in potentially attenuating NF‐κB activity, modulating cytokine storms, and mitigating the onset of ARDS in individuals diagnosed with COVID‐19 pneumonia. 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 They have also undertaken exhaustive and methodical analyses, both in the form of systematic reviews and original articles. Their initial review study delved into potential drugs that might exert a positive influence on the progression and outcomes of COVID‐19, including NAC. 5 Following this, their efforts have been directed toward conducting RCTs aimed at assessing the effectiveness and safety of versatile drugs like NAC. Building upon the conclusions of the present RCT, we postulate that the utilization of oral or IV NAC, as appropriate, could potentially enhance oxygen saturation, mitigate inflammation by reducing CRP levels, and contribute to a reduction in mortality rates.
5. CONCLUSIONS
In the context of the current absence of a definitive gold‐standard therapy for COVID‐19 and its associated complications, the potential of multipotential drugs endowed with anti‐inflammatory, antioxidant, and antiapoptotic attributes emerges as a promising avenue when appropriately administered. Our study stands as one of the most meticulously designed RCTs to date, with the purpose of assessing the safety and efficacy of NAC in the treatment of COVID‐19 patients.
The pivotal outcomes gleaned from this RCT highlight that, upon study conclusion, the NAC group exhibited a noteworthy additional reduction in CRP levels (p = .008). Notably, within the atazanavir/ritonavir + HCQ + NAC group, there were no reported instances of mortality, suggesting a potential for the combined administration of these medications to mitigate mortality risk. Furthermore, both the atazanavir/ritonavir + HCQ group and the atazanavir/ritonavir + HCQ + NAC group displayed the highest levels of oxygen saturation at the study's termination, alongside a substantial elevation in oxygen saturation subsequent to the initiation of the intervention, including NAC (p < .05).
Considering the insights derived from this RCT, we can affirm that oral NAC, when appropriately indicated, holds the potential to enhance oxygen saturation levels, temper the trajectory of inflammation (via CRP reduction), and contribute to a decrease in mortality risk among hospitalized COVID‐19 patients. Notably, NAC may exhibit heightened efficacy as a prophylactic or adjunctive therapy in cases of stable nonsevere COVID‐19, with a particularly positive role in augmenting oxygen saturation levels and expediting the reduction of CRP and associated inflammation.
6. LIMITATIONS
In this randomized clinical trial evaluating the efficacy and safety of oral NAC in COVID‐19 patients undergoing the routine antiviral and HCQ protocol, we recognize several limitations that warrant consideration when interpreting our findings.
-
1.
Low sample size: A prominent limitation of our study is the relatively modest sample size within each treatment group. The restricted number of participants compromises the statistical power of our analysis and may hinder the detection of subtle treatment effects. As such, caution must be exercised when generalizing our results to broader patient populations.
-
2.
Heterogeneous baseline characteristics: The differences in background characteristics among the treatment groups introduce variability that might confound our results. We acknowledge that variations in demographics, medical history, and comorbidities can influence treatment responses, thereby limiting the direct comparability of outcomes. Although we have reported these disparities transparently, the challenge of unequal baseline data remains a weakness in our study design.
-
3.
Limited serial laboratory parameter evaluations: The importance of serially evaluating laboratory parameters, including TNF and IL‐6, as integral components of response‐to‐treatment criteria, is acknowledged. Unfortunately, financial constraints hindered the incorporation of these tests, which would have provided comprehensive insights into the autoinflammatory response to treatments. While acknowledging this limitation, we have focused on assessing feasible parameters within the confines of our available resources.
Despite these limitations, our study offers valuable insights into the potential benefits and challenges of incorporating NAC into the routine antiviral and HCQ protocol for COVID‐19 patients. We emphasize our commitment to transparency by candidly discussing these limitations, enabling readers to interpret our results within the context of these constraints. While our findings contribute to the current understanding, we acknowledge the need for future investigations with larger and more homogenous samples to establish more robust conclusions regarding the role of NAC in COVID‐19 treatment protocols.
AUTHOR CONTRIBUTIONS
Azadeh Goodarzi and Najmolsadat Atefi conceived and planned the intervention. Taghi Riahi, Niloofar Khodabandehloo, Mahshid Talebi Taher and Niloufar Najar Nobari carried out the intervention. Zeinab Mahdi and Amir Baghestani gathering the datas. Amir Baghestani, Zeinab Mahdi, Rohollah Valizadeh, and Farnoosh Seirafianpour contributed to the interpretation of the results. Rohollah Valizadeh analyzed the data. Farnoosh Seirafianpour took the lead in writing the manuscript. All authors contributed to the preparation of data and the finalization of this article. All the figures have been produced by the authors of this article and are personal data.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The authors would like to thank staff of the Rasool Akram Medical Complex Clinical Research Development Center (RCRDC) specially Mrs. Farahnaz Nikkhah for their technical and editorial assistance.
Atefi N, Goodarzi A, Riahi T, et al. Evaluation of the efficacy and safety of oral N‐acetylcysteine in patients with COVID‐19 receiving the routine antiviral and hydroxychloroquine protocol: a randomized controlled clinical trial. Immun Inflamm Dis. 2023;11:e1083. 10.1002/iid3.1083
Trial registration: The trial was registered in Iranian Registry of Clinical Trials (IRCT) (#IRCT20200623047897N1; https://en.irct.ir/trial/49277, ethical code #IR.IUMS.REC.1399.206).
REFERENCES
- 1. Jorge‐Aarón R‐M, Rosa‐Ester M‐P. N‐acetylcysteine as a potential treatment for COVID‐19. Future Med. 2020;15:959‐969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan JFW, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395(10223):514‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jayaweera M, Perera H, Gunawardana B, Manatunge J. Transmission of COVID‐19 virus by droplets and aerosols: a critical review on the unresolved dichotomy. Environ Res. 2020;188:109819. 10.1016/j.envres.2020.109819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID‐19 infection? Respir Med. 2020;8(4):e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhat SA, Singh G, Bhat WF, Borole K, Khan AA. Coronavirus disease‐2019 and its current scenario—a review. Clinical eHealth. 2021;4:67‐73. 10.1016/j.ceh.2021.09.002 [DOI] [Google Scholar]
- 6. Seirafianpour F, Sodagar S, Pour Mohammad A, et al. Cutaneous manifestations and considerations in COVID‐19 pandemic: a systematic review. Dermatol Ther. 2020;33(6):e13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aslan A, Aslan C, Zolbanin NM, Jafari R. Acute respiratory distress syndrome in COVID‐19: possible mechanisms and therapeutic management. Pneumonia. 2021;13(1):14. 10.1186/s41479-021-00092-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krynytska I, Marushchak M, Birchenko I, Dovgalyuk A, Tokarskyy O. COVID‐19‐associated acute respiratory distress syndrome versus classical acute respiratory distress syndrome (a narrative review). Iran J Microbiol. 2021;13(6):737‐747. 10.18502/ijm.v13i6.8072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pfortmueller CA, Spinetti T, Urman RD, Luedi MM, Schefold JC. COVID‐19‐associated acute respiratory distress syndrome (CARDS): current knowledge on pathophysiology and ICU treatment—a narrative review. Best Pract Res Clin Anaesthesiol. 2021;35(3):351‐368. 10.1016/j.bpa.2020.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Long B, Carius BM, Chavez S, et al. Clinical update on COVID‐19 for the emergency clinician: presentation and evaluation. Am J Emerg Med. 2022;54:46‐57. 10.1016/j.ajem.2022.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu B, Huang S, Yin L. The cytokine storm and COVID‐19. J Med Virol. 2021;93(1):250‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baud D, Qi X, Nielsen‐Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID‐19 infection. Lancet Infect Dis. 2020;20(7):773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Daneshfar M, Dadashzadeh N, Ahmadpour M, et al. Lessons of mortality following COVID‐19 epidemic in the United States especially in the geriatrics. J Nephropharmacol. 2020;10(1):e06. [Google Scholar]
- 14. Tavakolpour S, Aryanian Z, Seirafianpour F, et al. A systematic review on efficacy, safety, and treatment‐durability of low‐dose rituximab for the treatment of pemphigus: special focus on COVID‐19 pandemic concerns. Immunopharmacol Immunotoxicol. 2021;43:507‐518. [DOI] [PubMed] [Google Scholar]
- 15. Dass E. Brief review of N‐acetylcysteine as antiviral agent: potential application in COVID‐19. J Biomed Pharm Res. 2020;9(3):69‐73. [Google Scholar]
- 16. Alamdari DH, Moghaddam AB, Amini S, et al. Application of methylene blue‐vitamin C–N‐acetyl cysteine for treatment of critically ill COVID‐19 patients, report of a phase‐I clinical trial. Eur J Pharmacol. 2020;885:173494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andreou A, Trantza S, Filippou D, Sipsas N, Tsiodras S. COVID‐19: the potential role of copper and N‐acetylcysteine (NAC) in a combination of candidate antiviral treatments against SARS‐CoV‐2. In Vivo. 2020;34(suppl 3):1567‐1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hamad MNM. MNa theory: triple therapy to COVID‐19: minocycline, N‐acetylcysteine and aspirin. Saudi J Biomed Res. 2020;5(4):53‐55. [Google Scholar]
- 19. Jaiswal N, Bhatnagar M, Shah H. N‐acetycysteine: a potential therapeutic agent in COVID‐19 infection. Med Hypotheses. 2020;144:110133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ahmed N, Chakrabarty A, Guengerich FP, Chowdhury G. Protective role of glutathione against peroxynitrite‐mediated DNA damage during acute inflammation. Chem Res Toxicol. 2020;33(10):2668‐2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Assimakopoulos SF, Marangos M. N‐acetyl‐cysteine may prevent COVID‐19‐associated cytokine storm and acute respiratory distress syndrome. Med Hypotheses. 2020;140:109778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hasan MJ. N‐acetylcysteine in severe COVID‐19: the possible mechanism. Int J Infect. 2020;7(4). [Google Scholar]
- 23. Hatami N, Kalani N, Javdani F, et al. N‐acetyl cysteine (NAC) and COVID‐19 treatment: new hopes in old medication. Int J Multidiscip Res Anal. 2020;3(9):122. [Google Scholar]
- 24. Shi Z, Puyo CA. N‐acetylcysteine to combat COVID‐19: an evidence review. Ther Clin Risk Manag. 2020;16:1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van Hecke O, Lee J. N‐acetylcysteine: a rapid review of the evidence for effectiveness in treating COVID‐19. 2020.
- 26. Poe FL, Corn J. N‐acetylcysteine: a potential therapeutic agent for SARS‐CoV‐2. Med Hypotheses. 2020;143:109862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ocampo Rojas SJ. COVID‐19 neurologic manifestation. Rev Fac Cien Med Univ Nac Cordoba. 2020;77(2):130. [DOI] [PubMed] [Google Scholar]
- 28. Carothers C, Birrer K, Vo M. Acetylcysteine for the treatment of suspected remdesivir‐associated acute liver failure in COVID‐19: a case series. Pharmacotherapy. 2020;40(11):1166‐1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sarkar S, Rapista N, Jean L‐G. Corona virus disease‐19‐induced acute liver failure leading to severe metabolic acidosis. Chest. 2020;158(4):A1002. [Google Scholar]
- 30. El‐Serafi I, Remberger M, El‐Serafi A, et al. The effect of N‐acetyl‐l‐cysteine (NAC) on liver toxicity and clinical outcome after hematopoietic stem cell transplantation. Sci Rep. 2018;8(1):8293. 10.1038/s41598-018-26033-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hołyńska‐Iwan I, Wróblewski M, Olszewska‐Słonina D, Tyrakowski T. [The application of N‐acetylcysteine in optimization of specific pharmacological therapies]. Pol Merkur Lekarski. 2017;43(255):140‐144. [PubMed] [Google Scholar]
- 32. Cadegiani FA. Repurposing existing drugs for COVID‐19: an endocrinology perspective. BMC Endocr Disord. 2020;20(1):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. De Flora S, Balansky R, La Maestra S. Rationale for the use of N‐acetylcysteine in both prevention and adjuvant therapy of COVID‐19. FASEB J. 2020;34(10):13185‐13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fregatti P, Gipponi M, Giacchino M, et al. Breast cancer surgery in the COVID‐19 pandemic: validation of a preventive program for patients and health care workers. In Vivo. 2021;35(1):635‐639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Alencar JCG, Moreira CL, Müller AD, et al. Double‐blind, randomized, placebo‐controlled trial with N‐acetylcysteine for treatment of severe acute respiratory syndrome caused by coronavirus disease 2019 (COVID‐19). Clin Infect Dis. 2021;72(11):e736‐e741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Daid SS, Toribio AD, Lakshmanan S, Sadda A, Epstein A. Spontaneous intraparenchymal hepatic hemorrhage as a sequela of COVID‐19. Cureus. 2020;12(9):e10447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Elgamasy S, Kamel MG, Ghozy S, Khalil A, Morra ME, Islam SMS. First case of focal epilepsy associated with SARS‐coronavirus‐2. J Med Virol. 2020;92(10):2238‐2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goodnough R, Canseco K. Truncated IV acetylcysteine treatment duration has potential to safely preserve resources during the COVID‐19 pandemic. Clin Toxicol. 2021;59(1):69. [DOI] [PubMed] [Google Scholar]
- 39. Goodnough R, Canseco K. Response to comment on truncated IV acetylcysteine treatment duration has potential to safely preserve resources during the COVID‐19 pandemic. Clin Toxicol. 2021;59(1):78‐79. [DOI] [PubMed] [Google Scholar]
- 40. Horowitz RI, Freeman PR. Three novel prevention, diagnostic, and treatment options for COVID‐19 urgently necessitating controlled randomized trials. Med Hypotheses. 2020;143:109851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ibrahim H, Perl A, Smith D, et al. Therapeutic blockade of inflammation in severe COVID‐19 infection with intravenous N‐acetylcysteine. Clin Immunol. 2020;219:108544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Laforge M, Elbim C, Frère C, et al. Tissue damage from neutrophil‐induced oxidative stress in COVID‐19. Nat Rev Immunol. 2020;20(9):515‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Luo P, Luo P, Liu Y, Liu D, Li J. Perspectives for the use of N‐acetylcysteine as a candidate drug to treat COVID‐19. Mini Rev Med Chem. 2021;21(3):268‐272. [DOI] [PubMed] [Google Scholar]
- 44. Meletis CD, Wilkes K. Immune competence and minimizing susceptibility to COVID‐19 and other immune system threats. Altern Ther Health Med. 2020;26(S2):94‐99. [PubMed] [Google Scholar]
- 45. Nasi A, McArdle S, Gaudernack G, et al. Reactive oxygen species as an initiator of toxic innate immune responses in retort to SARS‐CoV‐2 in an ageing population, consider N‐acetylcysteine as early therapeutic intervention. Toxicol Rep. 2020;7:768‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wong A. Comment on truncated IV acetylcysteine treatment duration has potential to safely preserve resources during the COVID‐19 pandemic. Clin Toxicol. 2021;59(1):77‐78. [DOI] [PubMed] [Google Scholar]
- 47. Zhou N, Yang X, Huang A, Chen Z. The potential mechanism of N‐acetylcysteine in treating COVID‐19. Curr Pharm Biotechnol. 2021;22:1584‐1590. [DOI] [PubMed] [Google Scholar]
- 48. Mohanty RR, Padhy BM, Das S, Meher BR. Therapeutic potential of N‐acetyl cysteine (NAC) in preventing cytokine storm in COVID‐19: review of current evidence. Eur Rev Med Pharmacol Sci. 2021;25(6):2802‐2807. [DOI] [PubMed] [Google Scholar]
- 49. Bourgonje AR, Offringa AK, van Eijk LE, et al. N‐acetylcysteine and hydrogen sulfide in coronavirus disease 2019. Antioxid Redox Signal. 2021;35(14):1207‐1225. [DOI] [PubMed] [Google Scholar]
- 50. Mashayekhi F, Seirafianpour F, Pour Mohammad A, Goodarzi A. Severe and life‐threatening COVID‐19‐related mucocutaneous eruptions: a systematic review. Int J Clin Pract. 2021;75(12):e14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Horowitz RI, Freeman PR, Bruzzese J. Efficacy of glutathione therapy in relieving dyspnea associated with COVID‐19 pneumonia: a report of 2 cases. Respir Med Case Rep. 2020;30:101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mohamadi M, Goodarzi A, Aryannejad A, et al. Geriatric challenges in the new coronavirus disease‐19 (COVID‐19) pandemic: a systematic review. Med J Islam Repub Iran. 2020;34:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Seirafianpour F, Mozafarpoor S, Fattahi N, Sadeghzadeh‐Bazargan A, Hanifiha M, Goodarzi A. Treatment of COVID‐19 with pentoxifylline: could it be a potential adjuvant therapy? Dermatol Ther. 2020;33(4):e13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shokraee K, Mahdavi H, Panahi P, et al. Accuracy of chest computed tomography and reverse transcription polymerase chain reaction in diagnosis of 2019 novel coronavirus disease; a systematic review and meta‐analysis. Immunopathol Persa. 2021;7(2):e36. [Google Scholar]
- 55. Sadeghzadeh‐Bazargan A, Behrangi E, Goodarzi A. Systemic retinoids in the COVID‐19 era–are they helpful, safe, or harmful? A comprehensive systematized review. Iran J Dermatol. 2020;23(suppl 1):9‐12. [Google Scholar]
- 56. Sadeghzadeh‐Bazargan A, Behrangi E, Goodarzi A. Cytokine storm and probable role of immunoregulatory drugs in COVID‐19: a comprehensive review. Iranian J Dermatol. 2020;23(Suppl.1(COVID‐19)):13‐18. [Google Scholar]
- 57. Goodarzi A. A comprehensive review on COVID‐19 infection and comorbidities of various organs. Acta Med Iranica. 2021;4‐14. [Google Scholar]
- 58. Kalantari S, Sadeghzadeh‐Bazargan A, Ebrahimi S, et al. The effect of influenza vaccine on severity of COVID‐19 infection: an original study from Iran. Med J Islam Repub Iran. 2021;35(1):865‐869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kooranifar S, Sadeghipour A, Riahi T, Goodarzi A, Tabrizi S, Davoody N. Histopathologic survey on lung necropsy specimens of 15 patients who died from COVID‐19: a large study from Iran with a high rate of anthracosis. Med J Islam Repub Iran. 2021;35(1):481‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yüce M, Filiztekin E, Özkaya KG. COVID‐19 diagnosis—a review of current methods. Biosens Bioelectron. 2021;172:112752. 10.1016/j.bios.2020.112752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nobari NN, Montazer F, Seirafianpour F, Goodarzi A. Histopathologic changes and cellular events of organs systems in COVID‐19. J Cell Mol Anesth. 2021;6(1):81‐88. [Google Scholar]
- 62. Tadayon Najafabadi B, Rayner DG, Shokraee K, et al. Obesity as an independent risk factor for COVID‐19 severity and mortality. Cochrane Database Syst Rev. 2023;5(5):CD015201. 10.1002/14651858.CD015201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Riahi T, Sadeghzadeh‐Bazargan A, Shokri S, et al. The effect of opium on severity of COVID‐19 infection: an original study from Iran. Med J Islam Repub Iran. 2021;35(1):870‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]


