Abstract
The cell wall of a yeast cell forms a barrier for various proteinaceous and nonproteinaceous molecules. Nisin, a small polypeptide and a well-known preservative active against gram-positive bacteria, was tested with wild-type Saccharomyces cerevisiae. This peptide had no effect on intact cells. However, removal of the cell wall facilitated access of nisin to the membrane and led to cell rupture. The roles of individual components of the cell wall in protection against nisin were studied by using synchronized cultures. Variation in nisin sensitivity was observed during the cell cycle. In the S phase, which is the phase in the cell cycle in which the permeability of the yeast wall to fluorescein isothiocyanate dextrans is highest, the cells were most sensitive to nisin. In contrast, the cells were most resistant to nisin after a peak in expression of the mRNA of cell wall protein 2 (Cwp2p), which coincided with the G2 phase of the cell cycle. A mutant lacking Cwp2p has been shown to be more sensitive to cell wall-interfering compounds and Zymolyase (J. M. Van der Vaart, L. H. Caro, J. W. Chapman, F. M. Klis, and C. T. Verrips, J. Bacteriol. 177:3104–3110, 1995). Here we show that of the single cell wall protein knockouts, a Cwp2p-deficient mutant is most sensitive to nisin. A mutant with a double knockout of Cwp1p and Cwp2p is hypersensitive to the peptide. Finally, in yeast mutants with impaired cell wall structure, expression of both CWP1 and CWP2 was modified. We concluded that Cwp2p plays a prominent role in protection of cells against antimicrobial peptides, such as nisin, and that Cwp1p and Cwp2p play a key role in the formation of a normal cell wall.
Nisin is an antimicrobial peptide produced by lactococci and has been used in consumer products for many years (30). Although this lantibiotic is inhibitory to microorganisms, it is harmless to humans (15, 16). Nisin is the first antimicrobial peptide with “generally recognized as safe” status in the United States for use in processed cheese; in addition, its use in various food products is allowed in several countries (9). Nisin is also of interest to the pharmaceutical industry (10).
Nisin is known to inhibit the growth of a number of gram-positive bacteria and also the outgrowth of spores of bacilli and clostridia (15, 16). Furthermore, the gram-negative bacterium Escherichia coli becomes sensitive to nisin when its outer membrane is made permeable by osmotic shock (20). Inhibition of the growth of other gram-negative bacteria can be achieved by simultaneous treatment with nisin and an agent which modifies and chelates the outer membrane, such as EDTA (29). These findings are consistent with the notion that nisin acts on the cytoplasmic membrane. Indeed, the main antimicrobial activity of nisin seems to rely on the ability of the compound to form pores in the cytoplasmic membrane, which leads to a loss of small intracellular molecules and ions and a collapse of the proton motive force (1, 6, 14, 20, 24, 25, 34). To exert its antimicrobial activity, nisin does not seem to require a specific receptor but instead requires a sufficient trans-negative electrical membrane potential (24, 25). Driessen et al. concluded that nisin acts as an anion carrier in the absence of anionic phospholipids (12). It has been suggested that pore formation by nisin in vivo involves local perturbation of the bilayer structure and trans-membrane-potential-dependent reorientation from a surface-bound configuration to a membrane-inserted configuration. In this membrane-inserted form the hydrophilic side of nisin and the attached lipid head groups face the center of a water-filled pore. The hydrophobic surface of nisin and the fatty acid chains of the lipids point to the lipid bilayer (31).
Nisin has no antimicrobial effect on yeasts and filamentous fungi. These organisms each have a rigid cell wall, a complex structure consisting of glucan cross-linked with chitin and cell wall proteins (4, 18). The processing of mannoproteins is complex and has been partially characterized in yeasts (19, 21). A similar mechanism has been suggested for filamentous fungi (4). Because mannoproteins are generally considered one of the key wall components which determine cell wall porosity (8, 18), they may represent a major barrier preventing free permeation of nisin through the cell wall and thus access to the cytosolic membrane.
Initial experiments indicated that a yeast cell is prone to the antimicrobial activity of nisin in certain stages of the cell cycle, suggesting that cell cycle-regulated components of the cell envelope are involved. Recently, Caro et al. showed that specific cell wall mannoproteins are expressed in different stages of the cell cycle (7). In this study we assessed the importance of individual mannoproteins, β-1,3-glucan, and chitin in conferring resistance to nisin upon yeast cells. In addition, below we present data describing the role of cell wall protein 1 (Cwp1p) and Cwp2p in the structuring of a normal yeast wall.
MATERIALS AND METHODS
Strains, probes, and media.
The E. coli strain used in this study was JM109 {endA1 recA1 gyrA96 thi hsdR17 (rK− mK+) relA1 supE44 (lac-proAB) [F′ traD36 proAB lacqZM15]} (35), which was grown in Luria broth (26) supplemented with 100 μg of ampicillin/ml when appropriate. The Saccharomyces cerevisiae strains used were SU50 (YT6-2-1 L) (MATa cir0 leu2-3,112 his4-519 can1) and SU51 (MATa cir+ leu2-3,112 his4-519 can1) (13). Deletion mutants cwp1Δ cwp2Δ and cwp1cwp2Δ derived from SU50 were kindly provided by J. M. van der Vaart (32). Deletion mutant cwh53Δ and its parent, AR27 (MATa ura3-52), were a kind gift from A. F. J. Ram (23). Deletion mutant pmt1Δ and its parent, SEY6210 (MATa leu2-3,112 ura3-52 his-Δ200 lys2-801 trp1-Δ901 suc-Δ9), were a gift from J. P. Bourdineaud (3). Yeast strains were maintained on YPD agar (1% yeast extract, 2% Bacto Peptone, 2% glucose, 1.5% Bacto Agar) or synthetic minimal medium agar containing 0.7% yeast nitrogen base, 2% glucose, 1.5% Bacto Agar, and amino acids as necessary (27). The yeast cells used for all assays were precultured on YPD agar.
Plasmids pUR2984-CWP1, pUR2984-CWP2, pUR2984-SED1, and pUR2984-TIP1 were kind gifts from J. M. van der Vaart (32). Plasmids pSB4 containing the FKS1 gene and plasmid pCHS3 containing the CHS3 gene were kind gifts from A. F. J. Ram (23) and J. H. Vossen (22). Plasmid pH2A was kindly provided by H. Sillje (33).
Probe preparation.
Plasmids were digested with NheI and HindIII. The DNA fragments containing part of the CWP1 gene (642 bp), part of the CWP2 gene (204 bp), part of the SED1 gene (690 bp), or part of the TIP1(435 bp) gene were isolated from an agarose gel. pH2A was cut with SacI, and pCHS3 was cut with BamHI, which resulted in probes H2A (2.3 kb) and CHS3 (2.8 kb). The probe fragment of the FKS1 gene (2.1 kb) was isolated from pSB4 as a ClaI-XhoI fragment. All fragments were purified by QIAEX 150 gel extraction. The specific DNA probes were randomly labelled by using [α32-P]dCTP (Amersham) as a substrate (26).
Reagents.
Nisin was obtained from Aplin & Barret (Dorset, United Kingdom). Yeast nitrogen base, Bacto Peptone, Bacto Yeast Extract, and Bacto Agar were obtained from Difco Laboratories (Detroit, Mich.). DNA restriction enzymes were purchased from New England Biolabs Inc. (Beverly, Mass.) and Boehringer Mannheim Biochemicals (Mannheim, Germany). α-Factor was obtained from Bachem Feinchemikalien AG. 4′,6-Diamidino-2-phenylindole (DAPI) was purchased from Sigma Chemical Co. (St. Louis, Mo.). Propidium iodide (PI), Calcofluor White, and FUN1 were obtained from Molecular Probes Inc., European BV (Leiden, The Netherlands). The [α32-P]dCTP used for DNA probe labelling and the Hybond-N membrane used for Northern blotting were obtained from Amersham (Arlington Heights, Ill.). Zymolyase 100T was obtained from Seikagaku Kogyo Co. (Tokyo, Japan).
Analytical procedures.
The confocal scanning laser microscope (CSLM) used consisted of a Zeiss Axioplan inverted microscope, a Bio-Rad model MRC-1024 system, and Lasersharp software. The objective used was a 1.5× zoom objective (magnification, ×63) with an image width of 110 μm. The software used to determine the percentage of PI-positive cells was the Leica Q500 MC software, as adapted by Aat Don (Department of Analytical & Information Sciences, Unilever, Vlaardingen, The Netherlands).
Yeast spheroplast generation.
Yeast cells were harvested by centrifugation and washed twice in 10 mM Tris-HCl (pH 7.4) and once in spheroplasting buffer (50 mM Na2CO3 [pH 7.4], 1 M sorbitol). The washed cells were resuspended in 10 ml of spheroplasting buffer, and 20 μl of β-mercaptoethanol was added. After 10 min of incubation at room temperature, 200 μl of Zymolyase 100T (5 mg/ml) was added, and the preparation was incubated at 37°C for an additional 40 min. The quality of spheroplast formation was assessed by diluting the preparation in deminerilized water (dH2O) and determining the percentage of spheroplasts that lysed.
Yeast culture synchronization.
Yeast cells were grown in YPD medium for 14 h at 25°C to an optical density at 620 nm (OD620) of 0.3. Subsequently, the culture was incubated with α-factor at a final concentration of 4 mg/ml at 25°C for 2.5 h. After induction, the cells were washed twice in YPD medium and grown under the same conditions for further analysis. The time that cells were transferred to fresh medium was considered zero time. Samples were taken every 30 min and used to determine the level of synchronization, the nisin sensitivity (determined by calculating the percentage of cells that were stained with PI), and the mRNA levels of several proteins (normalized to the level of ACT1).
The cell cycle stage was assessed by using fluorescence microscopy. Yeast cells were fixed in 0.13% formaldehyde, centrifuged, and resuspended in 96% (vol/vol) ethanol. Cell nuclei were visualized by staining cells in the dark with 1 μg of DAPI per ml for 15 min (17). Subsequently, after two washes with dH2O, the four cell-cycle stages were identified by using fluorescence microscopy.
Nisin sensitivity.
Yeast cells harvested from a synchronous culture were resuspended to an OD620 of 1.0. Then 100 μl of the suspension was incubated with 10 μg of nisin per ml at pH 4 at room temperature for 2 min. In the experiments in which the nisin sensitivities of cells and protoplasts were compared, a nisin concentration of 80 μg/ml was used. In the experiments performed with cell wall mutants, a concentration range of 0 to 50 μg/ml was assessed. After the peptide was removed, the pellet was suspended in 90 μl of dH2O and incubated with 5 μl of 100 μM FUN1 at 30°C for 20 min and then with 5 μl of a 1-mg/ml PI solution for 10 min under the same conditions (5). After staining, the cells were washed twice with dH2O to remove the excess dye and were analyzed with the CSLM system.
RNA isolation and Northern hybridization.
RNA was isolated by the hot phenol extraction method without the use of glass beads, as described elsewhere (1a). Basically, cells were lysed in TES solution (10 mM Tris-HCl [pH 7.5], 10 mM EDTA, 0.5% sodium dodecyl sulfate) and vigorously vortexed after incubation in water-saturated phenol at 65°C for 1 h. The aqueous phase was extracted once with phenol and once with chloroform. The RNA was precipitated with ethanol.
For Northern blotting (7, 17), 10 μg of RNA was loaded onto a 1% RNA agarose gel system containing formaldehyde and formamide. After blotting, the RNA was cross-linked to Hybond-N+ membranes by UV radiation. Northern hybridizations were performed in the presence of 50% formamide at 42°C by using α32-P-labelled gene fragments. The blots were washed at 42°C with decreasing concentrations of SSC (1× SSC is 0.15 M NaCl plus 15 mM sodium citrate, pH 7.0) down to 0.5× SSC in the presence of 0.1% sodium dodecyl sulfate. Hybridization signals were quantified by scanning autoradiograms in the linear range of the films. Levels of expression were normalized to actin levels.
RESULTS
Yeast wall forms a barrier for small antifungal peptides.
In order to determine whether the yeast cell wall forms a barrier to nisin, we first incubated cells with EDTA and dithiothreitol as described by de Nobel et al. (11) in order to increase the wall permeability. We observed that treatment of log-phase yeast cells with EDTA and/or dithiothreitol made them significantly more sensitive (by a factor of 2 to 4) to treatment with small antimicrobial peptides, such as nisin, as measured by the increase in the percentage of PI-positive cells. To study the inferred barrier function of the yeast wall for nisin in more detail, we removed the cell wall by incubation with a wall-lytic enzyme preparation and incubated the spheroplasts with nisin. Spheroplasts did not stain with Calcofluor White but were stained when they were incubated with the viability dye FUN1. Cells with damaged membranes were stained red due to uptake of PI. Yeast spheroplasts rapidly lysed when they were incubated in the presence of nisin at concentrations which hardly affected intact cells (10 to 80 μg/ml). Apparently, the cell wall normally forms a barrier for nisin. As the composition of the cell wall varies during the cell cycle, we set out to analyze the nisin sensitivity of yeast cells during the cycle.
Nisin sensitivity during the yeast cell cycle.
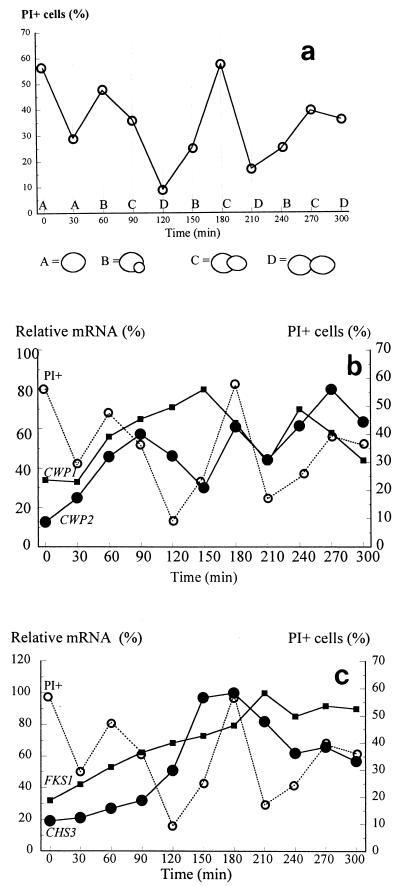
After incubation of a yeast culture with α-factor, the synchronous growth of this culture was checked (Fig. 1). Three synchronous consecutive cell cycles were observed. Confirmation of the cell cycle progression was obtained by measuring the fluctuation in H2A mRNA levels. The level of the mRNA of H2A, a prominent cell cycle marker gene which is actively transcribed in the S phase, peaked at 60, 150, and 240 min after the transfer to fresh medium without α-factor. This is consistent with the observation that cells had small buds at these time points.
FIG. 1.
S. cerevisiae synchronized by α-factor. Line A, nonbudding cells; line B, cells with small buds; line C, budding cells with migrating nuclei; line D, large budded binuclear cells.
The nisin sensitivity of the synchronous culture is shown in Fig. 2a. In the first cycle high percentages of PI-positive cells were recorded, and the culture was dominated by cells with small buds. In the second and third generations, however, cells with migrated nuclei seemed to be most sensitive to nisin. Perhaps the cells in the first cycle still suffered from direct effects of α-factor on the structure of their cell walls.
FIG. 2.
Correlation of cyclic nisin sensitivity with expression of genes coding for cell wall proteins and proteins involved in yeast cell wall biosynthesis. The levels of expression were determined relative to the level of expression of the ACT1 gene, which did not vary during the cell cycle. (a) Effect of nisin as assessed by determining the percentage of PI-positive cells. The different stages in the cell cycle are indicated at the bottom. (b) Expression of the mRNA of the CWP1 (■) and CWP2 (•) genes and nisin sensitivity (○) through three generations of synchronous growth. (c) Expression of the mRNA of the FKS1 (■) and CHS3 (•) genes and nisin sensitivity (○) through three generations of synchronous growth.
Figure 2b shows the transcription of CWP1 and CWP2 in relation to culture sensitivity to nisin. Maximum transcription of CWP1 was observed during the second and third generations, at the stage in the cell cycle when cells with small buds dominated the culture (150 and 240 min). Essentially similar results were obtained for SED1 (data not shown). The level of the mRNA of CWP2, on the other hand, started to peak in the first generation at the G2 phase of the cycle (90 min). A similar trend was observed in the second and third cycles (180 and 270 min). Finally, the level of TIP1 expression started high, dropped, and subsequently peaked early in the G1 phase at cell cycle stages just before cell division (data not shown).
Figure 2c shows the pattern of expression of FKS1, the gene coding for the catalytic subunit of the β-1,3-glucan-synthesizing complex, which is expressed when cells are cultured in glucose-containing media. Clearly, treatment with α-factor down-regulated expression of this gene. After release of the G1 block, the mRNA levels gradually increased again. A similar situation occurs with chitin synthase 3 gene expression. Chs3p is responsible for chitin synthesis in the chitin ring and in lateral walls.
The maximal levels of CWP1 and SED1 mRNA in the second and third generations were followed by maximal cell sensitivity to nisin. In contrast, peaks in CWP2 transcription were followed by maximal cell resistance to nisin. No clear correlation of TIP1 expression with nisin sensitivity was observed, although a tendency towards a high level of expression being followed by a high level of resistance to nisin was noted. Nor was there a correlation between the levels of expression of FKS1 and CHS3 and the cyclic sensitivity to nisin. Thus, we concluded that glucan and chitin levels as such were not important in the protection of yeast cells from nisin, whereas Cwp2p seemed to be very important in conferring resistance to nisin upon yeast cells. These inferred roles were further substantiated by an analysis of nisin sensitivity in which various knockout yeast mutants were used. We analyzed a cwp1Δ mutant yeast strain, a cwp2Δ mutant yeast strain, a cwp1cwp2Δ mutant yeast strain, a pmt1Δ mutant yeast strain, and a cwh53(fks1)Δ mutant yeast strain.
Sensitivity of yeast cell wall mutants to nisin.
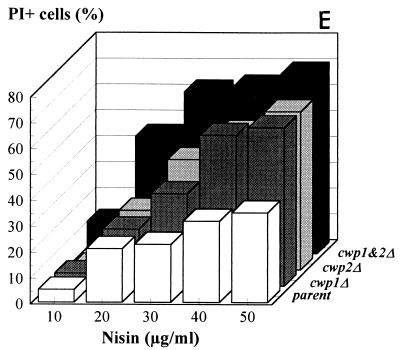
Figure 3 shows that logarithmically grown cwp1Δ cells were clearly more sensitive to the peptide than wild-type yeast cells were. cwp2Δ cells were even more sensitive to nisin. Finally, almost all of the cells of the double mutant were sensitive to nisin, as shown by their massive uptake of PI. Figure 3E shows the results in a bar diagram for nisin concentrations up to 50 μg/ml. A similar analysis was subsequently performed with a pmt1Δ strain. Bourdineaud et al. have recently shown that this strain contains exceptionally low amounts of glucanase-extractable mannoproteins in its cell wall and accordingly has increased wall permeability, as shown by its hypersensitivity to Zymolyase treatment (3). However, pmt1Δ cells were not hypersensitive to nisin (Fig. 4). As Cwp2p does occur in the walls of pmt1Δ cells, although at low levels, we concluded that Cwp2p plays a more crucial role than other glucanase-extractable mannoproteins in structuring the cell wall in such a way that the cell is protected against nisin. Fks1p-deficient cells, whose walls have a much lower β-1,3-glucan level, did not exhibit increased nisin sensitivity, confirming that glucan layers as such do not play a major role in the prevention of nisin permeation through the cell wall (data not shown).
FIG. 3.
Nisin sensitivity of wild-type and cell wall protein-deficient yeast strains at an OD620 of 1.0. (A through D) Images obtained with the CSLM of membrane integrity and cell viability after treatment with nisin (30 μg/ml). (A) Parent. (B) cwp1Δ. (C) cwp2Δ. (D) cwp1cwp2Δ. (E) Percentages of cells affected by nisin (0 to 50 μg/ml), as inferred from the images shown in panels A through D.
FIG. 4.
Nisin sensitivity of pmt1Δ and its parent, SEY6210. The values were deduced from images similar to those shown in Fig. 3A through D.
Regulation of CWP1 and CWP2 expression in cell wall mutants.
To investigate the proposed important function of CWP1 and CWP2 in the biogenesis of a normal yeast cell wall, we analyzed the levels of CWP1 and CWP2 transcripts in various yeast cell wall mutants and their parents. Figure 5 shows the results. Clearly, in a Cwp1p-deficient strain the levels of expression of CWP2 were also lower than the levels of expression in the wild-type strain. In contrast, the transcription of CWP1 in a Cwp2p-deficient strain increased. This suggests that in the analysis of the antifungal effect of nisin on cwp1Δ described above, the effect of nisin on the mutant due to the absence of Cwp1p could be overestimated since the level of Cwp2p was also decreased. On the other hand, it was evident in the analogous analysis of cwp2Δ that the effect of nisin on this mutant could not be ascribed to a decrease in CWP1 expression.
FIG. 5.
Levels of mRNA of the cell wall protein-encoding genes CWP1 and CWP2 in several mutant strains and their corresponding parent strains. All values were normalized to the levels of expression of the ACT1 gene. Subsequently, the level of expression of CWP2 in SU50 was defined as 100%. All other levels of expression were calculated relative to this value.
Interestingly, in the Pmt1p-deficient strain expression of CWP2 was induced significantly, whereas in the Fks1p-deficient strain expression of both CWP1 and CWP2 was induced moderately. We found that in the fks1Δ mutant, in addition to both cell wall protein genes, chitin synthase expression was also induced (data not shown). In this mutant the cell wall proteins are anchored mainly in the cell wall to the extra chitin (17c). Neither SED1 nor TIP1 was induced in these strains. Together, the data strongly suggest that Cwp2p and Cwp1p have an important structural function in the yeast cell wall (17a).
DISCUSSION
Our studies show that specific glucanase-extactable cell wall proteins with no known physiological function are crucial in conferring resistance to the antimicrobial peptide nisin upon yeast cells. As the yeast cell wall mannoproteins are heavily glycosylated and therefore determining their specific levels immunologically is very difficult, we chose to measure the level of transcription of the genes involved in the expression of cell wall proteins rather than work with the proteins themselves. Although transcription levels do not necessarily correlate with the presence of the components in the cell wall, we know from preliminary studies performed with green fluorescent protein fusions that Cwp1p and Cwp2p appear in the cell wall at distinct points in the cell cycle, in agreement with Northern analysis data (17b). High levels of transcription of Cwp2p just before the stage in the cell cycle when the cells were very resistant to nisin suggested that this protein protects the cell from nisin and similar peptides. Upon depletion of both Cwp2p and Cwp1p, the cells were very sensitive to nisin, as demonstrated by the high percentage of PI-positive cells. The fact that expression of CWP1 was induced in a cwp2Δ strain whereas CWP2 expression was not induced in a cwp1Δ strain and the fact that cwp2Δ cells were slightly more sensitive to nisin than cwp1Δ cells support the conclusion that the Cwp2p protein seems to be more important than the Cwp1p in conferring nisin resistance upon yeast cells. van der Vaart et al. (32) showed that the exponentially growing cwp2 deletion mutant is more sensitive to Calcofluor White, Congo red, and Zymolyase than the cwp1, tip1, and srp1 deletion mutants. Furthermore, depletion of Cwp2p resulted in a thinner electron-dense layer around the glucan layer. The importance of Cwp2p for normal cellular physiology is also underlined by the observation that overexpression of this protein can partially compensate for the lack of sphingolipids. Sphingolipids are necessary for the growth of S. cerevisiae bypass mutants at low pH values (28). Survival of these mutants at low pH values is enhanced by overexpression of the Cwp2 protein.
In addition to nisin, there are similar small, membrane-perturbing peptides, such as histatins, cecropins, and magainins, as well as synthetically modelled peptides (2). We studied the antifungal activity of synthetically produced peptides during the yeast cell cycle with the approach described in this paper and found a pattern similar to that observed for nisin (11a). Yeast cell wall proteins are also involved in resistance to somewhat larger membrane-active plant antimicrobial proteins. Yun et al. (36) recently showed that Pir cell wall proteins are induced in yeast cells in response to a challenge with the plant antifungal PR-5 protein osmotin.
We are currently constructing fusion proteins which consist of α-galactosidase, a protease-processing site, and various Cwps. These constructs should allow us to purify Cwps with (parts of) their glycosyl phosphatidyl inositol anchors for peptide binding studies. Furthermore, we plan to perform photolabelling experiments with nisin (and other peptides) in incubations with yeast cells. Complexes formed upon cross-linking of the peptides to the yeast wall will be analyzed in order to characterize the in vivo binding of the peptides to wall components. These two approaches should allow us to distinguish between a direct protective effect of Cwps against peptides through specific binding and indirect protection through an influence on the structural organization of the wall.
Finally, how yeast cells respond to a constant challenge with an antimicrobial peptide is not yet clear. Another issue is whether the age of yeast cells influences their sensitivity to peptides; this could explain the apparent sensitivity of some wild-type cells to nisin, as indicated by the data in Fig. 3A. Current studies are aimed at answering these questions.
ACKNOWLEDGMENT
John Chapman is gratefully acknowledged for his help during this study and for critical reading of the manuscript.
REFERENCES
- 1.Abee T, Rombouts F M, Hugenholtz J, Guihard G, Letellier L. Mode of action of nisin Z against Listeria monocytogenes Scott A grown at high and low temperatures. Appl Environ Microbiol. 1994;60:1962–1968. doi: 10.1128/aem.60.6.1962-1968.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates, Inc., and John Wiley & Sons, Inc.; 1990. [Google Scholar]
- 2.Bhakoo, M. September 1996. International patent WO 96/28468.
- 3.Bourdineaud J P, Van der Vaart J M, Donzeau M, deSampaio G, Verrips C T, Lauquin G J M. Pmt1 mannosyl transferase is involved in cell wall incorporation of several proteins in Saccharomyces cerevisiae. Mol Microbiol. 1998;27:85–98. doi: 10.1046/j.1365-2958.1998.00660.x. [DOI] [PubMed] [Google Scholar]
- 4.Brul S, King A, van der Vaart J M, Chapman J W, Klis F M, Verrips C T. The incorporation of mannoproteins in the cell wall of Saccharomyces cerevisiae and filamentous Ascomycetes. Antonie Leeuwenhoek. 1997;72:229–237. doi: 10.1023/a:1000429208049. [DOI] [PubMed] [Google Scholar]
- 5.Brul S, Nussbaum J, Dielbandhoesing S K. Fluorescent probes for wall porosity and membrane integrity in filamentous fungi. J Microbiol Methods. 1997;28:169–178. [Google Scholar]
- 6.Bruno M E C, Kaiser A, Montville T J. Depletion of proton motive force by nisin in Listeria monocytogenes cells. Appl Environ Microbiol. 1992;58:2255–2259. doi: 10.1128/aem.58.7.2255-2259.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caro L H P, Smits G J, van Egmond P, Chapman J W, Klis F M. Transcription of multiple cell wall protein-encoding genes in Saccharomyees cerevisiae is differentially regulated during the cell cycle. FEMS Microbiol Lett. 1998;161:345–349. doi: 10.1111/j.1574-6968.1998.tb12967.x. [DOI] [PubMed] [Google Scholar]
- 8.Cid V J, Durán A, del Rey F, Snyder M P, Nombela C, Sánchez M. Molecular basis of cell integrity and morphogenesis in Saccharomyces cerevisiae. Microbiol Rev. 1995;59:345–386. doi: 10.1128/mr.59.3.345-386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delves-Broughton J. Review: nisin and its application as a food preservative. J Soc Dairy Technol. 1990;43:73–76. [Google Scholar]
- 10.Delves-Broughton J, Balckburn P, Evans R J, Hugenholtz J. Applications of the bacteriocin, nisin. Antonie Leeuwenhoek. 1996;69:193–202. doi: 10.1007/BF00399424. [DOI] [PubMed] [Google Scholar]
- 11.de Nobel J G, Klis F M, Priem J, Minnik T, van Den Ende H. The glucanase-soluble mannoproteins limit cell wall porosity in Saccharomyces cerevisiae. Yeast. 1990;6:491–499. doi: 10.1002/yea.320060606. [DOI] [PubMed] [Google Scholar]
- 11a.Dielbandhoesing, S. K., et al. Unpublished data.
- 12.Driessen A J M, Van den Hooven H W, Kuiper W, Van de Kamp M, Sahl H-G, Konings R N H, Konings W N. Mechanistic studies of lantibiotic-induced permeabilization of phospholipid vesicles. Biochemistry. 1995;34:1606–1614. doi: 10.1021/bi00005a017. [DOI] [PubMed] [Google Scholar]
- 13.Erhart E, Hollenberg C P. The presence of a defective LEU2 gene on 2mm DNA recombinant plasmids of Saccharomyces cerevisiae is responsible for curing and high copy number. J Bacteriol. 1981;156:625–633. doi: 10.1128/jb.156.2.625-635.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao F H, Abee T, Konings W N. Mechanism of the peptide antibiotic nisin in liposomes and cytochrome c oxidase-containing proteoliposomes. Appl Environ Microbiol. 1991;57:2164–2170. doi: 10.1128/aem.57.8.2164-2170.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurst A. Nisin. Adv Appl Microbiol. 1981;27:85–123. [Google Scholar]
- 16.Hurst A, Hoover D G. Antimicrobials in food. New York, N.Y: Marcel Dekker, Inc.; 1993. pp. 369–394. [Google Scholar]
- 17.Kaiser C, Michealis S, Mitchell A. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 17a.Kapteyn, J. C., H. Vanden Ende, and F. M. Klis. The contribution of cell wall proteins to the organisation of the yeast cell wall. Biochim. Biophys. Acta, in press. [DOI] [PubMed]
- 17b.Klis, F., J. C. Kapteyn, and J. G. de Nobel. Unpublished data.
- 17c.Klis, F., J. C. Kapteyn, and J. G. de Nobel. Personal communication.
- 18.Klis F M, Caro L H P, Vossen J H, Kapteyn J C, Ram A F J, Monteijn R C, Van Berkel M A A, Van Den Ende H. Identification and characterisation of a major building block in the cell wall of Saccharomyces cerevisiae. Biochem Soc Trans. 1997;25:856–860. doi: 10.1042/bst0250856. [DOI] [PubMed] [Google Scholar]
- 19.Kollar R, Reinhold B, Petráková E, Yeh H J C, Ashwell G, Drgonová J, Kapteyn J C, Klis F M, Cabib E. Architecture of the yeast cell wall. J Biol Chem. 1997;272:17762–17775. doi: 10.1074/jbc.272.28.17762. [DOI] [PubMed] [Google Scholar]
- 20.Kordel M, Sahl H-G. Susceptibility of bacterial, eukaryotic and artificial membranes to the disruptive action of the cationic peptides Pep5 and nisin. FEMS Microbiol Lett. 1986;34:139–144. [Google Scholar]
- 21.Lu C-F, Kurjan J, Lipke P N. A pathway for cell wall anchorage of Saccharomyces cerevisiae a-agglutinin. Mol Cell Biol. 1994;14:4825–4833. doi: 10.1128/mcb.14.7.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pammer M, Briza P, Ellinger A, Schuster T, Stucka R, Feldmann H, Breitenbach M. DIT101 (CSD2, CAL1), a cell cycle-regulated yeast gene required for synthesis of chitin in cell walls and chitosan in spore walls. Yeast. 1992;8:1089–1099. doi: 10.1002/yea.320081211. [DOI] [PubMed] [Google Scholar]
- 23.Ram A F J, Wolters A, Ten Hopen R, Klis F M. A new approach for isolating cell wall mutants in Saccharomyces cerevisiae by screening for hypersensitivity to Calcofluor White. Yeast. 1994;10:1019–1030. doi: 10.1002/yea.320100804. [DOI] [PubMed] [Google Scholar]
- 24.Ruhr E, Sahl H-G. Mode of action of the peptide antibiotic nisin and influence on the membrane potential of whole cells and cytoplasmic and artificial membrane vesicles. Antimicrob Agents Chemother. 1985;27:841–845. doi: 10.1128/aac.27.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahl H-G. Pore formation in bacterial membranes by cationic lantibiotics. In: Jung G, Sahl H-G, editors. Nisin and novel lantibiotics. Leiden, The Netherlands: ESCOM Science Publishers; 1991. pp. 347–358. [Google Scholar]
- 26.Sambrook J, Maniatis T, Fritsch E F. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 27.Sherman F, Fink G R, Hicks J B. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1986. [Google Scholar]
- 28.Skrzypek M, Lester R L, Dickson R C. Suppressor gene analysis reveals an essential role for sphingolipids in transport of glycosylphosphatidylinositol-anchored proteins in Saccharomyces cerevisiae. J Bacteriol. 1997;179:1513–1520. doi: 10.1128/jb.179.5.1513-1520.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevens K A, Sheldon B W, Klapes N A, Klaenhammer T R. Nisin treatment for inactivation of Salmonella species and other gram-negative bacteria. Appl Environ Microbiol. 1991;57:3613–3615. doi: 10.1128/aem.57.12.3613-3615.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taniguchi M, Hoshino K, Urasaki H, Fujii M. Continuous production of an antibiotic polypeptide (nisin) by Lactococcus lactis using a bioreactor coupled to a microfiltration module. J Ferment Bioeng. 1994;77:704–708. [Google Scholar]
- 31.Van den Hooven H. Structure elucidation of the lantibiotic nisin in aqueous solution and in membrane-like environments. Ph.D. thesis. Nijmegen, The Netherlands: University of Nijmegen; 1996. [Google Scholar]
- 32.Van der Vaart J M, Caro L H, Chapman J W, Klis F M, Verrips C T. Identification of three mannoproteins in the cell wall of Saccharomyces cerevisiae. J Bacteriol. 1995;177:3104–3110. doi: 10.1128/jb.177.11.3104-3110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White J H M, Barker D, Nurse P, Johnston L H. Periodic transcription as a means of regulating gene expression during the cell cycle: contrasting modes of expression of DNA ligase genes in budding and fission yeast. EMBO J. 1986;5:1705–1709. doi: 10.1002/j.1460-2075.1986.tb04414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winkowski K, Bruno M E C, Montville T. Correlation of bioenergetic parameters with cell death in Listeria monocytogenes cells exposed to nisin. Appl Environ Microbiol. 1994;60:4186–4188. doi: 10.1128/aem.60.11.4186-4188.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yanisch-Perron C, Vieria J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 36.Yun D-J, Zhao Y, Pardo J M, Narasimhan M L, Damsz B, Lee H, Abad L R, D’Urzo M P, Hasegawa P M, Bressan R A. Stress proteins on the yeast cell surface determine resistance to osmotin, a plant antifungal protein. Proc Natl Acad Sci USA. 1997;94:7082–7087. doi: 10.1073/pnas.94.13.7082. [DOI] [PMC free article] [PubMed] [Google Scholar]