Abstract
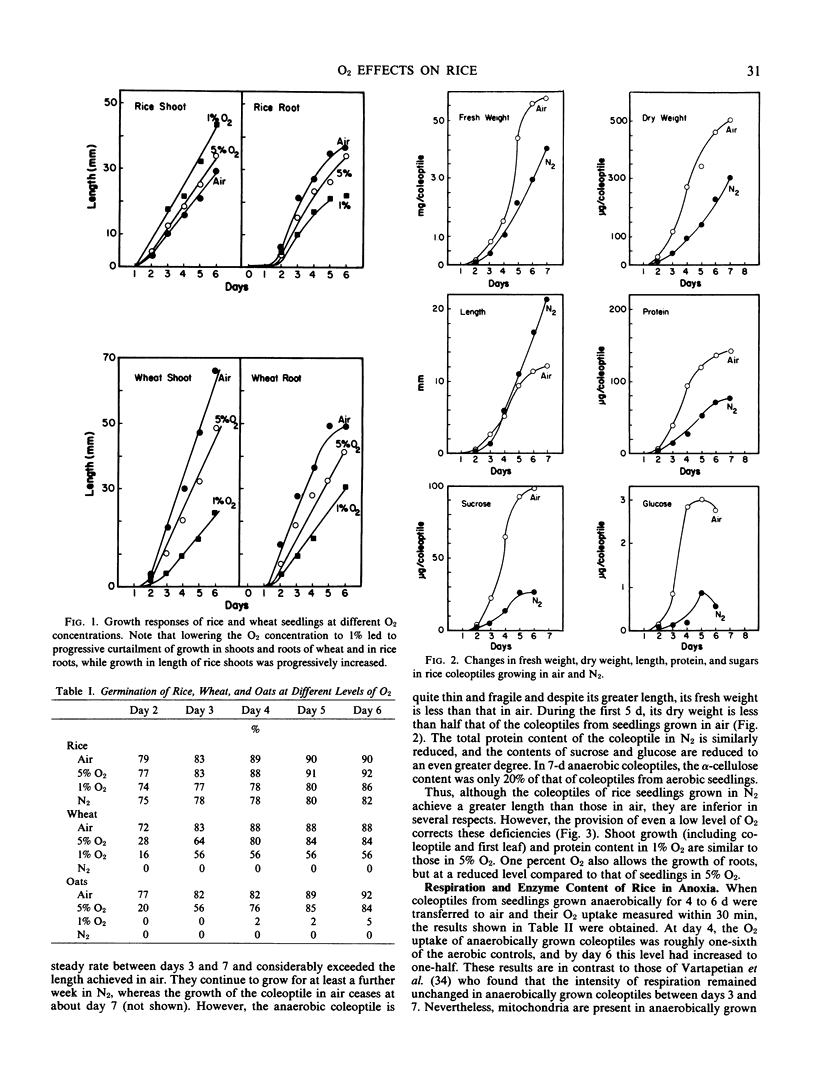
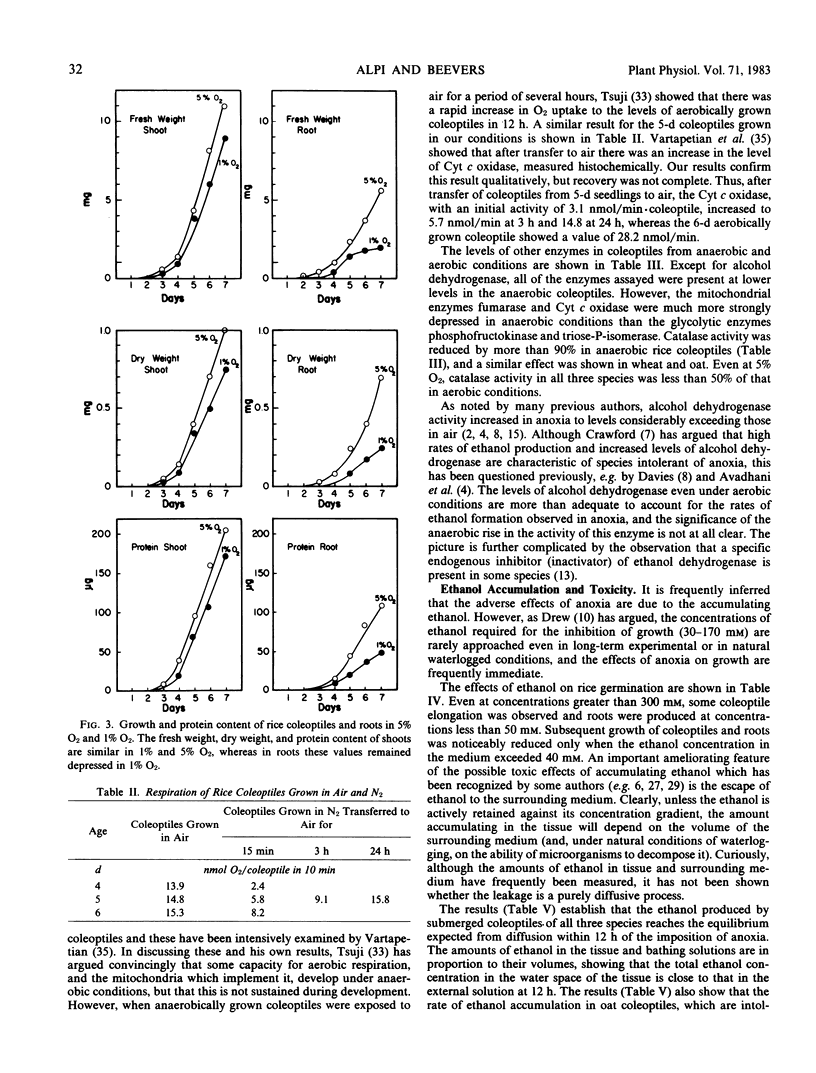
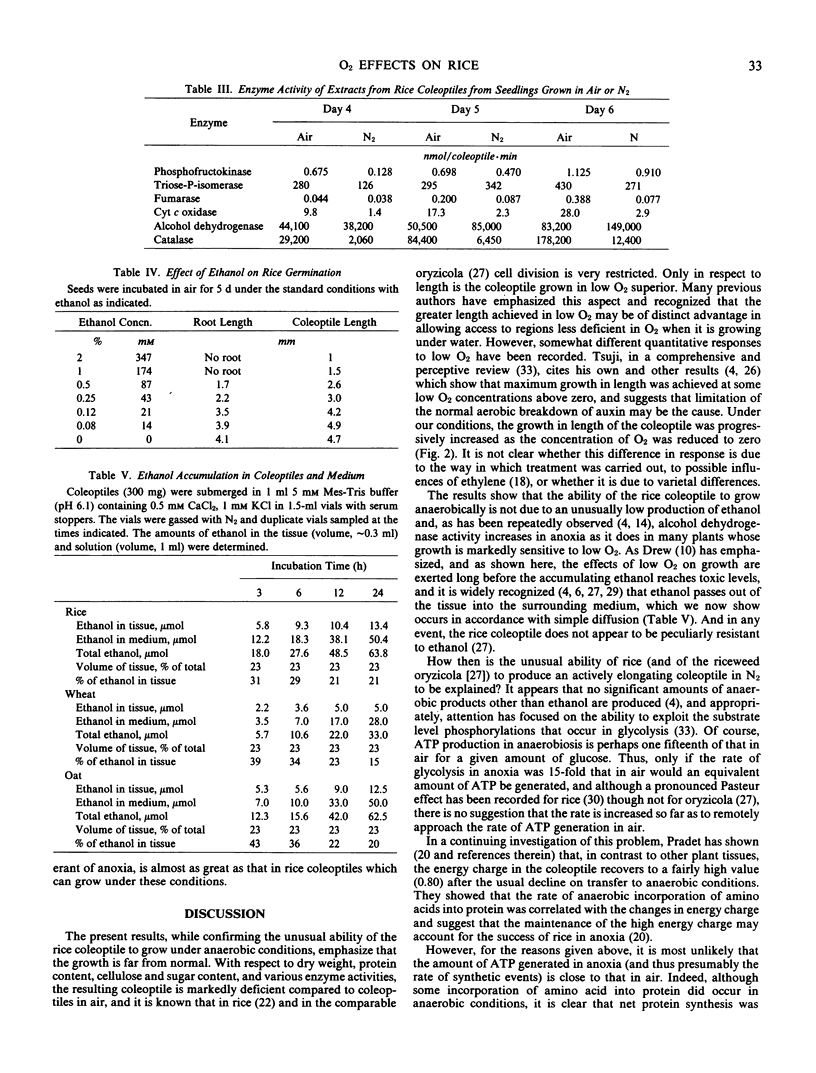
The ability of rice, wheat, and oat seedlings to germinate and grow as the O2 concentration was lowered to zero was compared. The germination of rice was completely unaffected by O2 supply, whereas that of oats and wheat was strongly retarded at levels below 5% O2. In contrast to the coleoptiles of oats and wheat and to roots of all three species where growth was progressively diminished as the O2 concentration was lowered, that of the rice coleoptile was progressively increased. However, the dry weight and content of protein, sugars, and cellulose were all depressed in the rice coleoptile in anoxia, and the levels of several respiratory enzymes, particularly those of mitochondria, were also much lower than those of the coleoptiles grown in air. In 1% O2, the growth of the rice coleoptile was similar to that in air. The effect of ethanol concentration on germination and growth of rice was measured. Coleoptile growth was reduced when the ethanol concentration exceeded 40 millimolarity, and root growth was somewhat more sensitive. Coleoptiles of all three species grown in air were transferred to N2, and ethanol accumulation was measured over 24 hours. The rate of ethanol accumulation in oats was close to that in rice, and in all three species the amounts of ethanol lost to the surrounding medium were those expected from simple diffusion from the tissue. The ability of the rice coleoptile to grow in anoxia is apparently not due to a particularly low rate of ethanol formation or to unusual ethanol tolerance. Any explanation of the success of rice in anoxia must encompass the much lower rate of ATP synthesis than that in air and account for the biochemical deficiencies of the coleoptile.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APP A. A., MEISS A. N. Effect of aeration on rice alcohol dehydrogenase. Arch Biochem Biophys. 1958 Sep;77(1):181–190. doi: 10.1016/0003-9861(58)90054-7. [DOI] [PubMed] [Google Scholar]
- Ho D. T., Scandalios J. G. Regulation of Alcohol Dehydrogenases in Maize Scutellum during Germination. Plant Physiol. 1975 Jul;56(1):56–59. doi: 10.1104/pp.56.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Developmental changes in endosperm of germinating castor bean independent of embryonic axis. Plant Physiol. 1974 Sep;54(3):277–279. doi: 10.1104/pp.54.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobr M. J., Beevers H. Gluconeogenesis in the castor bean endosperm: I. Changes in glycolytic intermediates. Plant Physiol. 1971 Jan;47(1):48–52. doi: 10.1104/pp.47.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mocquot B., Prat C., Mouches C., Pradet A. Effect of anoxia on energy charge and protein synthesis in rice embryo. Plant Physiol. 1981 Sep;68(3):636–640. doi: 10.1104/pp.68.3.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opik H. Effect of anaerobiosis on respiratory rate, cytochrome oxidase activity and mitochondrial structures in coleoptiles of rice (Oryza sativa L.). J Cell Sci. 1973 May;12(3):725–739. doi: 10.1242/jcs.12.3.725. [DOI] [PubMed] [Google Scholar]
- Osmond C. B., Akazawa T., Beevers H. Localization and properties of ribulose diphosphate carboxylase from castor bean endosperm. Plant Physiol. 1975 Feb;55(2):226–230. doi: 10.1104/pp.55.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- Rumpho M. E., Kennedy R. A. Anaerobic Metabolism in Germinating Seeds of Echinochloa crus-galli (Barnyard Grass) : METABOLITE AND ENZYME STUDIES. Plant Physiol. 1981 Jul;68(1):165–168. doi: 10.1104/pp.68.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simcox P. D., Reid E. E., Canvin D. T., Dennis D. T. Enzymes of the Glycolytic and Pentose Phosphate Pathways in Proplastids from the Developing Endosperm of Ricinus communis L. Plant Physiol. 1977 Jun;59(6):1128–1132. doi: 10.1104/pp.59.6.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]


