Abstract
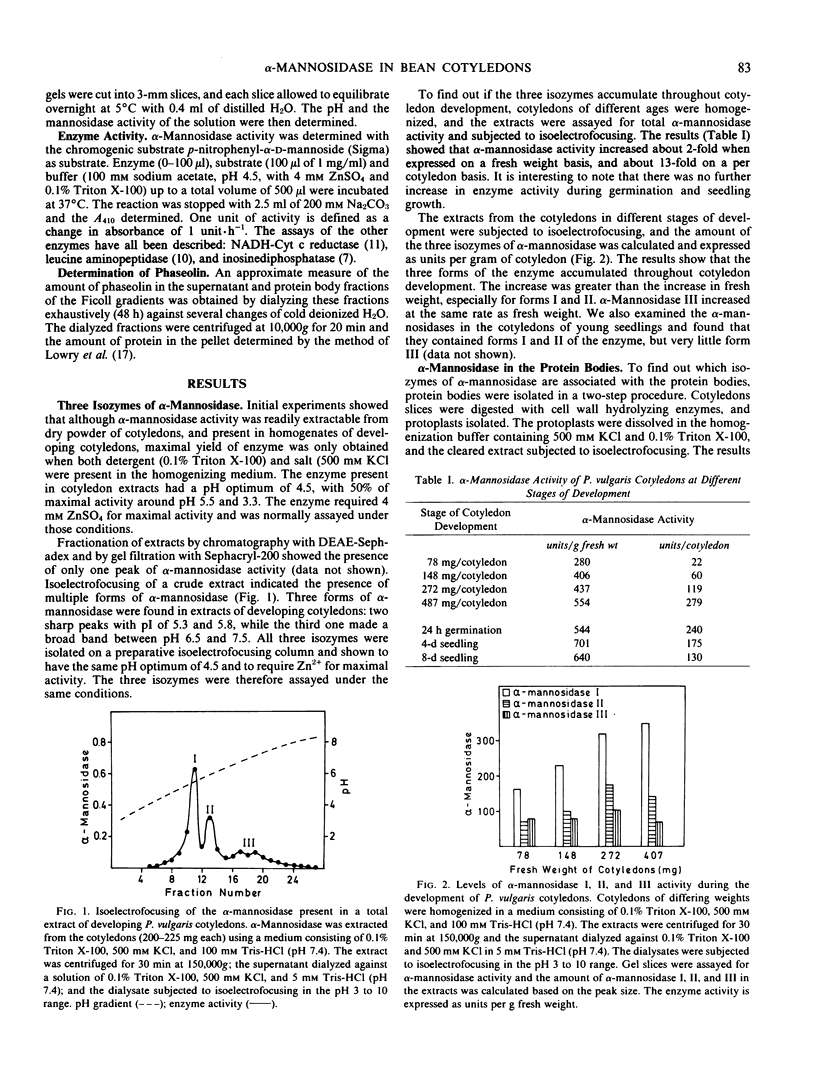
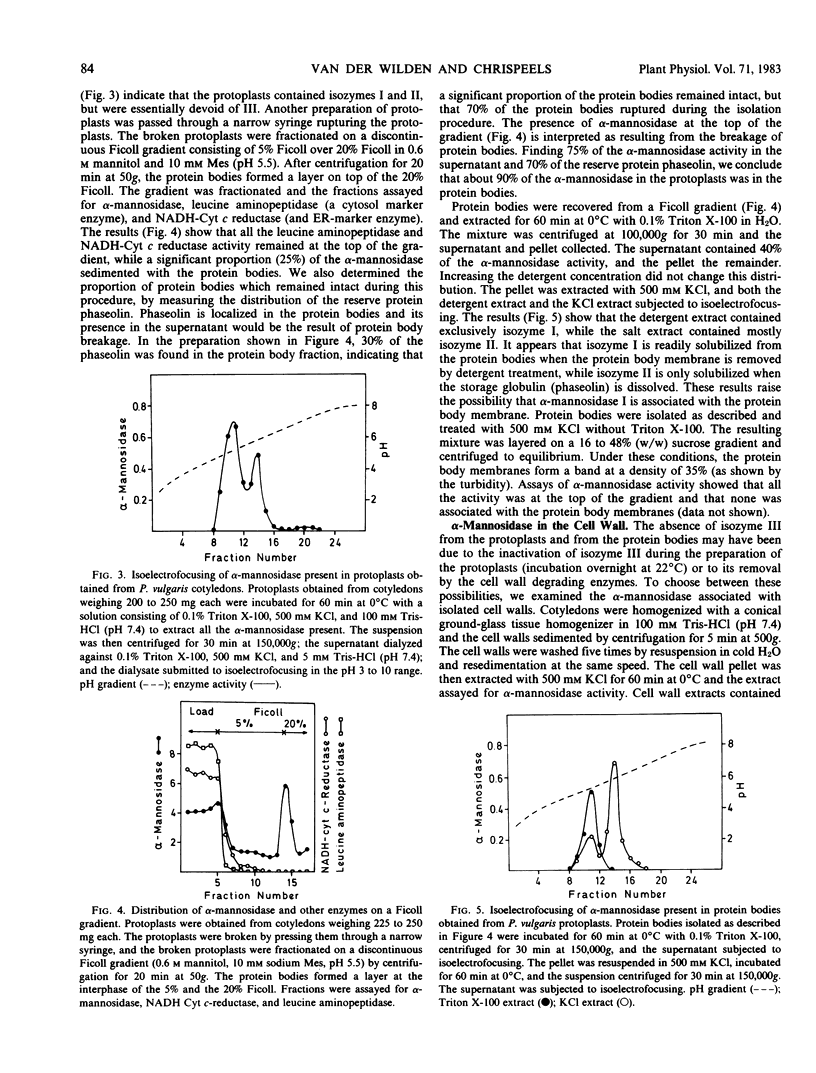
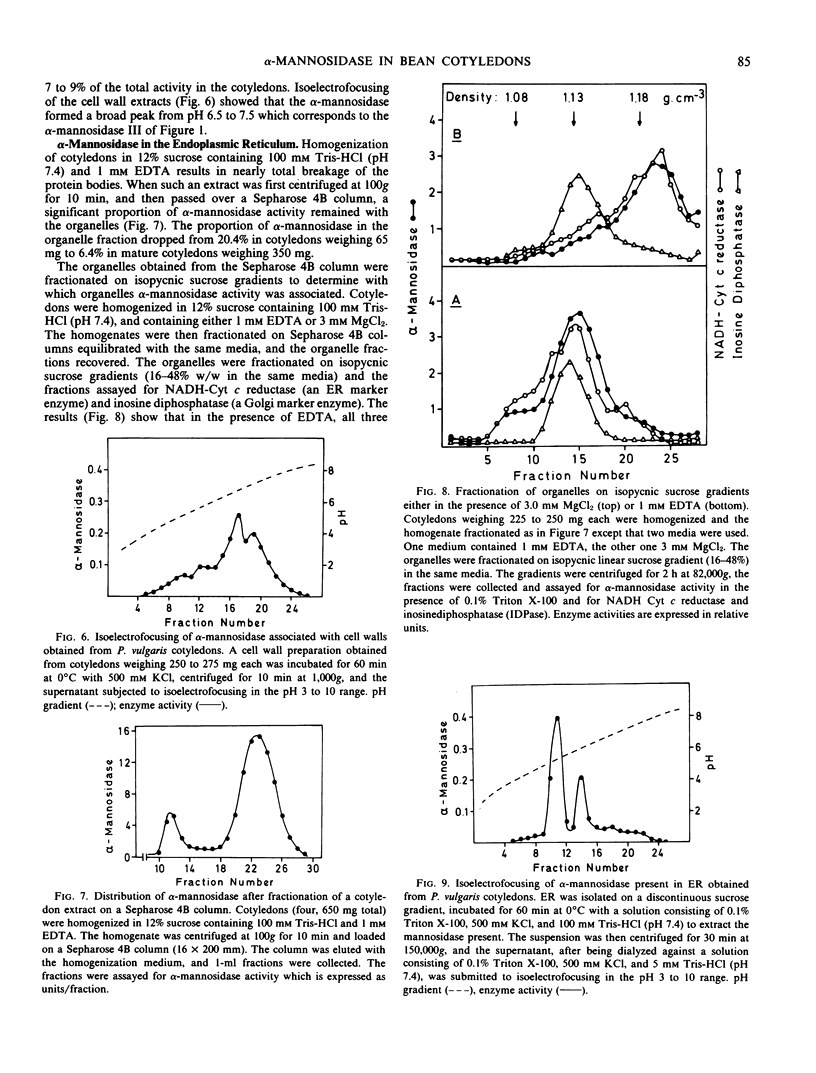
Cotyledons of maturing Phaseolus vulgaris seeds contain three isozymes of α-mannosidase which can be separated by isoelectrofocusing. They have isoelectric points of 5.3, 5.8, and 6.5 to 7.5 and were named I, II, and III in order of ascending pI. All three had an acid pH optimum (4.5) and required Zn2+ for maximal activity. Isozymes I and II were present in the protein bodies. Together they accounted for 85% of the total activity. Isozyme III was essentially absent from isolated protoplasts but could be extracted from isolated cell walls. All three isozymes were also found to be associated with the endoplasmic reticulum, and the proportion of the total activity in this fraction decreased from 20% in immature cotyledons to 6% in mature cotyledons. The results are interpreted as evidence that newly synthesized α-mannosidase is sequestered in the lumen of the ER prior to its transport to the protein bodies or the cell wall.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal K. M., Bahl O. P. Glycosidases of Phaseolus vulgaris. II. Isolation and general properties. J Biol Chem. 1968 Jan 10;243(1):103–111. [PubMed] [Google Scholar]
- Baumgartner B., Tokuyasu K. T., Chrispeels M. J. Localization of vicilin peptidohydrolase in the cotyledons of mung bean seedlings by immunofluorescence microscopy. J Cell Biol. 1978 Oct;79(1):10–19. doi: 10.1083/jcb.79.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles D. J., Kauss H. Characterization, enzymatic and lectin properties of isolated membranes from Phaseolus aureus. Biochim Biophys Acta. 1976 Sep 7;443(3):360–374. doi: 10.1016/0005-2736(76)90456-9. [DOI] [PubMed] [Google Scholar]
- Chrispeels M. J., Bollini R. Characteristics of Membrane-Bound Lectin in Developing Phaseolus vulgaris Cotyledons. Plant Physiol. 1982 Nov;70(5):1425–1428. doi: 10.1104/pp.70.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Boulter D. Control of storage protein metabolism in the cotyledons of germinating mung beans: role of endopeptidase. Plant Physiol. 1975 Jun;55(6):1031–1037. doi: 10.1104/pp.55.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Higgins T. J., Craig S., Spencer D. Role of the endoplasmic reticulum in the synthesis of reserve proteins and the kinetics of their transport to protein bodies in developing pea cotyledons. J Cell Biol. 1982 Apr;93(1):5–14. doi: 10.1083/jcb.93.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris N., Chrispeels M. J. Histochemical and biochemical observations on storage protein metabolism and protein body autolysis in cotyledons of germinating mung beans. Plant Physiol. 1975 Aug;56(2):292–299. doi: 10.1104/pp.56.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nagahashi J., Beevers L. Subcellular Localization of Glycosyl Transferases Involved in Glycoprotein Biosynthesis in the Cotyledons of Pisum sativum L. Plant Physiol. 1978 Mar;61(3):451–459. doi: 10.1104/pp.61.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus E. alpha-Mannosidase from Phaseolus vulgaris. Composition and structural properties. Eur J Biochem. 1977 Feb 15;73(1):155–161. doi: 10.1111/j.1432-1033.1977.tb11302.x. [DOI] [PubMed] [Google Scholar]
- Van der Wilden W., Gilkes N. R., Chrispeels M. J. The Endoplasmic Reticulum of Mung Bean Cotyledons: ROLE IN THE ACCUMULATION OF HYDROLASES IN PROTEIN BODIES DURING SEEDLING GROWTH. Plant Physiol. 1980 Sep;66(3):390–394. doi: 10.1104/pp.66.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Wilden W., Herman E. M., Chrispeels M. J. Protein bodies of mung bean cotyledons as autophagic organelles. Proc Natl Acad Sci U S A. 1980 Jan;77(1):428–432. doi: 10.1073/pnas.77.1.428. [DOI] [PMC free article] [PubMed] [Google Scholar]


