Abstract
Key Clinical Message
The presentation of posterior reversible encephalopathy syndrome (PRES) as the initial presenting sign of acute lymphoblastic leukemia is unusual, as PRES is more often a complication of therapy. This case highlights the importance of maintaining a broad differential diagnosis for pediatric hypertension and its complications.
Abstract
A 6‐year‐old male presented with a seizure‐like episode. Evaluation revealed hypertension and brain imaging showed findings consistent with posterior reversible encephalopathy syndrome. Complete blood count showed lymphoblasts, and the cause of his hypertension was determined to be renal infiltration of leukemia cells due to B‐cell acute lymphoblastic leukemia.
Keywords: ALL, emergency medicine, nephrology, oncology, pediatric hypertension, pediatrics and adolescent medicine, PRES, renal leukemic infiltrate
This case is an unusual presentation of a new diagnosis of B‐ALL and an important reminder of the differential diagnosis for abnormal neurologic findings and hypertension. PRES as a presenting symptom for hematologic malignancies has rarely been reported and is identified by MRI T2 prolongation and gyral swelling in the posterior brain, as shown.
1. INTRODUCTION
We report the case of a 6‐year‐old male who presented with a seizure‐like episode. He was subsequently noted to have hypertension. An evaluation for the cause of seizures was pursued. Brain magnetic resonance imaging (MRI) revealed findings indicative of posterior reversible encephalopathy syndrome (PRES), and renal ultrasound showed increased echogenicity and size bilaterally. A repeat complete blood count was done with a differential, showing abnormal lymphoblasts. He was ultimately diagnosed with B‐cell acute lymphoblastic leukemia (B‐ALL), which is the most common childhood cancer. The presentation of PRES as the initial sign of disease is unusual, as it is more often a complication of chemotherapy. We report this case to share this unique presentation and to highlight the importance of evaluating for underlying causes of hypertension in the setting of PRES.
2. CASE REPORT
A 6‐year‐old male presented to the Pediatric Emergency Department (PED) with a chief complaint of a seizure‐like episode as well as non‐bloody, non‐bilious emesis earlier the same day. His past medical history was notable only for seasonal allergies. Family reported he woke up from sleep with emesis and was unable to stand because of lightheadedness. This was followed by eye fluttering and unresponsiveness for approximately 1 min. The family denied any shaking of his extremities, bladder or bowel incontinence, or tongue biting. After the episode, he developed a headache with photophobia and vision change, and the family went to the PED. By the time of presentation, he had returned to his neurologic baseline without further emesis.
Additional history revealed intermittent vomiting in the mornings without nausea or abdominal pain over the preceding month. He had no history of prior seizure‐like activity. Family denied fevers, change in appetite, previous episodes of altered mental status, lightheadedness, cough, congestion, abdominal pain, constipation, diarrhea, hematuria, edema, urinary incontinence, or urinary retention. He had normal growth and development without regression of language or motor skills, and his vaccination status was current. Family history revealed his maternal uncle and first maternal cousin had a history of brain tumors of unknown type but no history of seizures.
On arrival at the PED, he appeared ill but non‐toxic. Vital signs showed blood pressure of 161/102 mmHg, heart rate ranged between 90 and 110 beats/min, temperature of 37.9°C, respiratory rate of 16 breaths/min, and weight at the 60th percentile. His mental status was normal for age. All cranial nerves were intact. He had normal tone, no clonus, and 2+ reflexes in his upper and lower extremities, though he had bilateral positive Babinski signs with upgoing toes. Abdominal exam revealed distension in his suprapubic area but otherwise no abdominal tenderness nor organomegaly. The remainder of the examination was unremarkable.
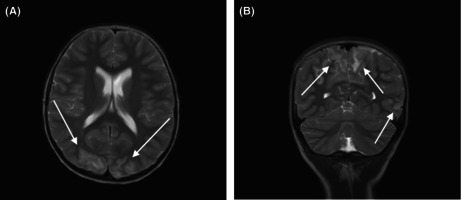
Due to concerns for cerebral involvement, imaging was obtained in the PED. A computerized tomography (CT) scan of his head showed a left hemisphere hypodense region but with artifact. To further characterize the findings, a brain MRI with diffusion‐weighted imaging (DWI) was obtained and concerning for bilateral parieto‐occipital subcortical T2 prolongation and swelling. He had bilateral areas of white matter vasogenic edema in the posterior cerebral hemispheres, particularly the parieto‐occipital regions, consistent with PRES (Figure 1).
FIGURE 1.

(A) Axial and coronal T2‐weighted images show cortical and subcortical T2 prolongation and gyral swelling in the posterior occipital (A), parietal and left temporal lobes (B), typical of PRES. The abnormalities were inconspicuous on CT performed the same day (not shown). There is no evidence of hemorrhage or diffusion abnormality. (B) Axial and coronal T2‐weighted images show cortical and subcortical T2 prolongation and gyral swelling in the posterior occipital (A), parietal and left temporal lobes (B), typical of PRES. The abnormalities were inconspicuous on CT performed the same day (not shown). There is no evidence of hemorrhage or diffusion abnormality.
Based on his eye‐fluttering, there was concern for focal seizures, and his significant lethargy suggested a post‐ictal state. He was admitted to the pediatric intensive care unit (PICU), and electroencephalography (EEG) demonstrated ongoing subclinical seizures. Specifically, per the EEG report, one subclinical seizure showed ictal onset in the right occipital region, whereas the second subclinical seizure originated in the left occipital region. He was progressively managed with lorazepam, levetiracetam, and fosphenytoin, which aborted his seizure activity.
Given that PRES is often related to hypertension, further investigation into the source of his elevated blood pressure was pursued. Complete blood count (without differential) and basic metabolic panel were notable for a white blood cell count of 29.7 × 106/mL, hemoglobin of 12 g/dL, platelet count of 265 × 106/mL, blood urea nitrogen of 16 mg/dL, and creatinine of 1.3 mg/dL. Urinalysis showed 30 mg/dL of protein but no blood or other abnormalities. A urine drug screen was negative. A lumbar puncture was performed, which showed 0 nucleated cells, 0 red blood cells, protein of 43 mg/dL, and glucose of 69 mg/dL. A renal ultrasound showed bilaterally increased echogenicity and marked enlargement of the kidneys (both 11.6 cm in length, approximately 5 standard deviations above the mean for age), 1 no abnormal vasculature on Doppler, and a distended bladder. A transthoracic echocardiogram (TTE) showed no left ventricular hypertrophy and was otherwise within normal limits. A funduscopic exam showed no retinal hemorrhages or papilledema.
A distended bladder, as seen in this patient, is not a common presentation of hypertensive emergency. Ultimately, this patient's urinary retention resolved with treatment of his acutely elevated blood pressure, suggesting that his urinary retention may have been related to the neurologic injury itself. Thereafter, the acute kidney injury (AKI) was likely not purely obstructive, since the AKI persisted after resolution of his urinary retention. Microscopic examination of the urine showed no red blood cells, and both anti‐streptolysin and C3 complement levels were normal, all reassuring against glomerulonephritis. His renal ultrasound with Doppler was inconsistent with renal artery stenosis, and he had no anemia suggestive of microangiopathy. His hypertension was not thought to be long‐standing, given the absence of left ventricular hypertrophy on TTE.
In the PICU, a nicardipine drip was initiated, which was gradually transitioned to oral amlodipine, atenolol, minoxidil, a clonidine patch, and as needed hydralazine. The patient stabilized but still had an unknown etiology for his hypertension and renal enlargement. He had no signs or symptoms of infection and had a negative blood culture, respiratory pathogen panel, and tick studies. He continued to be afebrile without antibiotics. Before transfer to the floor, a repeat complete blood count was obtained. This time, the lab was ordered with a differential, which showed blasts, and preliminary flow cytometry revealed abnormal B lymphoblasts consistent with a diagnosis of B‐ALL.
As part of the patient's diagnostic evaluation for leukemia, he required a bone marrow biopsy, port placement, and lumbar puncture with intrathecal cytarabine. Then, he began treatment with chemotherapy for standard risk B‐ALL with induction chemotherapy including dexamethasone, vincristine, pegaspargase, and intrathecal methotrexate. He was at risk for tumor lysis syndrome with initiation of his therapy in the setting of recent AKI, but he did not have any major electrolyte disturbances or complications. His AKI was resolved with chemotherapy initiation.
His imaging findings of PRES resolved within 2 weeks of his initial presentation (Figure 2). The patient was able to be weaned from anti‐seizure medications, which is often the case after radiological and clinical recovery. The recommended chemotherapy regimen posed risks of neurotoxicity from intrathecal and intravenous methotrexate, which can include seizures, encephalopathy, or stroke‐like symptoms. During his induction phase, two doses of oral leucovorin, a folinic acid, were added after each intrathecal methotrexate. His family was educated on the symptoms of PRES, and he continues to be monitored frequently as part of his routine care for B‐ALL for both neurologic symptoms as well as for blood pressure elevation. While surveillance neuroimaging is not recommended, there will be a low threshold to pursue neuroimaging if symptoms arise suddenly or persist. He continues to follow up with nephrology and neurology and has been weaned from his anti‐hypertensive medications and seizure medications without any further neurological or renal complications.
FIGURE 2.

Axial FLAIR obtained 11 days later shows near‐total resolution of symmetric bilateral occipital signal abnormality.
3. DISCUSSION
This case highlights the importance of initially ruling out the most dangerous etiologies of the chief complaint while continuing evaluation until a unifying diagnosis can be made for all symptoms. This patient's presentation with occasional morning emesis, headache with vision change, and altered mental status raises concern for increased intracranial pressure (ICP), which is acutely dangerous. Increased ICP leading to herniation would be supported by Cushing's Triad (hypertension, bradycardia, and alterations in respiratory rate), as well as papilledema, pupillary changes, or vision changes. Increased ICP can result from traumatic brain injury, intracranial hemorrhage, central nervous system infections, ischemic stroke, sinovenous thrombosis, neoplasm, hydrocephalus, vasculitis, idiopathic intracranial hypertension, and hypertensive encephalopathy. This patient had hypertension but not bradycardia or irregular respirations; however, in cases of unclear intracranial involvement, imaging such as a non‐contrast CT scan of the head or brain MRI is recommended to look for causes of increased ICP. Brain MRI has added sensitivity for several of the listed conditions, and diffusion‐weighted imaging is able to distinguish infarction from vasogenic edema. 2
In this case, brain imaging led to the diagnosis of PRES, of which the leading cause is hypertension. PRES is synonymous with reversible posterior leukoencephalopathy syndrome, reversible posterior cerebral edema syndrome, posterior leukoencephalopathy syndrome, hyper‐perfusion encephalopathy, and brain capillary leak syndrome. In summary, it is a clinical, radiographic syndrome of various causes that are grouped together based on neuroimaging findings. The pathophysiology is thought to be a combination of disordered cerebral autoregulation and endothelial dysfunction, such that hyper‐perfusion or direct injury results in dysfunction of the blood‐brain barrier and subsequent extravasation of fluid and blood products into the brain parenchyma. 3 , 4 , 5 Similar to the presentation in this patient, PRES can present with headaches that are constant, diffuse, and moderate to severe in severity. Confusion can range from mildly altered mental status to coma. Vision changes include auras, hallucinations, and blindness, although funduscopic exam is often normal. Tonic–clonic seizures can also occur, 6 though this patient presented with focal rather than generalized seizures. Babinski sign may or may not be present, but other focal neurologic deficits are rare. 7
CT findings may be subtle or even normal. MRI is more sensitive and should be performed if there is clinical suspicion, even in the absence of CT findings. Diffusion abnormalities smaller than the area of fluid‐attenuated inversion recovery (FLAIR) hyperintensity, patchy enhancement, and hemorrhage (focal hemorrhage, microhemorrhage, or convexity subarachnoid hemorrhage) are occasionally seen and are associated with worse clinical outcomes. In PRES, in addition to the occipital lobes, the frontal lobes, temporal lobes, and cerebellum are commonly affected. Usually, the MRI abnormality is not confined to a single vascular territory, 8 a feature that differentiates it from ischemic stroke. Infarcts are more likely to be unilateral, show reduced diffusivity on DWI, and involve gray and white matter.
The leading pathology implicated in the development of PRES is hypertension. When patients present with PRES, however, up to half of patients may have normal blood pressure but with transient, rapid rises and fluctuations. 9 , 10 The numerical value is important, as is the percentage over the patient's baseline blood pressure. 11 This patient had no known diagnosis of hypertension prior to presentation. Clinical practice guidelines from 2017 suggest that 30 mm Hg above the 95th percentile for the systolic blood pressure is the threshold at which emergency management should be pursued, 12 as was initiated in this patient.
The hypertension in this case was severe and presented with neurologic consequences (seizures, headache, vision change, altered mental status) due to PRES as well as urinary retention. In the setting of a hypertensive emergency, intravenous medication is usually indicated. 13 Due to the frequency of monitoring for titration, this is typically done in the PICU setting. Within the first 2–6 h, the blood pressure is lowered by no more than 25% of the patient's increase above the 95th percentile blood pressure. 13 If there is too rapid a decrease in blood pressure, there may be a reduction in blood pressure below the auto‐regulatory range, leading to cerebral, coronary, or renal ischemia. 14 The best initial agents to use are those that are easily titratable, such as nicardipine and labetalol. 15 Sodium nitroprusside continuous infusion lowers blood pressure as well but is avoided in AKI due to the potential for accumulation of its metabolite, thiocyanate. Like nicardipine, clevidipine is a dihydropyridine calcium channel blocker that is delivered by continuous intravenous infusion, but its effect may be more rapid and its titration more precise. Clevidipine use in pediatric critical care, while still relatively novel, appears effective at blood pressure reduction with a good safety profile. 16
Renal parenchymal and reno‐vascular causes of hypertension are the leading etiologies of hypertensive crises in pediatric patients. 12 Thus, in addition to laboratory studies such as a basic metabolic panel and urinalysis, a renal ultrasound is an initial imaging modality for broad assessment of the kidneys and urinary tract. It allows the identification of collecting system obstruction, cysts, tumors, and parenchymal abnormalities. If suspicion of reno‐vascular hypertension persists after initial work‐up, further investigation of the renal vasculature can be pursued. Doppler evaluation is readily available, although identification of stenosis of accessory, branch, and intrarenal renal arteries may be limited with this modality; CT and MRI have better and more specific rates of detection but may require sedation in younger age groups. 17 Generally, these can be delayed until after blood pressure is better controlled. Consequences of long‐standing hypertension, such as left ventricular hypertrophy, can be diagnosed via TTE, which would show thickened muscle tissue in the left ventricle and provide a better understanding of the duration of hypertension. Although electrocardiogram may be more readily obtained, it is insufficiently sensitive to detect left ventricular hypertrophy in pediatric patients. 18 If renal parenchymal disease is not identified, blood can be sent for renin and aldosterone levels, which may assist in identifying reno‐vascular hypertension.
This patient's kidneys were markedly enlarged for age and showed heterogeneously increased echogenicity of both kidneys. This indicates renal parenchymal disease as the source of his AKI, rather than a pre‐renal cause, such as dehydration. In this case, the patient's malignancy led to renal leukemic infiltrates, which caused the renal enlargement, AKI, and severe hypertension. While it is not uncommon to have leukemic infiltrates, 19 AKI secondary to leukemic infiltration is seen in less than 1% of ALL. 20 , 21 In cases of acute tubular necrosis in patients with leukemia and lymphoma, the most common precipitators include sepsis and nephrotoxic chemotherapy agents, 22 , 23 neither of which occurred in this patient. AKI with extensive leukemic infiltrates is likely multifactorial but involves tubular compression and disruption of the microvasculature by infiltrating leukemia cells, an apt setting for AKI. Histology may show tubular degenerative changes, but tubular changes are not always seen, even with significant renal dysfunction. 24 , 25 A hallmark of AKI due to tumor infiltration of the kidney is prompt improvement in kidney function after chemotherapy, coupled with tumor responsiveness and normalization of kidney size. 20 , 23 , 26
Disrupted renal blood flow by leukemic infiltrates also causes renin‐mediated hypertension. 22 In preclinical studies, a seven‐fold increase in renin content has been demonstrated in renal parenchyma adjacent to non‐perfused tissue compared to remote parenchyma, underscoring the dramatic reactivity of renal tissue to disruption of normal perfusion. 27 In one study, among over 300 children with ALL, the findings of renal leukemic infiltrates and AKI both correlated significantly with the occurrence of hypertension. 28 To our knowledge, there have been no previous reports documenting PRES from renal leukemic infiltration and AKI, as occurred in this patient. Similar to our case, an adult hemodialysis patient with previously well‐controlled hypertension developed severe hypertension (210/110), coincident with documented enlargement of kidneys and bone marrow biopsy diagnostic of T‐cell ALL. 29 Blood pressure improved with treatment of this adult patient's leukemia. Of note, while leukemic infiltration of the kidneys tends to occur in late stages of ALL and has been associated with a poor prognosis in some, 30 the patient in our case is doing well over 18 months into his therapy.
Acute lymphoblastic leukemia is the most common childhood malignancy, accounting for 25% of new childhood cancer diagnoses. Most commonly, presenting symptoms are a result of leukocytosis or cytopenias, such as pallor, fever, bleeding/bruising, bone pain, hepatosplenomegaly, and/or lymphadenopathy. Interestingly, this patient did not have any of these symptoms. In pediatric oncology, PRES most often occurs as a complication of chemotherapy, rather than the presentation of a new diagnosis. PRES can be seen as a result of hypertension associated with steroid courses for induction chemotherapy and/or due to synergistic neurotoxicity of multiple chemotherapeutic agents. 31 PRES has been shown to be a complication of all of the following chemotherapy regimens: VEGF inhibitors, cisplatin/platinum‐based agents, cyclosporine A, cytarabine, gemcitabine, INF‐a, ipilimumab, methotrexate, rituximab, tacrolimus, sirolimus, tyrosine kinase inhibitors, and vincristine. 32 PRES as a presenting symptom for hematologic malignancies has rarely been reported. 33
4. CONCLUSION
This case is an unusual presentation of a new diagnosis of B‐ALL and an important reminder of the differential diagnosis for abnormal neurologic findings and hypertension. While PRES is a known complication of the treatment of B‐ALL, it is rarely in the presenting constellation of symptoms of leukemia. Furthermore, to our knowledge, there have been no previous reports documenting PRES from renal leukemic infiltration and AKI. It is important to keep a broad differential diagnosis for hypertension in mind and to investigate further based on the history and physical exam, in addition to any prior records of blood pressure and renal function. Finally, this case highlights the importance of obtaining a differential with a complete blood count, as cancer in children does not always present with classic symptoms or objective findings.
AUTHOR CONTRIBUTIONS
Jessica Hayes: Conceptualization; data curation; writing – original draft; writing – review and editing. Anne Byrd Mahoney: Writing – original draft; writing – review and editing. Claci Ayers: Writing – original draft; writing – review and editing. Asha Sarma: Writing – original draft; writing – review and editing. Kevin C. Ess: Writing – original draft; writing – review and editing. Tracy E. Hunley: Writing – original draft; writing – review and editing. Christine Moore Smith: Conceptualization; writing – original draft; writing – review and editing.
FUNDING INFORMATION
No funding to report.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest relevant to this article to disclose.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Hayes J, Mahoney AB, Ayers C, et al. A rare cause of posterior reversible encephalopathy syndrome: Acute lymphoblastic leukemia. Clin Case Rep. 2023;11:e8238. doi: 10.1002/ccr3.8238
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Rosenbaum DM, Korngold E, Teele RL. Sonographic assessment of renal length in normal children. Am J Roentgenol. 1984;142(3):467‐469. doi: 10.2214/ajr.142.3.467 [DOI] [PubMed] [Google Scholar]
- 2. Ay H, Buonanno FS, Schaefer PW, et al. Posterior leukoencephalopathy without severe hypertension: utility of diffusion‐weighted MRI. Neurology. 1998;51(5):1369‐1376. doi: 10.1212/wnl.51.5.1369 [DOI] [PubMed] [Google Scholar]
- 3. Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions [published correction appears in Lancet Neurol. 2015 Sep;14(9):874]. Lancet Neurol. 2015;14(9):914‐925. doi: 10.1016/S1474-4422(15)00111-8 [DOI] [PubMed] [Google Scholar]
- 4. Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494‐500. doi: 10.1056/NEJM199602223340803 [DOI] [PubMed] [Google Scholar]
- 5. Brightman MW, Klatzo I, Olsson Y, Reese TS. The blood‐brain barrier to proteins under normal and pathological conditions. J Neurol Sci. 1970;10(3):215‐239. doi: 10.1016/0022-510x(70)90151-6 [DOI] [PubMed] [Google Scholar]
- 6. Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. 2010;85(5):427‐432. doi: 10.4065/mcp.2009.0590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stott VL, Hurrell MA, Anderson TJ. Reversible posterior leukoencephalopathy syndrome: a misnomer reviewed. Intern Med J. 2005;35(2):83‐90. doi: 10.1111/j.1445-5994.2004.00750.x [DOI] [PubMed] [Google Scholar]
- 8. Lamy C, Oppenheim C, Méder JF, Mas JL. Neuroimaging in posterior reversible encephalopathy syndrome. J Neuroimaging. 2004;14(2):89‐96. doi: 10.1111/j.1552-6569.2004.tb00223.x [DOI] [PubMed] [Google Scholar]
- 9. Rabinstein AA, Mandrekar J, Merrell R, Kozak OS, Durosaro O, Fugate JE. Blood pressure fluctuations in posterior reversible encephalopathy syndrome. J Stroke Cerebrovasc Dis. 2012;21(4):254‐258. doi: 10.1016/j.jstrokecerebrovasdis.2011.03.011 [DOI] [PubMed] [Google Scholar]
- 10. Gao B, Lyu C, Lerner A, McKinney AM. Controversy of posterior reversible encephalopathy syndrome: what have we learnt in the last 20 years? J Neurol Neurosurg Psychiatry. 2018;89:14‐20. doi: 10.1136/jnnp-2017-316225 [DOI] [PubMed] [Google Scholar]
- 11. Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2(2):161‐192. doi: 10.1161/01.str.15.3.413 [DOI] [PubMed] [Google Scholar]
- 12. Flynn JT, Kaelber DC, Baker‐Smith CM, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents [published correction appears in Pediatrics. 2017 Nov 30] [published correction appears in Pediatrics. 2018 Sep;142(3)]. Pediatrics. 2017;140(3):e20171904. doi: 10.1542/peds.2017-1904 [DOI] [PubMed] [Google Scholar]
- 13. Seeman T, Hamdani G, Mitsnefes M. Hypertensive crisis in children and adolescents. Pediatr Nephrol. 2019;34(12):2523‐2537. doi: 10.1007/s00467-018-4092-2 [DOI] [PubMed] [Google Scholar]
- 14. Strandgaard S, Paulson OB. Cerebral autoregulation. Stroke. 1984;15(3):413‐416. doi: 10.1161/01.str.15.3.413 [DOI] [PubMed] [Google Scholar]
- 15. Belsha CW. Pediatric hypertension in the emergency department. Ann Emerg Med. 2008;51(3 Suppl):S21‐S23. doi: 10.1016/j.annemergmed.2007.11.009 [DOI] [PubMed] [Google Scholar]
- 16. Wu M, Ryan KR, Rosenthal DN, Jahadi O, Moss J, Kwiatkowski DM. The use of clevidipine for hypertension in pediatric patients receiving mechanical circulatory support. Pediatr Crit Care Med. 2020;21(12):e1134‐e1139. doi: 10.1097/PCC.0000000000002562 [DOI] [PubMed] [Google Scholar]
- 17. de Oliveira Campos JL, Bitencourt L, Pedrosa AL, et al. Renovascular hypertension in pediatric patients: update on diagnosis and management. Pediatr Nephrol. 2020;36(12):3853‐3868. doi: 10.1007/s00467-021-05063-2 [DOI] [PubMed] [Google Scholar]
- 18. Woroniecki RP, Kahnauth A, Panesar LE, Supe‐Markovina K. Left ventricular hypertrophy in pediatric hypertension: a mini review. Front Pediatr. 2017;5:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rose A, Slone S, Padron E. Relapsed acute lymphoblastic leukemia presenting as acute renal failure. Case Rep Nephrol. 2019;2019:7913027. doi: 10.1155/2019/7913027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bunchman TE, Gale GB, O'Connor DM, Salinas‐Madrigal L, Chu JY. Renal biopsy diagnosis of acute lymphocytic leukemia. Clin Nephrol. 1992;38(3):142‐144. [PubMed] [Google Scholar]
- 21. Lundberg WB, Cadman ED, Finch SC, Capizzi RL. Renal failure secondary to leukemic infiltration of the kidneys. Am J Med. 1977;62(4):636‐642. doi: 10.1016/0002-9343(77)90427-2 [DOI] [PubMed] [Google Scholar]
- 22. Luciano RL, Brewster UC. Kidney involvement in leukemia and lymphoma. Adv Chronic Kidney Dis. 2014;21(1):27‐35. doi: 10.1053/j.ackd.2013.07.004 [DOI] [PubMed] [Google Scholar]
- 23. Rosner M, Perazella M. Acute kidney injury in patients with cancer. N Engl J Med. 2017;376(18):1770‐1781. doi: 10.1056/NEJMra1613984 [DOI] [PubMed] [Google Scholar]
- 24. Lommatzsch SE, Bellizzi AM, Cathro HP, Rosner MH. Acute renal failure caused by renal infiltration by hematolymphoid malignancy. Ann Diagn Pathol. 2006;10(4):230‐234. doi: 10.1016/j.anndiagpath.2005.09.015 [DOI] [PubMed] [Google Scholar]
- 25. Obrador GT, Price B, O'Meara Y, Salant DJ. Acute renal failure due to lymphomatous infiltration of the kidneys. J Am Soc Nephrol. 1997;8(8):1348‐1354. doi: 10.1681/ASN.V881348 [DOI] [PubMed] [Google Scholar]
- 26. Buoeva A, Bouvier R. Precursor B‐cell lymphoblastic leukemia as a cause of a bilateral nephromegaly. Pediatr Nephrol. 2005;20(5):679‐682. doi: 10.1007/s00467-004-1740-5 [DOI] [PubMed] [Google Scholar]
- 27. Correa‐Rotter R, Hostetter TH, Manviel JC, Rosenberg ME. Renin expression in renal ablation. Hypertension. 1992;20:483‐490. doi: 10.1161/01.hyp.20.4.483 [DOI] [PubMed] [Google Scholar]
- 28. Olgar S, Yetgin S, Cetin M, Aras T. Can renal leukemic infiltration cause hypertension in children? J Pediatr Hematol Oncol. 2006;28(9):579‐584. doi: 10.1097/01.mph.0000212990.64435.b9 [DOI] [PubMed] [Google Scholar]
- 29. Turkman K, Altintepe L, Guney I, et al. Uncontrolled hypertension secondary to leukemic cell infiltration of kidneys in a hemodialysis patient. Int J Nephrol Renovasc Dis. 2010;3:65‐68. doi: 10.2147/ijnrd.s11077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sherief LM, Azab SF, Zakaria MM, et al. Renal presentation in pediatric acute leukemia: report of 2 cases [published correction appears in Medicine (Baltimore). 2015 Oct;94(40):1]. Medicine (Baltimore). 2015;94(37):e1461. doi: 10.1097/MD.0000000000001461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mescher C, Slungaard A. Posterior reversible encephalopathy syndrome in a postpartum woman with acute lymphoblastic leukaemia after intrathecal methotrexate. BMJ Case Rep. 2017;2017:bcr2017220429. doi: 10.1136/bcr-2017-220429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. How J, Blattner M, Fowler S, Wang‐Gillam A, Schindler SE. Chemotherapy‐associated posterior reversible encephalopathy syndrome: a case report and review of the literature. Neurologist. 2016;21(6):112‐117. doi: 10.1097/NRL.0000000000000105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patejdl R, Borchert K, Pagumbke H, et al. Posterior reversible encephalopathy syndrome (PRES): an unusual primary manifestation of a diffuse large B‐cell lymphoma. Clin Neurol Neurosurg. 2011;113:819‐821. doi: 10.1016/j.clineuro.2011.08.018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


