Abstract
Background
In patients undergoing percutaneous coronary intervention (PCI) in the SMART-CHOICE trial, P2Y12 inhibitor monotherapy after three months of dual antiplatelet therapy (DAPT) achieved clinical outcomes comparable to those of 12 months of DAPT. Nonetheless, the effects of sex on these outcomes remain unknown.
Methods
This open-label, non-inferiority, randomized study, conducted in 33 hospitals in South Korea, included 2,993 patients undergoing PCI with drug-eluting stents. Patients were randomly assigned to receive DAPT (aspirin plus a P2Y12 inhibitor) for three months then P2Y12 inhibitor alone for nine months, or DAPT for the entire 12 months. The primary endpoints were major adverse cardiac and cerebrovascular events (a composite of all-cause death, myocardial infarction, or stroke) 12 months after the index procedure. The bleeding endpoints were Bleeding Academic Research Consortium (BARC) bleeding types 2 to 5.
Results
Of the patients, 795 (26.6%) were women, who were older and had a higher prevalence of hypertension, diabetes, and dyslipidemia than men. The sexes exhibited comparable primary endpoints (adjusted hazard ratio [HR], 0.93; 95% confidence interval [CI], 0.55–1.55; P = 0.770) and bleeding endpoints (adjusted HR, 1.07; 95% CI, 0.63–1.81; P = 0.811). P2Y12 inhibitor monotherapy vs DAPT was associated with lower risk of BARC type 2 to 5 bleeding in women (adjusted HR, 0.40; 95% CI, 0.16–0.98; P = 0.045) but the difference was not statistically significant when using the Bonferroni correction. The primary endpoints were similar between treatment groups in both sexes.
Conclusion
In both sexes undergoing PCI, P2Y12 inhibitor monotherapy after three months of DAPT achieved similar risks of the primary endpoints and the bleeding events compared with prolonged DAPT. Therefore, the benefits of early aspirin withdrawal with ongoing P2Y12 inhibitors may be comparable in women and men.
Trial Registration
ClinicalTrials.gov Identifier: NCT02079194
Keywords: Women, Dual Antiplatelet Therapy, P2Y12 Inhibitor, Percutaneous Coronary Intervention, Drug-Eluting Stent
Graphical Abstract
INTRODUCTION
Dual antiplatelet therapy (DAPT) with aspirin plus a P2Y12 inhibitor is the current standard antiplatelet therapy after percutaneous coronary intervention (PCI) with drug-eluting stents.1,2 However, prolonged DAPT increases the risk of bleeding, which offsets the benefits of reducing recurrent ischemic events.3,4,5,6 Short-term DAPT, in contrast, is associated with an increased risk of myocardial infarction and stent thrombosis, as reported in meta-analyses.6,7 A recent trial, Comparison Between P2Y12 Antagonist Monotherapy and Dual Antiplatelet Therapy in Patients Undergoing Implantation of Coronary Drug-eluting Stents (SMART-CHOICE),8 revealed that among patients undergoing PCI, P2Y12 inhibitor monotherapy after three months of DAPT achieved noninferior rates of major adverse cardiac and cerebrovascular events compared to prolonged DAPT. Bleeding rates (Bleeding Academic Research Consortium [BARC] types 2 to 5) were significantly lower in the P2Y12 inhibitor monotherapy group than in the DAPT group. Nonetheless, it remains unclear whether these effects vary with sex. Further, while women have higher risk of ischemic and bleeding events after the early period of PCI than men,9,10,11 it remains unclear whether they are also at a greater risk of these events during the early period of P2Y12 inhibitor monotherapy after PCI with drug-eluting stents. Therefore, we performed a pre-specified secondary analysis to explore sex differences in the SMART-CHOICE population, and to evaluate possible associations between sex and clinical outcomes following administration of P2Y12 inhibitor monotherapy after three months of DAPT in patients undergoing PCI.
METHODS
Study design
The SMART-CHOICE trial was an investigator-initiated, multicenter, open-label, non-inferiority, randomized study performed at 33 sites in South Korea. The trial, described previously,8,12 was designed by the steering committee and coordinated by the Academic Clinical Research Organization of Samsung Medical Center (Seoul, Korea). The institutional review board of each participating center approved the trial protocol. The independent data and safety monitoring board oversaw trial safety. The SMART-CHOICE trial started on March 18, 2014, and follow-up was completed on July 19, 2018.
Study population and regimen
Eligible patients were aged 20 years or older, had one or more coronary artery stenoses of 50% or greater in the native coronary artery, and underwent PCI. Patients were randomly assigned to the P2Y12 inhibitor monotherapy group (aspirin plus a P2Y12 inhibitor for three months and thereafter a P2Y12 inhibitor alone) or the DAPT group (aspirin plus a P2Y12 inhibitor for at least 12 months) at a 1:1 ratio. Enrollment and random assignment were conducted at the index procedure or at the follow-up visit within three months after the index procedure.
Outcomes
The primary endpoints were major adverse cardiac and cerebrovascular events, defined as a composite of all-cause death, myocardial infarction, or stroke 12 months after the index procedure. Bleeding endpoints included BARC bleeding types of 2 to 5 at 12 months after the index procedure. The clinical events have previously been described in detail.8
Statistical analysis
We summarized the clinical and procedural characteristics by sex and the randomly assigned treatment group. Continuous variables were represented using means and standard deviations, while categorical variables were expressed as numbers and percentages. The analysis of the primary endpoint and occurrences of bleeding events was conducted on the intention-to-treat population. We used the Kaplan-Meier method to estimate the cumulative occurrence of the primary endpoints. If patients did not experience the primary endpoints within the timeframe from randomization to one year, their data was treated as incomplete either at the time of their demise or at their last known point of contact, whichever came first. We employed Cox proportional hazards models to determine hazard ratios (HRs) along with 95% confidence intervals (CIs). Additionally, Cox regression was employed to explore the links between sex and clinical outcomes. The models were adjusted for variables displaying baseline differences, including age, body-mass index, hypertension, diabetes mellitus, dyslipidemia, current smoking, previous revascularization, previous myocardial infarction, chronic renal failure, left ventricular ejection fraction, clinical presentation of ST-segment elevation myocardial infarction, transradial approach, multivessel disease, left main disease, left anterior descending artery disease, and thrombotic lesion. Treatment outcomes of P2Y12 inhibitor monotherapy or DAPT were evaluated based on sex, and formal interaction-testing using Cox regression was performed to assess association modification. To account for conducting multiple tests, we used the Bonferroni method. P values were two-sided, and statistical significance was set at P < 0.05. All statistical analyses were performed using R (version 4.2.2; R Foundation for Statistical Computing, Vienna, Austria). The data were analyzed between September and December 2022. This study was registered with ClinicalTrials.gov, Identifier: NCT02079194.
Ethics statement
The present study protocol was reviewed and approved by the Institutional Review Boards at Samsung Medical Center (IRB No. 2014-01-161) and all participating centers. Informed consent was submitted by all subjects when they were enrolled.
RESULTS
Clinical and procedural characteristics
In total, 2993 patients were enrolled, of whom 795 (26.6%) were women. Table 1 shows the baseline clinical and procedural characteristics according to sex. The women were older than the men (mean [SD] age in years: 69.7 [9.4] vs. 62.6 [10.5]) and were more likely to have a higher prevalence of hypertension, diabetes, and dyslipidemia. Conversely, women were less likely to be current smokers and have a clinical presentation of ST-segment elevation myocardial infarction, right coronary artery lesion, or thrombotic lesion characteristics (Table 1). No significant differences were noted in the extent of coronary artery disease, number of stents used, stent length, or type of drug-eluting stents between the sexes (Table 1). Table 2 shows the baseline clinical and procedural characteristics according to sex and randomized treatment assignment. Among the women, chronic renal failure and left main lesions were more prevalent in the DAPT group. Among the men, body mass index was higher in the DAPT group, and current smoking was more common in the P2Y12 inhibitor monotherapy group (Table 2). The other baseline clinical characteristics were well balanced between the treatment groups. Clopidogrel was used as the P2Y12 inhibitor monotherapy in 356 women (87.2%) and 809 men (74.5%). In the DAPT group, clopidogrel was used in 344 women (88.9%) and 832 men (74.9%).
Table 1. Baseline clinical and procedural characteristics by sex.
| Variables | Women (n = 795) | Men (n = 2,198) | P value | ||
|---|---|---|---|---|---|
| Age, yr | 69.7 (9.4) | 62.6 (10.5) | < 0.001 | ||
| Age ≥ 75 | 272 (34.2) | 300 (13.6) | < 0.001 | ||
| Body-mass index, kg/m2 | 24.4 ± 3.3 | 24.7 ± 3.1 | 0.029 | ||
| Comorbidities | |||||
| Hypertension | 562 (70.8) | 1,278 (58.1) | < 0.001 | ||
| Diabetes mellitus | 343 (43.2) | 779 (35.5) | < 0.001 | ||
| Dyslipidemia | 404 (51.0) | 948 (43.3) | < 0.001 | ||
| Current smoking | 44 (5.5) | 747 (34.0) | < 0.001 | ||
| Previous revascularization | 81 (10.2) | 268 (12.2) | 0.151 | ||
| Previous stroke | 62 (7.8) | 139 (6.3) | 0.178 | ||
| Previous myocardial infarction | 27 (3.4) | 100 (4.6) | 0.202 | ||
| Chronic renal failure | 28 (3.5) | 69 (3.1) | 0.681 | ||
| Left ventricular ejection fraction, % | 61.1 (11.2) | 59.5 (10.6) | 0.001 | ||
| Clinical presentation | |||||
| Stable angina | 332 (41.8) | 918 (41.8) | > 0.999 | ||
| Unstable angina | 281 (35.3) | 677 (30.8) | 0.022 | ||
| Non–ST-segment elevation myocardial infarction | 119 (15.0) | 350 (15.9) | 0.557 | ||
| ST-segment elevation myocardial infarction | 63 (7.9) | 251 (11.4) | 0.007 | ||
| Transradial approach | 558 (70.2) | 1,624 (73.9) | 0.050 | ||
| Multiple vessels disease | 379 (47.7) | 1,104 (50.2) | 0.233 | ||
| No. of lesion treated | 1.4 (0.6) | 1.4 (0.7) | 0.582 | ||
| Location of lesions | |||||
| Left main | 13 (1.6) | 45 (2.0) | 0.567 | ||
| Left anterior descending artery | 519 (65.3) | 1,334 (60.7) | 0.025 | ||
| Left circumflex | 205 (25.8) | 570 (25.9) | 0.973 | ||
| Right coronary artery | 251 (31.6) | 797 (36.3) | 0.020 | ||
| Lesion complexity | |||||
| Calcified | 132 (16.6) | 332 (15.1) | 0.353 | ||
| Bifurcation | 89 (11.2) | 291 (13.3) | 0.150 | ||
| Thrombotic | 44 (5.5) | 178 (8.1) | 0.022 | ||
| Use of intravascular ultrasonography | 199 (25.2) | 579 (26.4) | 0.532 | ||
| Treated lesions per patient | 0.412 | ||||
| 1 | 556 (69.9) | 1,550 (70.5) | |||
| 2 | 193 (24.3) | 487 (22.2) | |||
| 3 | 40 (5.0) | 137 (6.2) | |||
| ≥ 4 | 6 (0.8) | 24 (1.1) | |||
| Multilesion intervention | 239 (30.1) | 648 (29.5) | 0.793 | ||
| Multivessel intervention | 185 (23.3) | 520 (23.7) | 0.864 | ||
| No. of stents per patient | 0.184 | ||||
| 1 | 520 (65.5) | 1,441 (65.6) | |||
| 2 | 210 (26.4) | 540 (24.6) | |||
| 3 | 51 (6.4) | 165 (7.5) | |||
| ≥ 4 | 13 (1.6) | 52 (2.4) | |||
| Stent length per patient, mm | 36.7 (21.4) | 38.3 (23.2) | 0.085 | ||
| Type of drug-eluting stents | 0.489 | ||||
| Cobalt-chromium everolimus eluting | 262 (33.0) | 789 (35.9) | |||
| Platinum-chromium everolimus eluting | 268 (33.7) | 699 (31.8) | |||
| Sirolimus-eluting with biodegradable polymer | 265 (33.3) | 707 (32.2) | |||
| Zotarolimus eluting | 0 | 1 (0.0) | |||
| Paclitaxel-cilostazol eluting | 0 | 1 (0.0) | |||
| Medications at discharge | |||||
| Aspirin | 792 (99.6) | 2,196 (99.9) | 0.103 | ||
| P2Y12 receptor inhibitor | |||||
| Clopidogrel | 689 (86.7) | 1,622 (73.8) | < 0.001 | ||
| Prasugrel | 9 (1.1) | 120 (5.5) | < 0.001 | ||
| Ticagrelor | 96 (12.1) | 456 (20.7) | < 0.001 | ||
| Statin | 755 (95.0) | 2,069 (94.2) | 0.457 | ||
| Angiotensin-converting enzyme inhibitor | 135 (17.0) | 392 (17.9 | 0.632 | ||
| Angiotensin receptor inhibitor | 325 (41.0) | 836 (38.1) | 0.164 | ||
| β-Blocker | 431 (54.3) | 1,147 (52.2) | 0.342 | ||
Values are presented as number (%) or mean ± standard deviation.
Table 2. Baseline clinical and procedural characteristics by sex and randomized treatment assignment.
| Variables | Women (n = 795) | Men (n = 2,198) | ||||||
|---|---|---|---|---|---|---|---|---|
| P2Y12 inhibitor monotherapy (n = 408) | Dual antiplatelet therapy (n = 387) | P value | P2Y12 inhibitor monotherapy (n = 1,087) | Dual antiplatelet therapy (n = 1,111) | P value | |||
| Age, yr | 69.7 ± 9.3 | 69.7 ± 9.6 | 0.956 | 62.7 ± 10.6 | 62.5 ± 10.4 | 0.622 | ||
| ≥ 75 | 132 (32.4) | 140 (36.2) | 0.289 | 150 (13.8) | 150 (13.5) | 0.888 | ||
| Body-mass index, kg/m2 | 24.4 ± 3.3 | 24.3 ± 3.3 | 0.708 | 24.5 ± 3.0 | 24.8 ± 3.1 | 0.032 | ||
| Comorbidities | ||||||||
| Hypertension | 288 (70.8) | 274 (70.8) | > 0.999 | 633 (58.2) | 645 (58.1) | 0.967 | ||
| Diabetes mellitus | 179 (44.0) | 164 (42.4) | 0.701 | 391 (36.0) | 388 (34.9) | 0.617 | ||
| Dyslipidemia | 203 (50.0) | 201 (52.1) | 0.609 | 470 (43.3) | 478 (43.3) | > 0.999 | ||
| Current smoking | 26 (6.4) | 18 (4.7) | 0.370 | 398 (36.7) | 349 (31.5) | 0.011 | ||
| Previous revascularization | 34 (8.4) | 47 (12.1) | 0.100 | 138 (12.7) | 130 (11.7) | 0.512 | ||
| Previous stroke | 35 (8.6) | 27 (7.0) | 0.472 | 64 (5.9) | 75 (6.8) | 0.461 | ||
| Previous myocardial infarction | 13 (3.2) | 14 (3.6) | 0.894 | 49 (4.5) | 51 (4.6) | > 0.999 | ||
| Chronic renal failure | 7 (1.7) | 21 (5.4) | 0.008 | 37 (3.4) | 32 (2.9) | 0.561 | ||
| Left ventricular ejection fraction, % | 61.3 ± 10.8 | 60.9 ± 11.5 | 0.570 | 59.5 ± 10.8 | 59.6 ± 10.4 | 0.869 | ||
| Clinical presentation | ||||||||
| Stable angina | 167 (40.9) | 165 (42.6) | 0.678 | 458 (42.1) | 460 (41.5) | 0.789 | ||
| Unstable angina | 145 (35.5) | 136 (35.1) | 0.966 | 322 (29.6) | 355 (32.0) | 0.244 | ||
| Non–ST-segment elevation myocardial infarction | 57 (14.0) | 62 (16.0) | 0.477 | 182 (16.7) | 168 (15.1) | 0.336 | ||
| ST-segment elevation myocardial infarction | 39 (9.6) | 24 (6.2) | 0.105 | 125 (11.5) | 126 (11.4) | 0.972 | ||
| Transradial approach | 289 (70.8) | 269 (69.5) | 0.741 | 802 (73.8) | 822 (74.0) | 0.951 | ||
| Multiple vessels disease | 198 (48.5) | 181 (46.8) | 0.671 | 551 (50.7) | 553 (49.8) | 0.699 | ||
| No. of lesion treated | 1.4 (0.6) | 1.4 (0.6) | 0.787 | 1.4 (0.7) | 1.4 (0.7) | 0.385 | ||
| Location of lesions | ||||||||
| Left main | 2 (0.5) | 11 (2.8) | 0.020 | 21 (1.9) | 24 (2.2) | 0.820 | ||
| Left anterior descending artery | 263 (64.5) | 256 (66.1) | 0.670 | 640 (58.9) | 694 (62.5) | 0.093 | ||
| Left circumflex | 115 (28.2) | 90 (23.3) | 0.132 | 284 (26.1) | 286 (25.7) | 0.875 | ||
| Right coronary artery | 125 (30.6) | 126 (32.6) | 0.613 | 399 (36.7) | 398 (35.8) | 0.699 | ||
| Lesion complexity | ||||||||
| Calcified | 72 (17.6) | 60 (15.5) | 0.474 | 163 (15.0) | 169 (15.2) | 0.942 | ||
| Bifurcation | 45 (11.0) | 44 (11.4) | 0.969 | 154 (14.2) | 137 (12.3) | 0.221 | ||
| Thrombotic | 26 (6.4) | 18 (4.7) | 0.365 | 84 (7.7) | 94 (8.5) | 0.590 | ||
| Use of intravascular ultrasonography | 105 (25.9) | 94 (24.4) | 0.684 | 267 (24.6) | 312 (28.2) | 0.068 | ||
| Treated lesions per patient | 0.995 | 0.807 | ||||||
| 1 | 287 (70.3) | 269 (69.5) | 778 (71.6) | 772 (69.5) | ||||
| 2 | 98 (24.0) | 95 (24.5) | 231 (21.3) | 256 (23.0) | ||||
| 3 | 20 (4.9) | 20 (5.2) | 66 (6.1) | 71 (6.4) | ||||
| ≥ 4 | 3 (0.7) | 3 (0.8) | 12 (1.1) | 12 (1.1) | ||||
| Multilesion intervention | 121 (29.7) | 118 (30.5) | 0.858 | 309 (28.4) | 339 (30.5) | 0.305 | ||
| Multivessel intervention | 93 (22.8) | 92 (23.8) | 0.808 | 244 (22.4) | 276 (24.8) | 0.204 | ||
| No. of stents per patient | 0.753 | 0.355 | ||||||
| 1 | 261 (64.0) | 259 (67.1) | 721 (66.3) | 720 (64.8) | ||||
| 2 | 112 (27.5) | 98 (25.4) | 263 (24.2) | 277 (24.9) | ||||
| 3 | 27 (6.6) | 24 (6.2) | 75 (6.9) | 90 (8.1) | ||||
| ≥ 4 | 8 (2.0) | 5 (1.3) | 28 (2.6) | 20 (1.8) | ||||
| Stent length per patient, mm | 37.1 (21.0) | 36.4 (21.9) | 0.626 | 38.2 (23.2) | 38.4 (23.3) | 0.907 | ||
| Type of drug-eluting stents | 0.951 | 0.534 | ||||||
| Cobalt-chromium everolimus eluting | 136 (33.3) | 126 (32.6) | 389 (35.8) | 400 (36.0) | ||||
| Platinum-chromium everolimus eluting | 138 (33.8) | 130 (33.6) | 351 (32.3) | 348 (31.3) | ||||
| Sirolimus-eluting with biodegradable polymer | 134 (32.8) | 131 (33.9) | 347 (31.9) | 360 (32.4) | ||||
| Zotarolimus eluting | 0 | 0 | 0 | 1 (0.0) | ||||
| Paclitaxel-cilostazol eluting | 0 | 0 | 0 | 1 (0.0) | ||||
| Medications at discharge | ||||||||
| Aspirin | 406 (99.5) | 386 (99.7) | > 0.999 | 1,086 (99.9) | 1,110 (99.9) | > 0.999 | ||
| P2Y12 receptor inhibitor | ||||||||
| Clopidogrel | 351 (86.0) | 338 (87.3) | 0.661 | 798 (73.0) | 824 (74.2) | 0.583 | ||
| Prasugrel | 5 (1.2) | 4 (1.0) | > 0.999 | 57 (5.2) | 63 (5.7) | 0.729 | ||
| Ticagrelor | 51 (12.5) | 45 (11.6) | 0.788 | 232 (21.3) | 224 (20.4) | 0.495 | ||
| Statin | 385 (94.4) | 370 (95.6) | 0.522 | 1,031 (94.8) | 1,038 (93.5) | 0.213 | ||
| Angiotensin-converting enzyme inhibitor | 69 (17.0) | 66 (17.1) | > 0.999 | 202 (18.6) | 190 (17.1) | 0.394 | ||
| Angiotensin receptor inhibitor | 178 (43.8) | 147 (38.0) | 0.109 | 423 (39.0) | 413 (37.2) | 0.435 | ||
| β-Blocker | 219 (53.8) | 212 (54.8) | 0.839 | 576 (53.0) | 571 (51.5) | 0.508 | ||
Values are presented as number (%) or mean ± standard deviation.
Sex-based outcomes
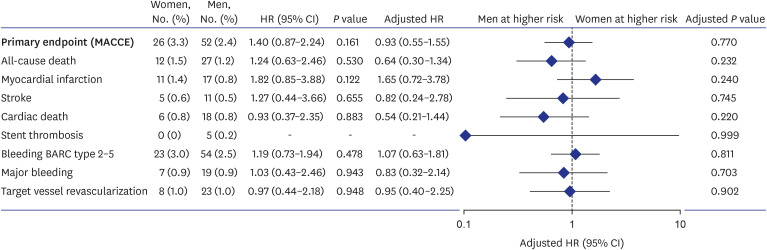
The incidence of primary endpoints at 12 months is shown in Fig. 1. No statistically significant differences were found between the sexes in the incidence of all-cause death, myocardial infarction, or stroke. There was no significant difference in the risk of bleeding between the sexes. After multivariable adjustment, these results remained unchanged, suggesting that the association between sex and risk was not significant (Fig. 1). No significant differences between men and women were observed in the incidence of BARC type 2 to 5 bleeding or major bleeding. After multivariate adjustment, these results remained unchanged.
Fig. 1. Primary composite endpoints and bleeding events 12 months after randomization based on sex. Men were used as the reference category. Adjusted HRs were calculated for age, Body-mass index, hypertension, diabetes mellitus, dyslipidemia, current smoking, previous revascularization, previous myocardial infarction, chronic renal failure, left ventricular ejection fraction, clinical presentation of ST-segment elevation myocardial infarction, transradial approach, multivessel disease, left main disease, left anterior descending artery disease, and thrombotic lesion. The primary composite endpoints and bleeding outcomes were assessed in the intention-to-treat cohort.
HR = hazard ratio, CI = confidence interval, BARC = Bleeding Academic Research Consortium, MACCE = major adverse cardiac and cerebrovascular events.
Outcomes based on sex and randomized treatment assignment
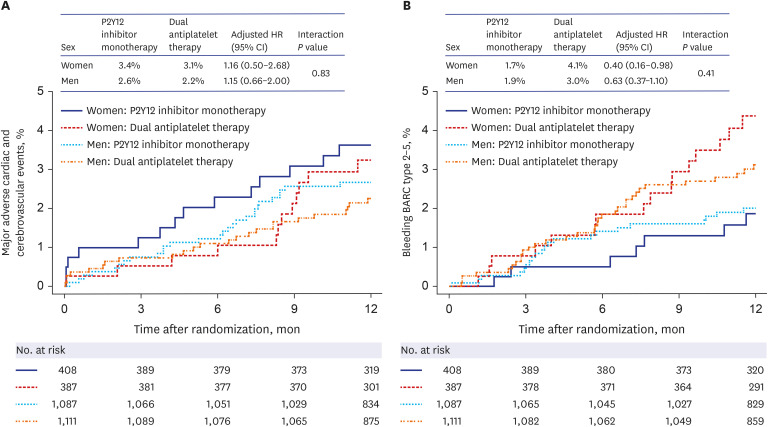
Table 3 shows the clinical outcomes based on sex and randomized assignment of the 12-month treatment regimen. In both sexes, the risks of the primary endpoints in the P2Y12 inhibitor monotherapy group were comparable with those in the DAPT group, with no significant association between randomized treatment assignment and sex (P for the interaction = 0.834) (Fig. 2A). In women, the risk of bleeding was lower in the P2Y12 inhibitor monotherapy group than in the DAPT group (7 patients [1.7%] vs. 16 patients [4.1%], respectively; adjusted HR, 0.40; 95% CI, 0.16–0.98; P = 0.045). However, the difference was not statistically significant when using the Bonferroni correction and no significant association between randomized treatment assignment and sex (P for interaction = 0.410) (Fig. 2B). In both sexes, P2Y12 inhibitor monotherapy and DAPT were associated with similar rates of target-vessel revascularization.
Table 3. Clinical outcomes by sex and randomized treatment assignment at 12 months after randomization.
| Variables | Women (n = 795), No. (%)a | Men (n = 2,198), No. (%)a | P for interactionc | Adjusted P for interactionc | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y12 inhibitor monotherapy (n = 408) | Dual antiplatelet therapy (n = 387) | HR (95% CI)c | P value | Adjusted HR (95% CI)c | P value | P2Y12 inhibitor monotherapy (n = 1,087) | Dual antiplatelet therapy (n = 1,111) | HR (95% CI)c | P value | Adjusted HR (95% CI)c | P value | |||
| Primary endpoint (MACCE)a | 14 (3.4) | 12 (3.1) | 1.14 (0.53–2.47) | 0.732 | 1.16 (0.50–2.68) | 0.727 | 28 (2.6) | 24 (2.2) | 1.20 (0.70–2.07) | 0.514 | 1.15 (0.66–2.00) | 0.620 | 0.922 | 0.834 |
| All-cause death | 7 (1.7) | 5 (1.3) | 1.36 (0.43–4.29) | 0.597 | 1.85 (0.51–6.77) | 0.353 | 14 (1.3) | 13 (1.2) | 1.10 (0.52–2.35) | 0.798 | 1.14 (0.53–2.48) | 0.734 | 0.761 | 0.875 |
| Myocardial infarction | 3 (0.7) | 8 (2.1) | 0.37 (0.10–1.38) | 0.139 | 0.38 (0.10–1.50) | 0.169 | 8 (0.7) | 9 (0.8) | 0.91 (0.35–2.36) | 0.847 | 0.83 (0.32–2.17) | 0.707 | 0.275 | 0.331 |
| Stroke | 4 (1.0) | 1 (0.3) | 3.95 (0.44–35.35) | 0.219 | 3.07 (0.32–29.76) | 0.332 | 7 (0.6) | 4 (0.4) | 1.79 (0.53–6.13) | 0.351 | 1.60 (0.46–5.55) | 0.459 | 0.542 | 0.534 |
| Cardiac death | 2 (0.5) | 4 (1.0) | 0.49 (0.09–2.69) | 0.413 | 0.54 (0.09–3.17) | 0.496 | 9 (0.8) | 9 (0.8) | 1.02 (0.41–2.58) | 0.960 | 0.97 (0.38–2.45) | 0.960 | 0.453 | 0.540 |
| Stent thrombosis | 0 | 0 | - | - | - | - | 3 (0.3) | 2 (0.2) | 1.53 (0.26–9.18) | 0.639 | 1.73 (0.24–12.69) | 0.590 | ||
| Bleeding BARC type 2-5 | 7 (1.7) | 16 (4.1) | 0.42 (0.17–1.03) | 0.058 | 0.40 (0.16–0.98) | 0.045 | 21 (1.9) | 33 (3.0) | 0.65 (0.38–1.12) | 0.122 | 0.63 (0.37–1.10) | 0.104 | 0.420 | 0.410 |
| Major bleedingb | 5 (1.2) | 2 (0.5) | 2.49 (0.48–12.81) | 0.276 | 2.21 (0.42–11.65) | 0.348 | 7 (0.6) | 12 (1.1) | 0.60 (0.24–1.52) | 0.278 | 0.58 (0.23–1.48) | 0.256 | 0.140 | 0.159 |
| Target vessel revascularization | 4 (1.0) | 4 (1.0) | 0.99 (0.25–3.94) | 0.984 | 1.02 (0.24–4.31) | 0.980 | 8 (0.7) | 15 (1.4) | 0.55 (0.12–1.29) | 0.169 | 0.44 (0.18–1.09) | 0.076 | 0.488 | 0.324 |
Data are presented for the intention-to-treat population. The percentages are Kaplan-Meier estimates.
HR = hazard ratio, CI = confidence interval, MACCE = major adverse cardiac and cerebrovascular events, BARC = Bleeding Academic Research Consortium.
aA composite of all-cause mortality, myocardial infarction, or stroke.
bBARC type 3 to 5 bleeding.
Primary endpoint is defined as a composite of all-cause death, myocardial infarction, or stroke at 12 months after the index procedure.
cModel adjusted for age, Body-mass index, hypertension, diabetes mellitus, dyslipidemia, current smoking, previous revascularization, previous myocardial infarction, chronic renal failure, left ventricular ejection fraction, clinical presentation of ST-segment elevation myocardial infarction, transradial approach, multivessel disease, left main disease, Left anterior descending artery disease, and thrombotic lesion.
Fig. 2. Primary composite endpoints and bleeding events based on sex and randomized treatment assignment. Kaplan-Meier estimates and adjusted HRs for the primary composite endpoints (A) and bleeding events (B) 12 months after randomization. Data were adjusted for age, body-mass index, hypertension, diabetes mellitus, dyslipidemia, current smoking, previous revascularization, previous myocardial infarction, chronic renal failure, left ventricular ejection fraction, clinical presentation of ST-segment elevation myocardial infarction, transradial approach, multivessel disease, left main disease, left anterior descending artery disease, and thrombotic lesion.
HR = hazard ratio, CI = confidence interval.
DISCUSSION
In this pre-specified subgroup analysis, there were substantial differences in baseline characteristics between the sexes, including significantly higher age and risk-factor prevalence in women than in men. During early P2Y12 inhibitor monotherapy after PCI with drug-eluting stents, the sexes exhibited similar incidences of primary composite endpoints. Although women experienced more bleeding events with prolonged DAPT after PCI, the difference was not statistically significant when using the Bonferroni correction. After adjusting for baseline and procedural characteristics, the results remained unchanged. No significant associations were observed between randomized treatment assignment and sex in terms of primary composite and bleeding endpoints.
Although sex-based studies have consistently shown that women have higher crude or unadjusted incidences of ischemic and bleeding events after PCI,9,10,11,13,14,15 several studies have not detected similar associations.16,17,18 These discrepancies might reflect differences in baseline comorbidities, particularly higher age and prevalence of diabetes, hypertension, and renal insufficiency in women, rather than biological factors.19,20,21 When potent P2Y12 inhibitors were administered from the early period after PCI and DAPT was maintained, the frequency of ischemic events was not further reduced, while bleeding events increased in frequency; this phenomenon was more prominent in women than in men.10,11,22 Recently, the Ticagrelor With Aspirin or Alone in High-Risk Patients After Coronary Intervention (TWILIGHT) study11 revealed that, among patients undergoing PCI, ticagrelor monotherapy after three months of DAPT achieved a reduction in bleeding events (BARC types 2, 3, or 5) without an increase in ischemic events, compared with continued DAPT. In the TWILIGHT subgroup analyses, relative to ticagrelor plus aspirin, ticagrelor plus placebo was associated with lower risk of BARC types 2, 3, or 5 bleeding in women (adjusted HR, 0.62; 95% CI, 0.42–0.92; P = 0.020) and men (adjusted HR, 0.57; 95% CI, 0.44–0.73; P < 0.001; P for interaction = 0.690).
The SMART-CHOICE trial of patients receiving current generation drug-eluting stents compared P2Y12 inhibitor monotherapy (clopidogrel in 76.9% of patients) after three months of DAPT, with DAPT for 12 months. The former regimen achieved noninferior results for the primary endpoints (major adverse cardiac and cerebrovascular events) 12 months after the index procedures, and was associated with a lower rate of bleeding. These results are important as they reveal the safety and efficacy of P2Y12 inhibitor monotherapy during the early period after PCI compared with those of DAPT.8 Compared with DAPT, P2Y12 inhibitor monotherapy three months after PCI was associated with a lower risk of bleeding. However, the results were not evaluated across sex subgroups, particularly for the early period after PCI with drug-eluting stents. This study found no sex differences in the primary endpoints and the bleeding endpoints of patients after PCI in randomized treatment assignment with prolonged DAPT. These results indicate that the benefits of early aspirin withdrawal with continuation of a P2Y12 inhibitor (primarily clopidogrel) were similar for both women and men.
This study has some limitations. Although the subgroup analysis was pre-specified, it should only be considered as hypothesis-generating research, and requires confirmation in future studies. Randomization was not stratified based on sex, and multiplicity was not accounted for, increasing the possibility of error in statistical decision-making. The female subgroup had fewer participants. There were substantial differences in baseline risks between the sexes, and some differences between the sex-specific treatment groups in patient characteristics, in terms of the prevalence of diabetes, hypertension, or dyslipidemia. Despite multivariate adjustments for baseline differences, we were unable to exclude residual confounding factors such as anemia and proton pump inhibitors that reduce the risk of gastrointestinal bleeding. Neither of the sex-specific subgroups was sufficiently powered to draw definitive conclusions regarding the effects of P2Y12 inhibitor monotherapy versus DAPT on the primary composite and bleeding endpoints. Furthermore, our findings are specific to the population in the early period after PCI with drug-eluting stents, and may not be extrapolated to a broader population of patients undergoing PCI.
In both sexes undergoing PCI, P2Y12 inhibitor monotherapy after three months of DAPT achieved similar risks of the primary endpoints and the bleeding events compared with prolonged DAPT. These findings suggest that the benefits of early aspirin withdrawal with continuation of a P2Y12 inhibitor are similar in women and men. Considering the limitations of the study, further research is needed to better understand how early P2Y12 inhibitor monotherapy affects clinical outcomes based on sex differences.
Footnotes
Funding: This study was supported by unrestricted grants from the Korean Society of Interventional Cardiology (grant 2013-3), Abbott Vascular, Biotronik, and Boston Scientific.
The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosure: Joo-Yong Hahn reports receiving grants from Abbott Vascular, Boston Scientific, Biotronik, Korean Society of Interventional Cardiology, and Medtronic; and speaker’s fees from AstraZeneca, Daiichi Sankyo, and Sanofi-Aventis. Hyeon-Cheol Gwon reports receiving research grants from Abbott Vascular, Boston Scientific, and Medtronic; and speaker’s fees from Abbott Vascular, Boston Scientific, and Medtronic. All other authors declare that they have no conflicts of interest.
Data Sharing Statement: Data sharing is not applicable because consent of subjected was not obtained for data sharing.
- Conceptualization: Shin ES, Hahn JY, Song YB, Gwon HC.
- Data curation: Shin ES, Kim B, Yang JH, Choi SH, Lee SH, Gwon HC.
- Formal analysis: Shin ES, Kim B, Song YB, Lee JM, Park TK, Gwon HC.
- Funding acquisition: Shin ES, Lee JM, Park TK, Yang JH, Choi SH, Lee SH.
- Investigation: Shin ES, Hahn JY, Song YB, Park TK, Lee SH.
- Methodology: Shin ES, Kim B, Song YB.
- Project administration: Hahn JY, Choi JH, Choi SH.
- Resources: Hahn JY, Song YB, Lee JM, Park TK, Choi JH, Choi SH, Lee SH, Gwon HC.
- Software: Shin ES, Kim B, Song YB.
- Supervision: Shin ES, Hahn JY, Gwon HC.
- Validation: Hahn JY.
- Visualization: Shin ES, Hahn JY, Song YB, Gwon HC.
- Writing - original draft: Shin ES, Her AY, Hahn JY, Song YB, Gwon HC.
- Writing - review & editing: Shin ES, Her AY, Gwon HC.
References
- 1.Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Thorac Cardiovasc Surg. 2016;152(5):1243–1275. doi: 10.1016/j.jtcvs.2016.07.044. [DOI] [PubMed] [Google Scholar]
- 2.Capodanno D, Alfonso F, Levine GN, Valgimigli M, Angiolillo DJ. ACC/AHA versus ESC guidelines on dual antiplatelet therapy: JACC guideline comparison. J Am Coll Cardiol. 2018;72(23 Pt A):2915–2931. doi: 10.1016/j.jacc.2018.09.057. [DOI] [PubMed] [Google Scholar]
- 3.Mehran R, Pocock SJ, Stone GW, Clayton TC, Dangas GD, Feit F, et al. Associations of major bleeding and myocardial infarction with the incidence and timing of mortality in patients presenting with non-ST-elevation acute coronary syndromes: a risk model from the ACUITY trial. Eur Heart J. 2009;30(12):1457–1466. doi: 10.1093/eurheartj/ehp110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ndrepepa G, Schuster T, Hadamitzky M, Byrne RA, Mehilli J, Neumann FJ, et al. Validation of the Bleeding Academic Research Consortium definition of bleeding in patients with coronary artery disease undergoing percutaneous coronary intervention. Circulation. 2012;125(11):1424–1431. doi: 10.1161/CIRCULATIONAHA.111.060871. [DOI] [PubMed] [Google Scholar]
- 5.Giustino G, Baber U, Sartori S, Mehran R, Mastoris I, Kini AS, et al. Duration of dual antiplatelet therapy after drug-eluting stent implantation: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2015;65(13):1298–1310. doi: 10.1016/j.jacc.2015.01.039. [DOI] [PubMed] [Google Scholar]
- 6.Palmerini T, Benedetto U, Bacchi-Reggiani L, Della Riva D, Biondi-Zoccai G, Feres F, et al. Mortality in patients treated with extended duration dual antiplatelet therapy after drug-eluting stent implantation: a pairwise and Bayesian network meta-analysis of randomised trials. Lancet. 2015;385(9985):2371–2382. doi: 10.1016/S0140-6736(15)60263-X. [DOI] [PubMed] [Google Scholar]
- 7.Palmerini T, Benedetto U, Biondi-Zoccai G, Della Riva D, Bacchi-Reggiani L, Smits PC, et al. Long-term safety of drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. J Am Coll Cardiol. 2015;65(23):2496–2507. doi: 10.1016/j.jacc.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Hahn JY, Song YB, Oh JH, Chun WJ, Park YH, Jang WJ, et al. Effect of P2Y12 inhibitor monotherapy vs dual antiplatelet therapy on cardiovascular events in patients undergoing percutaneous coronary intervention: the SMART-CHOICE randomized clinical trial. JAMA. 2019;321(24):2428–2437. doi: 10.1001/jama.2019.8146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehran R, Chandrasekhar J, Urban P, Lang IM, Windhoevel U, Spaulding C, et al. Sex-based outcomes in patients with a high bleeding risk after percutaneous coronary intervention and 1-month dual antiplatelet therapy: a secondary analysis of the LEADERS FREE randomized clinical trial. JAMA Cardiol. 2020;5(8):939–947. doi: 10.1001/jamacardio.2020.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chichareon P, Modolo R, Kerkmeijer L, Tomaniak M, Kogame N, Takahashi K, et al. Association of sex with outcomes in patients undergoing percutaneous coronary intervention: a subgroup analysis of the GLOBAL LEADERS randomized clinical trial. JAMA Cardiol. 2020;5(1):21–29. doi: 10.1001/jamacardio.2019.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogel B, Baber U, Cohen DJ, Sartori S, Sharma SK, Angiolillo DJ, et al. Sex differences among patients with high risk receiving ticagrelor with or without aspirin after percutaneous coronary intervention: a subgroup analysis of the TWILIGHT randomized clinical trial. JAMA Cardiol. 2021;6(9):1032–1041. doi: 10.1001/jamacardio.2021.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song YB, Oh SK, Oh JH, Im ES, Cho DK, Cho BR, et al. Rationale and design of the comparison between a P2Y12 inhibitor monotherapy versus dual antiplatelet therapy in patients undergoing implantation of coronary drug-eluting stents (SMART-CHOICE): A prospective multicenter randomized trial. Am Heart J. 2018;197:77–84. doi: 10.1016/j.ahj.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Yu J, Mehran R, Grinfeld L, Xu K, Nikolsky E, Brodie BR, et al. Sex-based differences in bleeding and long term adverse events after percutaneous coronary intervention for acute myocardial infarction: three year results from the HORIZONS-AMI trial. Catheter Cardiovasc Interv. 2015;85(3):359–368. doi: 10.1002/ccd.25630. [DOI] [PubMed] [Google Scholar]
- 14.Spirito A, Gragnano F, Corpataux N, Vaisnora L, Galea R, Svab S, et al. Sex-based differences in bleeding risk after percutaneous coronary intervention and implications for the academic research consortium high bleeding risk criteria. J Am Heart Assoc. 2021;10(12):e021965. doi: 10.1161/JAHA.121.021965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hess CN, McCoy LA, Duggirala HJ, Tavris DR, O’Callaghan K, Douglas PS, et al. Sex-based differences in outcomes after percutaneous coronary intervention for acute myocardial infarction: a report from TRANSLATE-ACS. J Am Heart Assoc. 2014;3(1):e000523. doi: 10.1161/JAHA.113.000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson ML, Peterson ED, Brennan JM, Rao SV, Dai D, Anstrom KJ, et al. Short- and long-term outcomes of coronary stenting in women versus men: results from the National Cardiovascular Data Registry Centers for Medicare & Medicaid services cohort. Circulation. 2012;126(18):2190–2199. doi: 10.1161/CIRCULATIONAHA.112.111369. [DOI] [PubMed] [Google Scholar]
- 17.Husted S, James SK, Bach RG, Becker RC, Budaj A, Heras M, et al. The efficacy of ticagrelor is maintained in women with acute coronary syndromes participating in the prospective, randomized, PLATelet inhibition and patient Outcomes (PLATO) trial. Eur Heart J. 2014;35(23):1541–1550. doi: 10.1093/eurheartj/ehu075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandiramani R, Cao D, Claessen BE, Sorrentino S, Guedeney P, Blum M, et al. Sex-related differences in patients at high bleeding risk undergoing percutaneous coronary intervention: a patient-level pooled analysis from 4 postapproval studies. J Am Heart Assoc. 2020;9(7):e014611. doi: 10.1161/JAHA.119.014611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin ES, Lee CW, Ahn JM, Lee PH, Chang M, Kim MJ, et al. Sex differences in left main coronary artery stenting: Different characteristics but similar outcomes for women compared with men. Int J Cardiol. 2018;253:50–54. doi: 10.1016/j.ijcard.2017.06.051. [DOI] [PubMed] [Google Scholar]
- 20.Shin ES, Jun EJ, Han JK, Kong MG, Kang J, Zheng C, et al. Sex-related impact on clinical outcomes of patients treated with drug-eluting stents according to clinical presentation: Patient-level pooled analysis from the GRAND-DES registry. Cardiol J. 2023;30(1):105–116. doi: 10.5603/CJ.a2021.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SH, Choi J, Chang YJ, Shin ES, Choi KH, Lee JM, et al. Sex difference in long-term clinical outcomes after percutaneous coronary intervention: a propensity-matched analysis of National Health Insurance data in Republic of Korea. Catheter Cardiovasc Interv. 2021;98(2):E171–E180. doi: 10.1002/ccd.29511. [DOI] [PubMed] [Google Scholar]
- 22.Schreuder MM, Badal R, Boersma E, Kavousi M, Roos-Hesselink J, Versmissen J, et al. Efficacy and safety of high potent P2Y12 inhibitors prasugrel and ticagrelor in patients with coronary heart disease treated with dual antiplatelet therapy: a sex-specific systematic review and meta-analysis. J Am Heart Assoc. 2020;9(4):e014457. doi: 10.1161/JAHA.119.014457. [DOI] [PMC free article] [PubMed] [Google Scholar]