Abstract
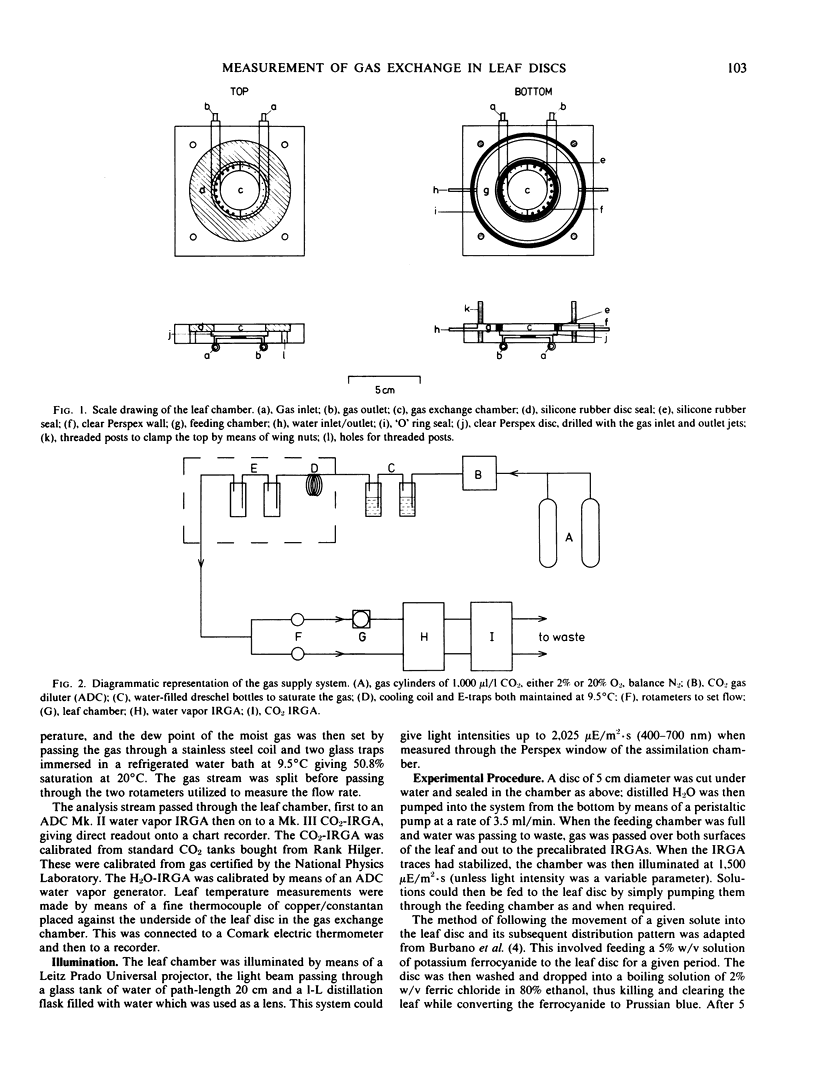
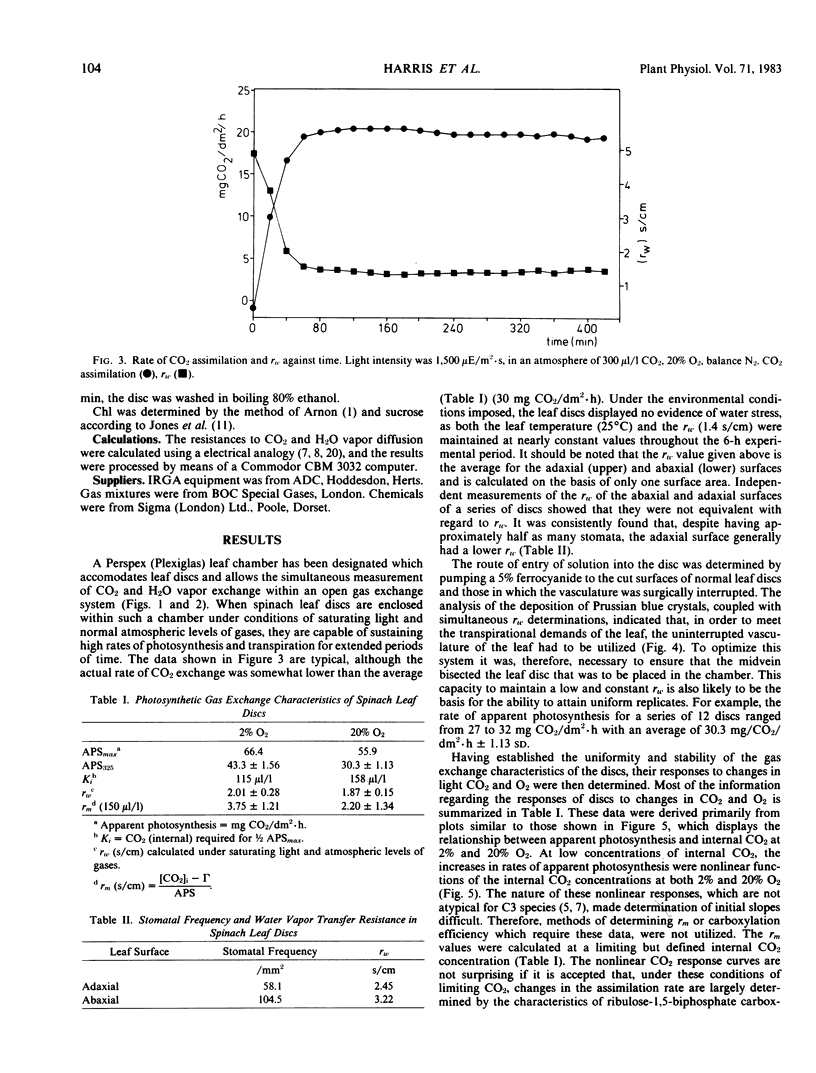
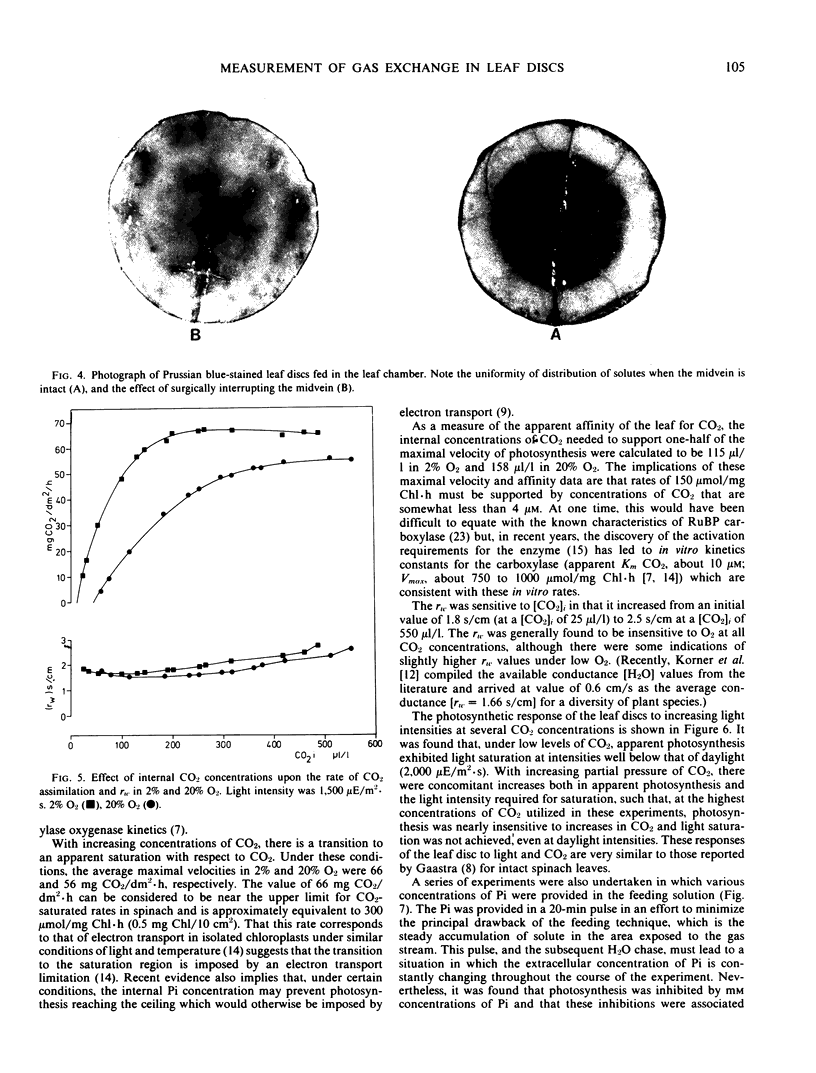
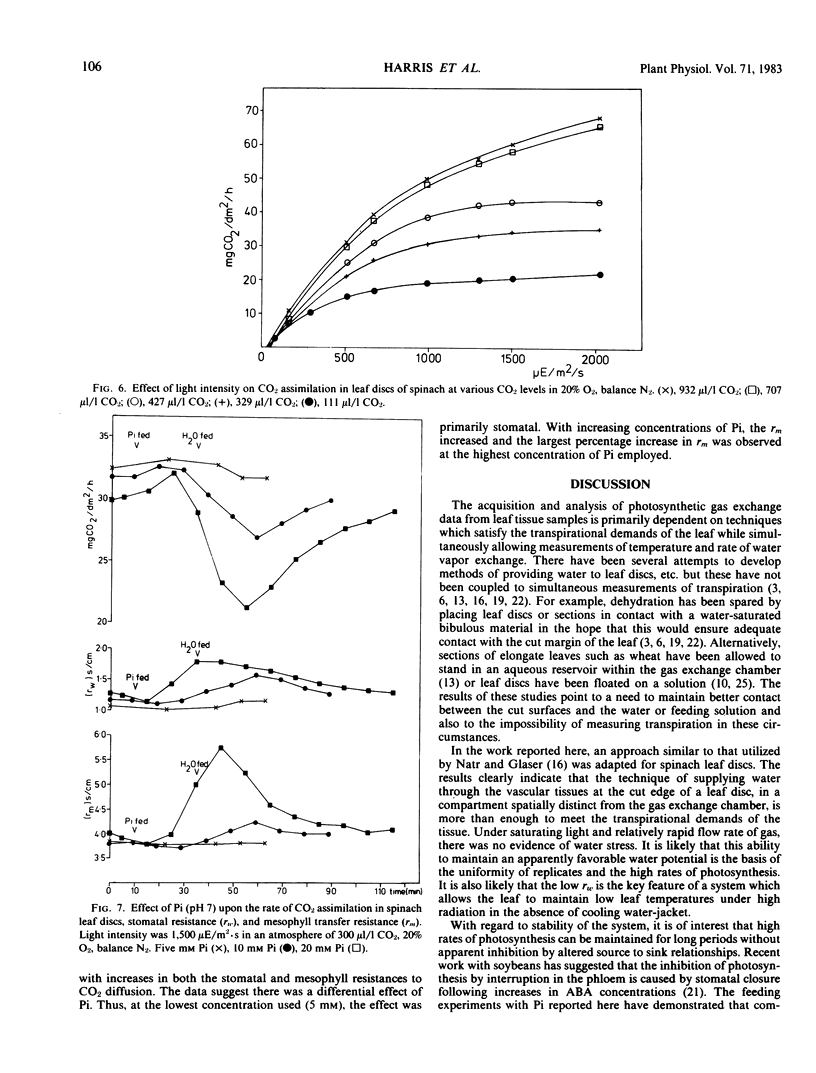
A leaf chamber has been designed which allows the measurement of both CO2 and water vapor exchange in Spinacia oleracea leaf discs. The center of the disc lies within a cylindrical gas chamber and its margins are enclosed within a cavity through which water or various metabolites can be pumped. In saturating light and normal atmospheres, the leaf discs have a relatively low resistance to H2O vapor transfer (rw = 1.87 seconds per centimeter) and can support high rates of photosynthesis for several hours. The abaxial surface of a disc had a higher resistance to water vapor transfer (rw = 3.22 seconds per centimeter) than the adaxial (rw = 2.45 seconds per centimeter) despite having a higher stomatal frequency (abaxial, 105/square millimeter; adaxial, 58/square millimeter). In 2% O2, the discs required an internal concentration of CO2 of 115 microliters per liter to support one-half of the maximal velocity of apparent photosynthesis (average value, 66 milligrams CO2 per square decimeter per hour). In 20% O2, the comparable values are 156 microliters per liter and 56 milligrams CO2 per square decimeter per hour. In air, apparent photosynthesis saturated at intensities (750 microeinsteins per square meter per second) well below that of daylight but, when the internal CO2 was raised to 700 to 900 microliters per liter, photosynthesis was not saturated even at daylight intensities (2025 microeinsteins per square meter per second). The distribution of Prussian blue crystals, formed after ferrocyanide feeding, showed that water entered the disc via the vasculature. When 25-minute pulses of orthophosphate were provided in the feeding solution, there were concentration-dependent increases in both rw and rm leading to inhibition of photosynthesis. The orthophosphate-dependent inhibitions were reversible.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canvin D. T., Berry J. A., Badger M. R., Fock H., Osmond C. B. Oxygen exchange in leaves in the light. Plant Physiol. 1980 Aug;66(2):302–307. doi: 10.1104/pp.66.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G. C., Cheesbrough J. K., Walker D. A. Effects of mannose on photosynthetic gas exchange in spinach leaf discs. Plant Physiol. 1983 Jan;71(1):108–111. doi: 10.1104/pp.71.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. G., Outlaw W. H., Lowry O. H. Enzymic assay of 10 to 10 moles of sucrose in plant tissues. Plant Physiol. 1977 Sep;60(3):379–383. doi: 10.1104/pp.60.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. Carbon dioxide assimilation by leaves, isolated chloroplasts, and ribulose bisphosphate carboxylase from spinach. Plant Physiol. 1975 Jun;55(6):1087–1092. doi: 10.1104/pp.55.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorimer G. H., Badger M. R., Andrews T. J. The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism, and physiological implications. Biochemistry. 1976 Feb 10;15(3):529–536. doi: 10.1021/bi00648a012. [DOI] [PubMed] [Google Scholar]
- Pallas J. E. An apparent anomaly in peanut leaf conductance. Plant Physiol. 1980 May;65(5):848–851. doi: 10.1104/pp.65.5.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setter T. L., Brun W. A., Brenner M. L. Effect of obstructed translocation on leaf abscisic Acid, and associated stomatal closure and photosynthesis decline. Plant Physiol. 1980 Jun;65(6):1111–1115. doi: 10.1104/pp.65.6.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]




