Patients with heart failure (HF) who have preserved ejection fraction (HFpEF) display elevation in left ventricular (LV) filling pressures during exercise leading to dyspnoea and increased morbidity and mortality.1 High LV filling pressures in HFpEF are caused in part by an extrinsic constraint on the heart mediated by the pericardium, which becomes amplified during exercise,2,3 impairing exercise capacity and increasing risk for HF hospitalization or death.4–6 We have previously shown in animal models and patients without HF undergoing cardiac surgery that pericardiotomy reduces this extrinsic restraint, abrogating the rise in LV filling pressures during volume loading.7–9 Here, we report results from the first clinical trial testing safety and efficacy of surgical pericardiotomy to treat patients with HFpEF.
Four individuals with HFpEF [LV ejection fraction (LVEF) ≥ 50%] with severe symptoms despite optimal medical therapy and elevated pulmonary capillary wedge pressure (PCWP) at rest or exercise were enrolled following written informed consent. The Mayo Clinic Institutional Review Board approved the study, and the trial was registered (NCT03923673). Participants underwent echocardiography, cardiac magnetic resonance (CMR) imaging, health status assessment using the Kansas City Cardiomyopathy Questionnaire overall summary score (KCCQ-OSS), and cardiopulmonary exercise testing. No statistical analyses were performed, and the results are purely descriptive.
Right heart catheterization was performed to measure haemodynamics before pericardiotomy at baseline and during volume loading with passive leg raise and 250 mL saline bolus. Participants then underwent surgical pericardiotomy using a thoracoscope to completely open the anterolateral pericardium overlaying the left ventricle. General endotracheal anaesthesia was administered through a double-lumen endotracheal tube. A radial arterial line was used for invasive blood pressure monitoring during the procedure. With the left lung deflated, a 3 cm left lateral thoracotomy incision was created, and the left pleural space was entered in the fourth intercostal space. The pericardium was opened 4 cm anterior and parallel to the left phrenic nerve using low voltage electrocautery. The pericardiotomy was extended longitudinally from the apex of the left ventricle to the proximal aortic reflection under direct vision using a 5 mm thoracoscope. A 26 Fr straight chest tube was inserted into the left pleural space through a separate wound placed anteriorly in the sixth intercostal space. The mini left thoracotomy was closed in layers, and the left lung was re-inflated. Haemodynamic assessments were then repeated at baseline and immediately following volume load with pericardium intact to assess diastolic reserve under general anaesthesia acutely. Following recovery and discharge, patients returned for clinical assessments at three and six months post-operatively. The primary safety outcome was major adverse cardiovascular and cerebrovascular events, and the primary efficacy outcome was the change in PCWP during volume loading following pericardiotomy.
Four patients (all women) aged 75 ± 4 years with body mass index of 34.8 ± 4.5 kg/m2 were enrolled. All had hypertension and were treated with diuretics and renin-angiotensin system inhibitors, two had diabetes, and one had a history of atrial fibrillation. Participants had severe HFpEF [all New York Heart Association class III, baseline peak oxygen consumption (VO2) 11.0 ± 2.0 mL/kg/min, baseline KCCQ-OSS 29 ± 9]. On invasive haemodynamic exercise testing prior to enrollment, 3 of 4 patients had normal resting PCWP. Overall mean PCWP increased from 15 ± 6.7 to 32 ± 3 mmHg, and mean pulmonary artery pressures increased from 27 ± 8 to 54 ± 6 mmHg. By echocardiography, patients had normal LVEF (60 ± 5%) and elevated E/e′ ratio (17 ± 12). Three patients had left atrial enlargement, and none had right ventricular dysfunction. By CMR, mean LVEF was 62 ± 7% with normal LV end-diastolic volume (LVEDV, 102 ± 32 mL) and RV end-diastolic volume (RVEDV, 105 ± 27 mL).
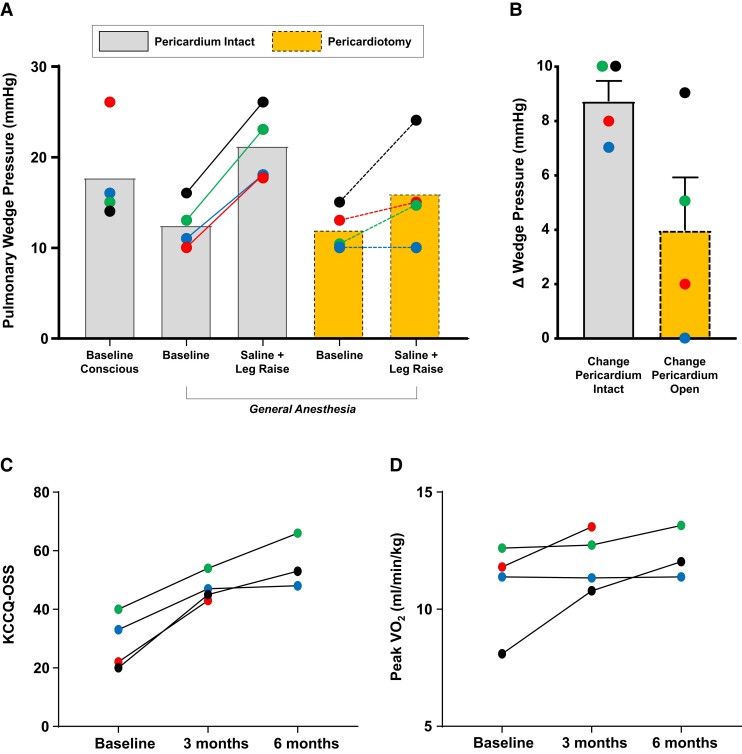
Baseline haemodynamics while conscious, before pericardiotomy, revealed mildly elevated PCWP (18 ± 6 mmHg), which decreased following induction with general anaesthesia (Figure 1A). With the pericardium intact under general anaesthesia, combined volume loading with passive leg raise and simultaneous 250 mL saline bolus increased PCWP from 13 ± 3 to 21 ± 4 mmHg (P < .001). All patients then underwent anterolateral pericardiotomy under thoracoscopic visualization without acute complication. After pericardiotomy, with the left lung re-inflated, PCWP was not significantly different than baseline values under general anaesthesia (Figure 1A). However, with repeat volume loading manoeuver, PCWP was lower as compared to values pre-pericardiotomy (16 ± 6 vs. 21 ± 4 mmHg), and the primary efficacy outcome of increase in PCWP with volume loading was significantly reduced compared with pericardium intact (ΔPCWP 4 ± 4 vs. 9 ± 2 mmHg; Figure 1B).
Figure 1.
(A) Pulmonary wedge pressures prior to and following induction with general anaesthesia, following volume load manoeuver, then again at rest and repeat volume load following pericardiotomy. (B) The primary efficacy endpoint of change in pulmonary wedge pressure with volume loading was significantly reduced by pericardiotomy. (C, D) Changes in Kansas City Cardiomyopathy Questionnaire overall summary score (KCCQ-OSS) and peak oxygen consumption (VO2) during upright cycle ergometry stress testing. Each coloured dot represents an individual patient at given time point
All patients returned at 3 months but one patient did not return for the 6-month study visit because of the COVID-19 pandemic (this participant reported no serious adverse events by telephone visit). At 3 months, there were increases in KCCQ-OSS following pericardiotomy (29 ± 9 to 47 ± 5) and in peak VO2 (11.0 ± 2.0 to 12.1 ± 1.3 mL/kg/min). These changes were maintained at the 6-month follow-up visit for KCCQ (31 ± 10 to 55 ± 8, n = 3) and peak VO2 (10.7 ± 2.3 to 12.3 ± 1.1 mL/kg/min, n = 3; Figure 1C and D). Among the 3 patients returning at 6 months, echocardiography showed no change in LVEF (66 ± 2%) or E/e′ ratio (16 ± 6). N-terminal pro-B-type natriuretic peptide levels were 638 ± 988 pg/mL at baseline, 1080 ± 1670 pg/mL at 3 months, and 318 ± 207 pg/mL at 6 months. By CMR, there was no change in LVEF (66 ± 2%) or heart volumes (LVEDV 92 ± 4 mL and RVEDV 91 ± 16 mL, mean changes +1 ± 28 mL and −14 ± 26 mL).
There were no major adverse cardiovascular, renal, or cerebrovascular events out to 6-month follow-up. Patients #1 and #2 developed post-operative atrial fibrillation while in hospital that was treated with amiodarone and cardioversion, along with 4 weeks of anticoagulation. There were three serious adverse events leading to hospitalization following discharge, one of which was judged related to study intervention, as Patient #1 developed pericarditis, a small to moderate inflammatory/exudative pericardial effusion anterior and inferior to the right ventricle on post-operative Day 14. While clinically-overt tamponade was not present, there was inward motion of the RV free wall during diastole suggesting possible early tamponade physiology, prompting treatment with pericardiocentesis, intrapericardial steroids, and colchicine. Following discharge, the patient had no recurrent adverse events.
In this first-in patient pilot study, we show evidence for central haemodynamic efficacy from surgical anterior pericardiotomy in patients with HFpEF, significantly mitigating the pathologic rise in LV filling pressures during volume loading. This salutary central cardiac effect may translate to other stresses that provoke symptoms, such as with exercise, though peripheral (non-cardiac) abnormalities also play an important role in many patients and would not be ameliorated through this approach. Long-term effects may not be reflected by acute haemodynamic changes, and these results must be considered as hypothesis-generating because of the small sample size and absence of blinding or sham control. The favourable signals observed in this pilot study call for future controlled trials to test the safety and efficacy of this novel interventional HF treatment in larger patient populations using less invasive, percutaneous pericardiotomy devices that can be applied in the catheterization laboratory, where thoracotomy or sternotomy is not required.
Declarations
Disclosure of Interest
B.A.B. receives research support from the National Institutes of Health (NIH) and the United States Department of Defense, as well as research grant funding from AstraZeneca, Axon, GlaxoSmithKline, Medtronic, Mesoblast, Novo Nordisk, and Tenax Therapeutics. B.A.B. has served as a consultant for Actelion, Amgen, Aria, BD, Boehringer Ingelheim, Cytokinetics, Edwards Lifesciences, Eli Lilly, Janssen, Merck, and Novo Nordisk. B.A.B. and S.J.A. are named inventors (US Patent no. 10,307,179) for the tools and approach for a minimally invasive pericardial modification procedure to treat heart failure.
Contributor Information
Barry A Borlaug, Department of Cardiovascular Medicine, Mayo Clinic and Foundation, 200 First Street SW, Rochester, MN 55905, USA.
Hartzell V Schaff, Department of Cardiovascular Surgery, Mayo Clinic, Rochester, MN 55906, USA.
Samuel J Asirvatham, Department of Cardiovascular Medicine, Mayo Clinic and Foundation, 200 First Street SW, Rochester, MN 55905, USA.
Katlyn E Koepp, Department of Cardiovascular Medicine, Mayo Clinic and Foundation, 200 First Street SW, Rochester, MN 55905, USA.
William J Mauermann, Department of Anesthesiology, Mayo Clinic, Rochester, MN 55906, USA.
Phillip G Rowse, Department of Cardiovascular Surgery, Mayo Clinic, Rochester, MN 55906, USA.
Data Availability
Data will be made available to researchers upon reasonable request from the corresponding author.
Funding
This research was supported by a grant from the Mayo Clinic Transform Practice Award to B.A.B. B.A.B. is also supported by R01 HL128526, R01 HL162828, and U01 HL160226, from the National Institutes of Health, and W81XWH2210245 from the US Department of Defense.
Ethical Approval
The Mayo Clinic Institutional Review Board approved the study, and the trial was registered (NCT03923673).
Pre-registered Clinical Trial Number
Clinical trial registration: NCT03923673.
References
- 1. Borlaug BA, Sharma K, Shah SJ, Ho JE. Heart failure with preserved ejection fraction: JACC scientific statement. J Am Coll Cardiol 2023;81:1810–34. 10.1016/j.jacc.2023.01.049 [DOI] [PubMed] [Google Scholar]
- 2. Parasuraman SK, Loudon BL, Lowery C, Cameron D, Singh S, Schwarz K, et al. Diastolic ventricular interaction in heart failure with preserved ejection fraction. J Am Heart Assoc 2019;8:e010114. 10.1161/JAHA.118.010114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borlaug BA, Reddy YNV. The role of the pericardium in heart failure: implications for pathophysiology and treatment. JACC Heart Fail 2019;7:574–85. 10.1016/j.jchf.2019.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reddy YNV, Olson TP, Obokata M, Melenovsky V, Borlaug BA. Hemodynamic correlates and diagnostic role of cardiopulmonary exercise testing in heart failure with preserved ejection fraction. JACC Heart Fail 2018;6:665–75. 10.1016/j.jchf.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Obokata M, Olson TP, Reddy YNV, Melenovsky V, Kane GC, Borlaug BA. Haemodynamics, dyspnoea, and pulmonary reserve in heart failure with preserved ejection fraction. Eur Heart J 2018;39:2810–21. 10.1093/eurheartj/ehy268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Omote K, Verbrugge FH, Sorimachi H, Omar M, Popovic D, Obokata M, et al. Central haemodynamic abnormalities and outcome in patients with unexplained dyspnoea. Eur J Heart Fail 2023;25:185–96. 10.1002/ejhf.2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Borlaug BA, Carter RE, Melenovsky V, DeSimone CV, Gaba P, Killu A, et al. Percutaneous pericardial resection: a novel potential treatment for heart failure with preserved ejection fraction. Circ Heart Fail 2017;10:e003612. 10.1161/CIRCHEARTFAILURE.116.003612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Borlaug BA, Schaff HV, Pochettino A, Pedrotty DM, Asirvatham SJ, Abel MD, et al. Pericardiotomy enhances left ventricular diastolic reserve with volume loading in humans. Circulation 2018;138:2295–7. 10.1161/CIRCULATIONAHA.118.036006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jain CC, Pedrotty D, Araoz PA, Sugrue A, Vaidya VR, Padmanabhan D, et al. Sustained improvement in diastolic reserve following percutaneous pericardiotomy in a porcine model of heart failure with preserved ejection fraction. Circ Heart Fail 2021;14:e007530. 10.1161/CIRCHEARTFAILURE.120.007530 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available to researchers upon reasonable request from the corresponding author.