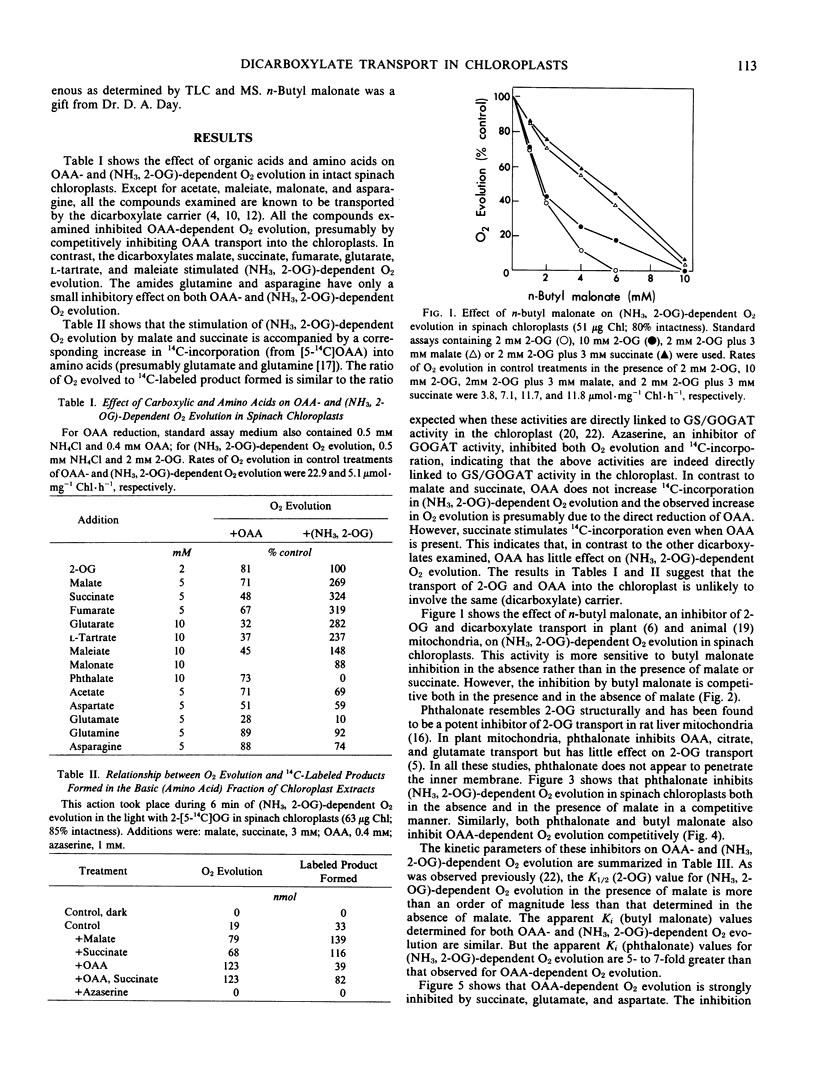
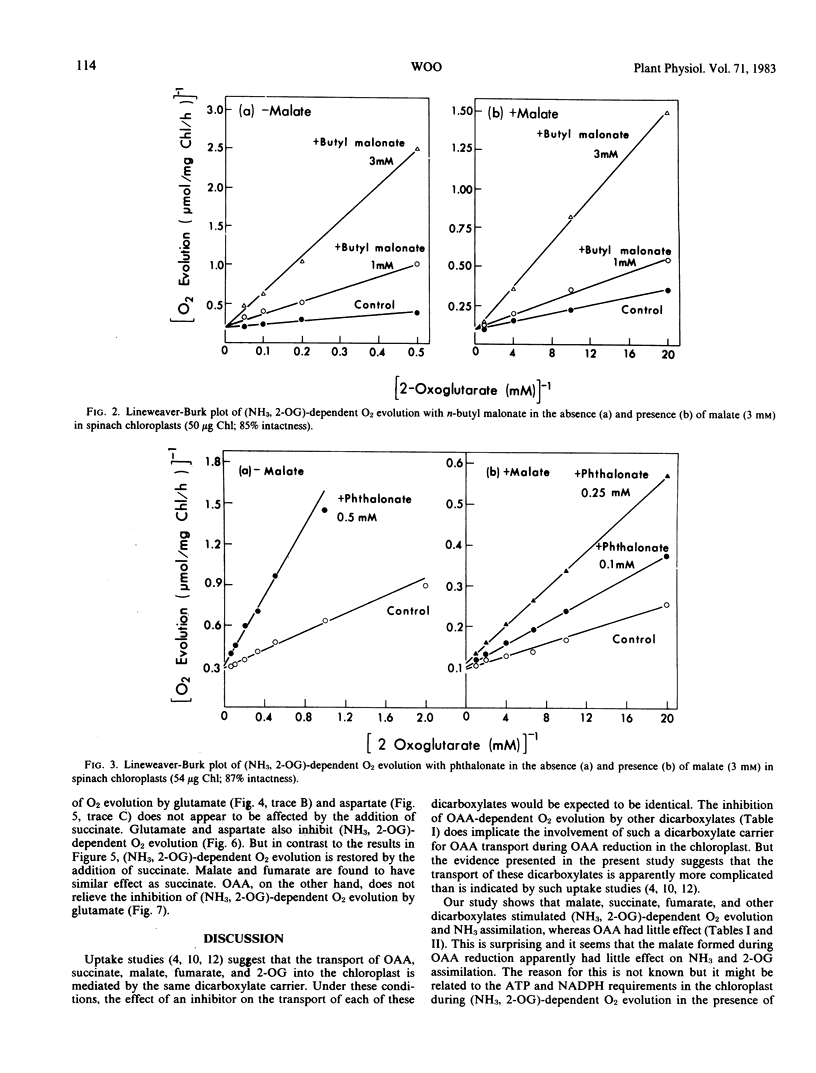
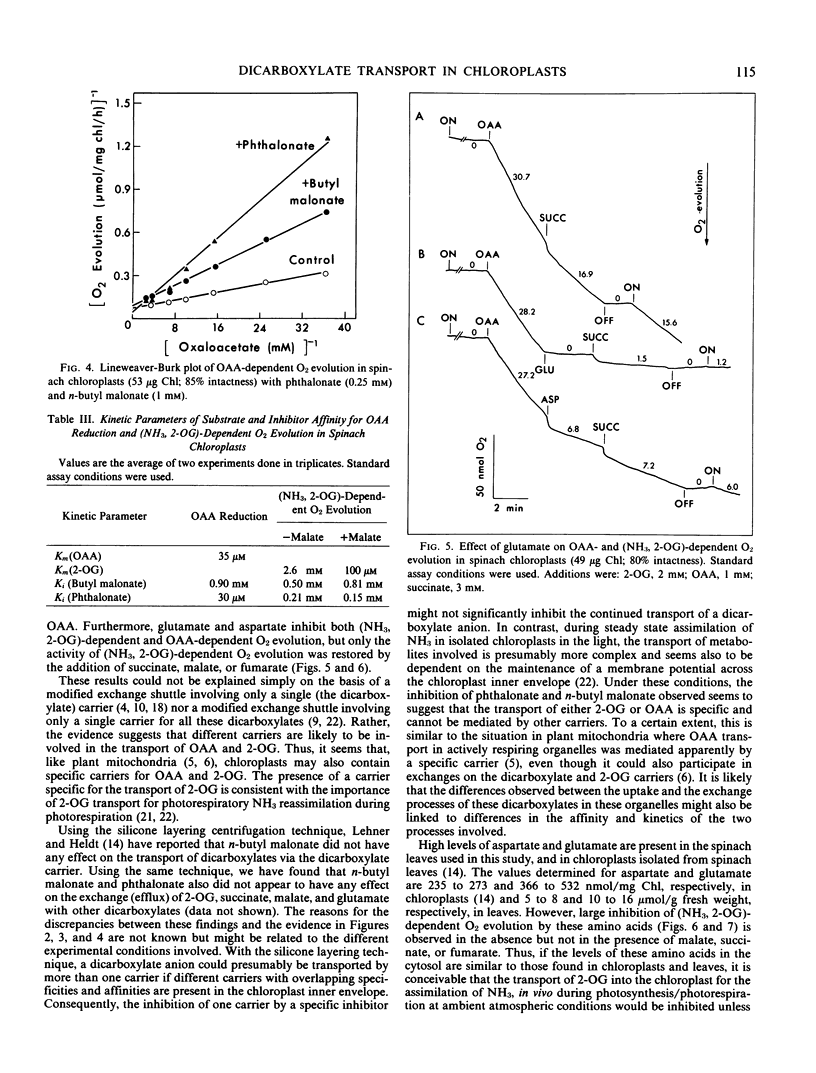
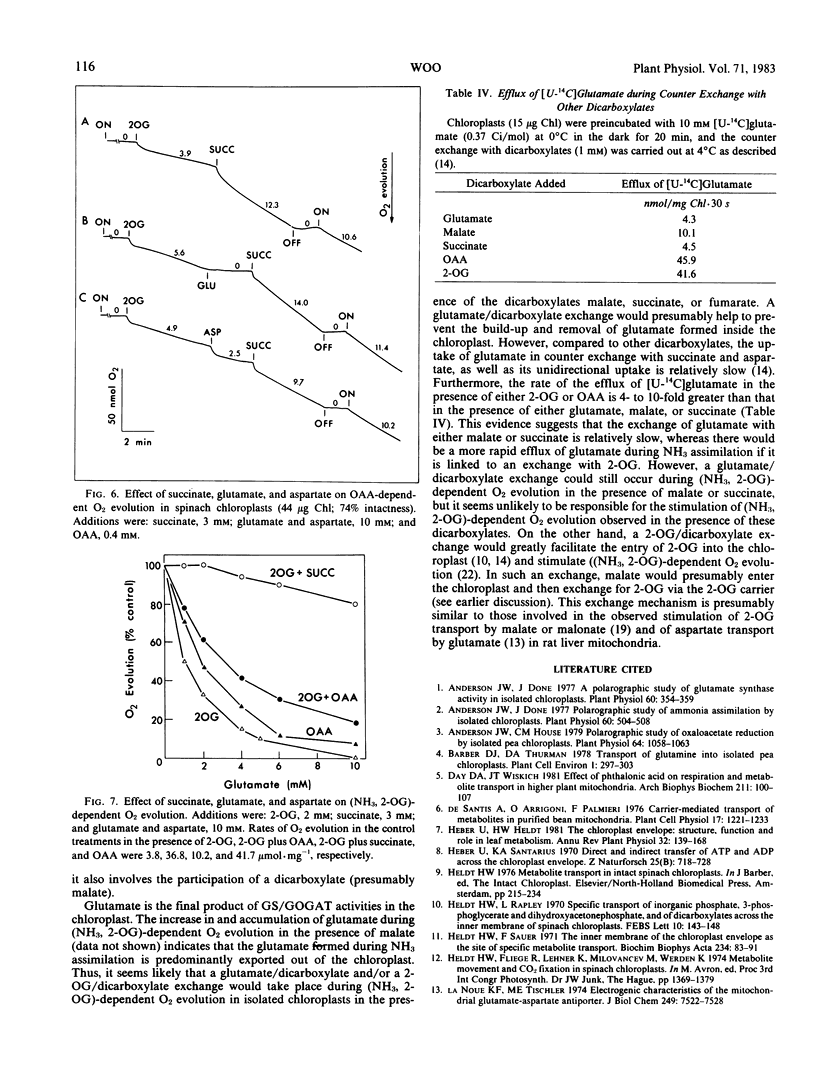
Abstract
The evolution of O2 in spinach chloroplasts in the presence of oxaloacetate (OAA) was inhibited by a wide range of dicarboxylates. In contrast, (ammonia, 2-oxoglutarate)-dependent O2 evolution was stimulated by malate, succinate, fumarate, glutarate, maleiate, and l-tartrate although OAA has little effect. This increase in O2 evolution was accompanied by a similar increase in 14C incorporation from [5-14C]oxoglutarate into amino acids which was sensitive to azaserine inhibition. Glutamate and aspartate inhibited (ammonia, 2-oxoglutarate)-dependent O2 evolution, but this inhibition was relieved by the addition of succinate, malate, or fumarate. OAA-dependent O2 evolution also was inhibited by glutamate and aspartate, but succinate, malate, or fumarate had little effect on this inhibition. Phthalonate and n-butyl malonate inhibited (ammonia, 2-oxoglutarate)-dependent O2 evolution competitively with respect to 2-oxoglutarate and uncompetitively with respect to malate. Both these inhibitors inhibited OAA-dependent O2 evolution competitively. This evidence suggests that different mechanisms might be involved in the transport of OAA, 2-oxoglutarate, and malate into the chloroplasts.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. W., Done J. A polarographic study of glutamate synthase activity in isolated chloroplasts. Plant Physiol. 1977 Sep;60(3):354–359. doi: 10.1104/pp.60.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. W., Done J. Polarographic study of ammonia assimilation by isolated chloroplasts. Plant Physiol. 1977 Oct;60(4):504–508. doi: 10.1104/pp.60.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. W., House C. M. Polarographic study of oxaloacetate reduction by isolated pea chloroplasts. Plant Physiol. 1979 Dec;64(6):1058–1063. doi: 10.1104/pp.64.6.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. A., Wiskich J. T. Effect of phthalonic acid on respiration and metabolite transport in higher plant mitochondria. Arch Biochem Biophys. 1981 Oct 1;211(1):100–107. doi: 10.1016/0003-9861(81)90434-3. [DOI] [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Rapley L. Specific transport of inorganic phosphate, 3-phosphoglycerate and dihydroxyacetonephosphate, and of dicarboxylates across the inner membrane of spinach chloroplasts. FEBS Lett. 1970 Oct 5;10(3):143–148. doi: 10.1016/0014-5793(70)80438-0. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Sauer F. The inner membrane of the chloroplast envelope as the site of specific metabolite transport. Biochim Biophys Acta. 1971 Apr 6;234(1):83–91. doi: 10.1016/0005-2728(71)90133-2. [DOI] [PubMed] [Google Scholar]
- LaNoue K. F., Tischler M. E. Electrogenic characteristics of the mitochondrial glutamate-aspartate antiporter. J Biol Chem. 1974 Dec 10;249(23):7522–7528. [PubMed] [Google Scholar]
- Lehner K., Heldt H. W. Dicarboxylate transport across the inner membrane of the chloroplast envelope. Biochim Biophys Acta. 1978 Mar 13;501(3):531–544. doi: 10.1016/0005-2728(78)90119-6. [DOI] [PubMed] [Google Scholar]
- Meijer A. J., von Woerkom G. M., Eggelte T. A. Phthalonic acid, an inhibitor of alpha-oxoglutarate transport in mitochondria. Biochim Biophys Acta. 1976 Apr 9;430(1):53–61. doi: 10.1016/0005-2728(76)90221-8. [DOI] [PubMed] [Google Scholar]
- Mitchell C. A., Stocking C. R. Kinetics and Energetics of Light-driven Chloroplast Glutamine Synthesis. Plant Physiol. 1975 Jan;55(1):59–63. doi: 10.1104/pp.55.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson B. H., Chappell J. B. The inhibition of malate, tricarboxylate and oxoglutarate entry into mitochondria by 2-n-butylmalonate. Biochem Biophys Res Commun. 1967 Jul 21;28(2):249–255. doi: 10.1016/0006-291x(67)90437-8. [DOI] [PubMed] [Google Scholar]
- Woo K. C., Osmond C. B. Stimulation of ammonia and 2-oxoglutarate-dependent o(2) evolution in isolated chloroplasts by dicarboxylates and the role of the chloroplast in photorespiratory nitrogen recycling. Plant Physiol. 1982 Mar;69(3):591–596. doi: 10.1104/pp.69.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]


