Abstract
Background and Aims
Interventional studies in pulmonary arterial hypertension completed to date have shown to be effective in symptomatic patients with significantly elevated mean pulmonary artery pressure (mPAP) (≥25 mmHg) and pulmonary vascular resistance (PVR) > 3 Wood Unit (WU). However, in health the mPAP does not exceed 20 mmHg and PVR is 2 WU or lower, at rest. The ESC/ERS guidelines have recently been updated to reflect this. There is limited published data on the nature of these newly defined populations (mPAP 21–24 mmHg and PVR >2–≤3 WU) and the role of comorbidity in determining their natural history. With the change in guidelines, there is a need to understand this population and the impact of the ESC/ERS guidelines in greater detail.
Methods
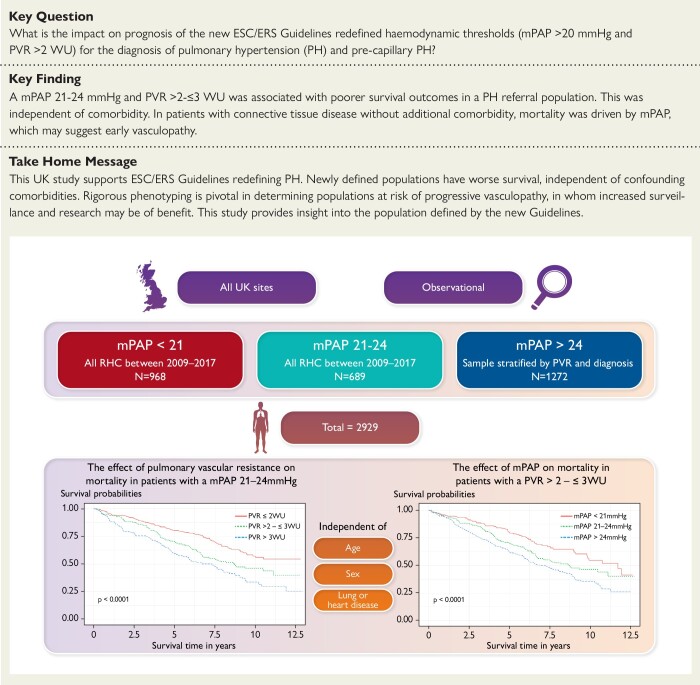
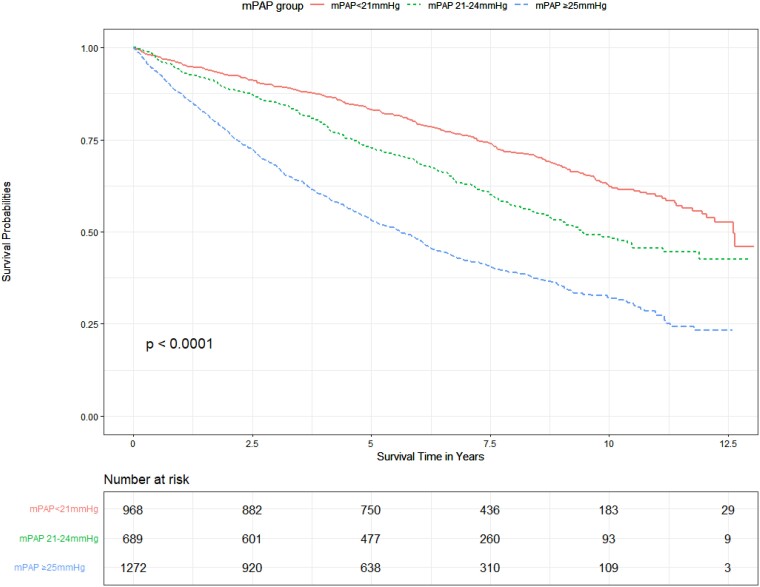
A retrospective nationwide evaluation of the role of pulmonary haemodynamics and comorbidity in predicting survival among patients referred to the UK pulmonary hypertension (PH) centres between 2009 and 2017. In total, 2929 patients were included in the study. Patients were stratified by mPAP (<21 mmHg, 21–24 mmHg, and ≥25 mmHg) and PVR (≤2 WU, > 2–≤3 WU, and >3 WU), with 968 (33.0%) in the mPAP <21 mmHg group, 689 (23.5%) in the mPAP 21–24 mmHg group, and 1272 (43.4%) in the mPAP ≥25 mmHg group.
Results
Survival was negatively correlated with mPAP and PVR in the population as a whole. Survival in patients with mildly elevated mPAP (21–24 mmHg) or PVR (>2–≤3WU) was lower than among those with normal pressures (mPAP <21 mmHg) and normal PVR (PVR ≤ 2WU) independent of comorbid lung and heart disease [hazard ratio (HR) 1.36, 95% confidence interval (CI) 1.14–1.61, P = .0004 for mPAP vs. HR 1.28, 95% CI 1.10–1.49, P = .0012 for PVR]. Among patients with mildly elevated mPAP, a mildly elevated PVR remained an independent predictor of survival when adjusted for comorbid lung and heart disease (HR 1.33, 95% CI 1.01–1.75, P = .042 vs. HR 1.4, 95% CI 1.06–1.86, P = .019). 68.2% of patients with a mPAP 21–24 mmHg had evidence of underlying heart or lung disease. Patients with mildly abnormal haemodynamics were not more symptomatic than patients with normal haemodynamics. Excluding patients with heart and lung disease, connective tissue disease was associated with a poorer survival among those with PH. In this subpopulation evaluating those with a mPAP of 21–24 mmHg, survival curves only diverged after 5 years.
Conclusions
This study supports the change in diagnostic category of the ESC/ERS guidelines in a PH population. The newly included patients have an increased mortality independent of significant lung or heart disease. The majority of patients in this new category have underlying heart or lung disease rather than an isolated pulmonary vasculopathy. Mortality is higher if comorbidity is present. Rigorous phenotyping will be pivotal to determine which patients are at risk of progressive vasculopathic disease and in whom surveillance and recruitment to studies may be of benefit. This study provides an insight into the population defined by the new guidelines.
Keywords: Mean pulmonary artery pressure, Pulmonary hypertension, Comorbidity, Haemodynamics
Structured Graphical Abstract
Structured Graphical Abstract.
Comorbidity and predictors of mortality among patients with mildly abnormal cardiovascular haemodynamics and the impact of the 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension—insights from EVIDENCE-PAH UK. mPAP, mean pulmonary artery pressure; PVR, pulmonary vascular resistance; RHC, right heart catheterization; WU, Wood Unit.
See the editorial comment for this article ‘Confirmation of survival prediction based on 2022 ESC/ERS pulmonary hypertension guidelines new haemodynamic thresholds’, by N. Galiè, M. Palazzini, and A. Manes, https://doi.org/10.1093/eurheartj/ehad672.
Introduction
Resting mean pulmonary artery pressure (mPAP) does not exceed 20 mmHg in health,1,2 and pulmonary vascular resistance (PVR) does not exceed 2 Wood Units (WU).3 For over four decades, the pulmonary hypertension (PH) community has used a cut off for mPAP ≥25 mmHg1 for the diagnosis of PH. The 2015 ESC/ERS guidelines added PVR >3 WU,4 alongside pulmonary artery wedge pressure (PAWP) ≤ 15 mmHg, for the diagnosis of pre-capillary PH and the 6th World Symposium on Pulmonary Hypertension proposed defining pre-capillary PH a mPAP >20 mmHg, PAWP ≤15 mmHg, and PVR ≥3 WU.3,5 This changing view of PH thresholds is reflected in the 2022 ESC/ERS guidelines, which have lowered the threshold further to PVR >2 WU,6 creating a need to understand the implications of this change in the newly defined PH and pre-capillary PH population.
The lowering of thresholds has been driven by several studies, particularly in specific subgroups, which found that mildly elevated pulmonary artery pressures are prognostic.7 For instance, in PH associated with connective tissue disease (CTD), mildly elevated pulmonary artery pressure is associated with poorer outcome and progression to classical vasculopathic pulmonary arterial hypertension (PAH).7,8 Similarly in chronic thromboembolic disease studies have shown an improvement in symptoms and quality of life (QoL) in patients who have received intervention.9 Furthermore, evidence from large cohort and smaller single-centre studies suggest that in all-comers prognosis is adversely affected if mPAP10–14 or PVR15 are even mildly elevated. These data show the prognostic relevance and level of unmet need among patients with mild haemodynamic PH, and we now need to fully characterize the phenotypic subtypes. To date, there have been no large multi-centre systematic studies of adequately phenotyped patients with mildly elevated pulmonary pressures and associated outcome among patients referred to PH centres.
EVIDENCE-PAH UK is a UK cohort national study that aims to phenotype and determine drivers of outcome in patients with mild elevations in pulmonary artery pressure and PVR. This study focuses on patients newly defined as having PH that was referred to and systematically evaluated in all UK tertiary PH centres between 2009 and 2017.
The aims of this article are (i) to evaluate survival in patients with mildly abnormal pulmonary haemodynamics referred to PH centres; (ii) to determine if comorbidity affects outcome; and (iii) to use cardiovascular haemodynamics from right heart catheterization to further stratify mild elevations in mPAP.
Methods
Study population
All seven adult tertiary PH centres across the UK were included in this study (Freeman Hospital, the Golden Jubilee Hospital, Hammersmith Hospital, Royal Brompton Hospital, Royal Free Hospital, Royal Hallamshire Hospital, and the Royal Papworth Hospital). Each centre maintains a comprehensive database of all right heart catheterizations, which is collected prospectively and submitted to the UK National Audit of Pulmonary Hypertension. Data collected prospectively for the UK national audit between January 2009 and December 2017 were analysed to determine the size and nature of the population with mildly elevated pulmonary haemodynamics and the associated impact on survival determined from the NHS digital database. Where additional clinical data were missing from the database, data were retrospectively collected on healthcare records review.
The primary study population comprised all patients in whom right heart catheterization showed a mPAP <21 mmHg and mPAP 21–24 mmHg irrespective of PAWP. For comparison, a stratified random sample of patients with a mPAP ≥25 mmHg was included. Sampling was performed by first stratifying patients into those amenable to treatment with advanced therapies (Groups 1 and 4 PH) and not amenable (Groups 2 and 3 PH). These two groups were further stratified by PVR (<2 WU, 2–<3 WU, ≥ 3 WU) resulting in six groups. These groups were then randomly sampled with equal numbers from each group and overall sample size determined by site feasibility. Exclusion criteria are detailed in the Supplementary data online, Table S1.
Haemodynamic data collected in all patients included mPAP, cardiac output (CO), PAWP, and PVR, calculated as mPAP-PAWP/CO (with thermodilution values used for CO, were possible).
Exercise capacity was assessed by either 6-minute walk test or incremental shuttle walk test. To make the two tests comparable, we utilized the percentage of predicted distance16,17 with equations found in Supplementary data online, Table S2.
Quality of life scores were collected by either Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR) or emPHASIS-10 scores (emPHASIS-10 scores were only introduced after 2015).
Population characteristic recording (defining confounders)
Contemporaneous baseline clinical data were collected from electronic health records no earlier than 1 year before the baseline catheterization or 3 months after the baseline catheterization. Clinical data and investigations included functional class, QoL scores, exercise tests, echocardiograms, lung function tests, lung imaging (computed tomography and perfusion scanning), and N-terminal pro-B-type natriuretic peptide. These data were also used to identify confounding comorbidities, which included co-existing lung18,19 and heart disease.20–22 Connective tissue disorders23 were identified as a contributing comorbidity that might lead to progression to Group 1 PAH. Definition of confounders is found in Table 1.
Table 1.
Definition of the comorbidities and confounders
| Lung disease was defined as an abnormality identified on lung function and/or imaging of the lungs and/or a multidisciplinary team (MDT) diagnosis of lung disease. |
| (i) A reduced FEV1/FVC ratio <.7, FEV1 < 60% predicted was considered obstructive lung disease.18,19 |
| (ii) A FEV1/FVC ratio >.7 and reduced FVC <70% was considered restrictive disease18,19 based on trial data specific to pulmonary hypertension. |
| (iii) A TLCO <45% in non-scleroderma population and <32% for the scleroderma population was considered as evidence of lung disease where a diagnosis of pulmonary veno-occlusive disease was not made. |
| Left heart disease was defined by recognized standards on echocardiography suggestive of left heart disease; diastolic dysfunction, systolic dysfunction, left ventricular hypertrophy, and significant valvular heart disease. |
| (i) A high probability of diastolic dysfunction was defined by enlarged left atrial size and mitral Doppler measurements suggestive of diastolic dysfunction (E/e′ ≥ 14, E/A ratio >2). Intermediate probability of diastolic dysfunction was defined as E/e′ > 14 and E/A > 2 on mitral Doppler measurements and no left atrial dilatation.20 |
| (ii) Systolic dysfunction was defined as a either LVEF <45%, (preferentially by biplane echocardiography), or if by visual estimation, moderate to severe LV systolic dysfunction. |
| (iii) Significant left ventricular hypertrophy was defined significant increase in wall thickness (IVSD >1.5 cm). |
| (iv) Significant valvular disease was based on echo parameters, more than moderate left-sided valvular stenosis, regurgitation, or clinical multidisciplinary decision. |
| (v) Where echo data were not available, MDT decision outcomes were used or if patients had ≥3 clinical cardiovascular risk factors; atrial fibrillation, arterial hypertension, diabetes, body mass index ≥30 kg/m2, and/or known coronary artery disease.21,22 |
| Connective tissue disease was defined by clinical diagnosis by American College of Rheumatology criteria and/or auto-immune profile.23 |
FEV1, forced expiratory volume in one second; FVC, forced vital capacity; LVEF, left ventricular ejection fraction; TLCO, transfer factor for carbon monoxide.
Mortality data
Data obtained from NHS Digital and the UK Office for National Statistics (ONS) provided mortality data. All causes of mortality were included with a cut-off date of 1st December 2021. Mortality was defined as time to death in days from index right heart catheterization to death.
Statistical analysis
Analyses were performed on R studio (for Microsoft Windows version 2022.02.2+485). Patients were grouped by their baseline haemodynamics on right heart catheterization mPAP <21 mmHg, 21–24 mmHg, and ≥25 mmHg and PVR (≤2 WU, PVR >2–≤3 WU, and PVR >3 WU), based on the 2022 ESC/ERS guidelines. It should be noted that the analysis of the PVR groups is slightly different to that indicated at recruitment as the study was designed prior to the latest guidelines. Continuous variables data are presented in medians and quartiles. Categorical variables data are presented in percentages. Comparison between mPAP groups in demographic and clinical characteristics at baseline, one-way analysis of variance was used for continuous variables. For categorical variables, Fisher’s exact test was used. We utilized two-sided P-values. Significance level is considered as P-value of <.05.
Kaplan–Meier curves were used to demonstrate unadjusted survival curves for all-cause mortality. Log-rank tests were used to compare unadjusted mPAP and PVR groups for significance with pairwise tests adjusted using the Benjamini–Hochberg method. Cox-proportional hazard regression was used to adjust mortality for potential confounders individually (defined in Table 1) as well as age and gender, expressed as hazard ratios (HR) with 95% confidence intervals (CI). The proportional hazards assumption was tested using by analysing the association between the Schoenfeld residuals and time. Analysis was run across all mPAP groups and PVR groups, we ran the same survival analysis adjusting for confounders in populations of interest; mPAP 21–24 mmHg and PVR >2–≤3 WU, as defined in the 2022 ESC/ERS guidelines.6
Results
Study population
A total of 2929 patients were included in the study, with 968 patients (33.0%) in the mPAP <21 mmHg group, 689 patients (23.5%) in the mPAP 21–24 mmHg group, and 1272 (43.4%) in the stratified mPAP ≥25 mmHg group. The PH diagnoses for patients within mPAP ≥25 mmHg are described in Supplementary data online, Table S3.
Baseline characteristics
The median age of the whole population was 65 (interquartile range 53–73) years with age increasing significantly with each increment in mPAP group (P < .000001; Table 2). Exercise performance evaluated using percentage predicted distance also worsened significantly with each increment of mPAP group (P < .000001; Table 2). The majority of QoL scores deteriorated significantly with incrementing mPAP group (P < .01; Table 2), with the exception for CAMPHOR symptoms scores (P = .06) that trended toward a difference (Table 2). Baseline invasive haemodyanmics demonstrate worsening haemodynamics with each incremental mPAP group, with an increasing right atrial pressure and total pulmonary resistance, decreasing mixed venous saturation, CO, cardiac index, stroke volume, and pulmonary arterial compliance (Table 3).
Table 2.
Baseline demographics
| mPAP <21 mmHg (n = 968) | mPAP 21–24 mmHg (n = 689) | mPAP ≥25 mmHg (n = 1272) | Overall (n = 2929) | P-value | |
|---|---|---|---|---|---|
| Age | 60.0 [48.0–71.0]a,b | 65.0 [54.0–73.0]c | 67.0 [57.0–74.0] | 65.0 [53.0–73.0] | <.000001 |
| Gender | |||||
| Female | 665 (68.7%) | 454 (65.9%) | 809 (63.6%) | 1928 (65.8%) | |
| Male | 303 (31.3%)b | 235 (34.1%) | 463 (36.4%) | 1001 (34.2%) | .04164 |
| Ethnicity | |||||
| White | 701 (80.1%) | 521 (81.2%) | 985 (83.8%) | 2207 (82.0%) | |
| Asian | 71 (8.1%) | 54 (8.4%) | 74 (6.3%) | 199 (7.4%) | |
| Black | 51 (5.8%) | 48 (7.5%) | 76 (6.5%) | 175 (6.5%) | |
| Other | 52 (5.9%) | 19 (3.0%) | 41 (3.5%) | 112 (4.2%) | P = NS |
| WHO functional class | |||||
| I | 73 (9.1%) | 23 (3.6%) | 10 (0.8%) | 106 (3.9%) | |
| II | 297 (36.8%) | 205 (32.3%) | 169 (13.6%) | 672 (25.0%) | |
| III | 418 (51.9%) | 390 (61.4%) | 964 (77.6%) | 1773 (66.1%) | |
| IV | 18 (2.2%) | 17 (2.7%) | 100 (8.0%) | 135 (5.0%) | P = NS |
| CAMPHOR | |||||
| Symptoms | 11.0 [6.5–17.0] | 11.0 [6–17.0] | 12.0 [8–17.0] | 12 [7.0–17.0] | .06478 |
| Activity | 9.00 [4–15.0]b | 11.0 [6–16.0]c | 12.0 [7–18.0] | 11.0 [6.0–16.0] | <.000001 |
| QoL | 9.00 [2.25–15.0]b | 8.00 [3–15.0]c | 10.0 [5–16.0] | 9.5 [4.0–16.0] | .00938 |
| emPHasis-10 score | 26.0 [15.0–34.0] | 23.0 [10–30.5]c | 28.0 [16–37.0] | 25.0 [15.0–35.0] | .01711 |
| % of predicted walking distance | 64.8 [45.49–80.42]a,b | 60.2 [39–77.6]c | 45.6 [27–66.4] | 55.9 [33.0–73.7] | <.000001 |
| Borg Dyspnoea scale | 3.00 [2.0–5.0]b | 3.00 [2.0–5.0]c | 4.00 [3.0–5.0] | 4.0 [2.0–5.0] | <.000001 |
For continuous variables expressed in median [IQR]. n and missing values can be found in Supplementary data online, Table S6.
CAMPHOR, Cambridge Pulmonary Hypertension Outcome Review; NS, not significant.
a P-value <.05 between mPAP <21 mmHg and mPAP 21–24 mmHg.
b P-value <.05 between mPAP <21 mmHg and mPAP ≥ 25 mmHg.
c P-value <.05 between mPAP 21–24 mmHg and mPAP ≥ 25 mmHg.
Table 3.
Cardiac haemodynamics
| mPAP <21 mmHg (n = 968) | mPAP 21–24 mmHg (n = 689) | mPAP ≥25 mmHg (n = 1272) | Overall (n = 2929) | |
|---|---|---|---|---|
| Right atrial pressure (mmHg) | 5.00 [3.0–7.0] | 6.00 [4.0–8.0] | 10.0 [7.0–14.0] | 7.00 [4.0–10.0] |
| Mean pulmonary artery pressure (mmHg) | 17.0 [15.0–19.0] | 23.0 [22.0–24.0] | 36.0 [29.0–46.0] | 24.0 [19.0–34.0] |
| Pulmonary arterial wedge pressure (mmHg) | 9.00 [7.0–11.0] | 12.0 [9.0–14.0] | 14.0 [10.0–19.0] | 11.0 [8.0–14.0] |
| Cardiac output (l/min) | 5.20 [4.2–6.3] | 5.10 [4.3–6.2] | 4.90 [3.7–6.3] | 5.10 [4.0–6.3] |
| Mixed venous oxygen saturation (%) | 73.0 [68.6–77.0] | 71.8 [67.3–75.5] | 67.7 [60.5–73.0] | 70.4 [65.0–75.0] |
| Pulmonary vascular resistance (WU) | 1.46 [1.1–2.0] | 2.12 [1.5–2.8] | 3.72 [2.4–7.9] | 2.27 [1.5–3.7] |
| Total pulmonary resistance (WU) | 3.23 [2.6–4.0] | 4.38 [3.6–5.3] | 7.26 [5.1–11.4] | 4.63 [3.4–7.0] |
| Cardiac index (l/min.m2) | 2.84 [2.4–3.4] | 2.77 [2.3–3.3] | 2.58 [2.1–3.2] | 2.73 [2.2–3.3] |
| Stroke volume (ml) | 69.1 [56.6–86.6] | 71.3 [57.7–88.9] | 64.8 [49.5–84.5] | 68.6 [54.0–86.1] |
| Stroke volume index (ml/m2) | 38.6 [31.9–46.2] | 39.0 [31.7–45.9] | 34.3 [26.7–43.3] | 37.4 [30.3–45.2] |
| Pulmonary arterial compliance (ml/mmHg) | 3.63 [2.8–4.9] | 3.23 [2.6–4.2] | 2.04 [1.2–2.9] | 2.78 [1.7–3.8] |
n and missing values can be found in Supplementary data online, Table S6.
In the mPAP 21–24 mmHg group, 68.2% (n = 437) had comorbid lung and/or left heart disease, compared to 51.4% (n = 466) in the mPAP <21 mmHg group and 78.8% (n = 975) in the stratified mPAP ≥25 mmHg group. Comorbid lung disease was present in 32.6% of mPAP <21 mmHg patients, 46.3% of mPAP 21–24 mmHg patients, and 54.2% of stratified mPAP ≥25 mmHg patients (P < .000001; Table 4). Left heart disease was present in 24.7%, 31.7%, and 49.5% of patients in the three mPAP groups, respectively (P < .000001). A detailed breakdown of the investigations can be found in Supplementary data online, Tables S4 and S5, with n and missing values in Supplementary data online, Table S6. CTD was present in 37.4%, 35.3%, and 29.6% in the three mPAP groups, respectively, with no significant difference between mPAP <21 mmHg and mPAP 21–24 mmHg (P = .38); however, significant difference between mPAP 21–24 mmHg and the stratified mPAP ≥25 mmHg (P = .02).
Table 4.
Presence of comorbidities
| mPAP <21 mmHg (n = 968) | mPAP 21–24 mmHg (n = 689) | mPAP ≥25 mmHg (n = 1272) | Overall | P-value | |
|---|---|---|---|---|---|
| Lung disease a | |||||
| No | 633 (67.4%) | 364 (53.7%) | 575 (45.8%) | 1572 (54.7%) | |
| Yes | 306 (32.6%)b,c | 314 (46.3%)d | 681 (54.2%) | 1301 (45.3%) | <.000001 |
| Left heart disease a | |||||
| No | 691 (75.2%) | 440 (68.3%) | 617 (50.5%) | 1748 (62.8%) | |
| Yes | 227 (24.7%)b,c | 204 (31.7%)d | 604 (49.5%) | 1035 (37.2%) | <.000001 |
| Lung and/or left heart disease a | |||||
| No | 440 (48.6%) | 204 (31.8%) | 262 (21.2%) | 906 (32.5%) | |
| Yes | 466 (51.4%)b,c | 437 (68.2%)d | 975 (78.8%) | 1878 (67.5%) | <.000001 |
| Connective tissue disease a | |||||
| No | 606 (62.6%) | 446 (64.7%) | 895 (70.4%) | 1947 (66.5%) | |
| Yes | 362 (37.4%)c | 243 (35.3%)d | 377 (29.6%) | 982 (33.5%) | .0003119 |
n and missing values can be found in Supplementary data online, Table S6.
aDefined in Table 1.
b P-value <.05 between mPAP <21 mmHg and mPAP 21–24 mmHg.
c P-value <.05 between mPAP <21 mmHg and mPAP ≥25mmHg.
d P-value <.05 between mPAP 21–24 mmHg and mPAP ≥25 mmHg.
Association between mPAP group and survival
During the observation period (median of 6.1 years of follow-up, range 0–13 years), there were 1383 deaths (47.2%). 30.8% of the mPAP <21 mmHg group, 43.3% of the mPAP 21–24 mmHg group, and 61.8% of the mPAP ≥25 mmHg group died during follow-up. Primary cause of death, obtained from death certification from NHS digital, can be found in Supplementary data online, Table S7.
Figure 1 shows the Kaplan–Meier curves for the three mPAP groups with worsening survival for each increment in mPAP group (pairwise comparison significant across all groups—P < .000001). At 1 year, 4.2% of patients had died in the mPAP <21 mmHg group, 6.5% in the mPAP 21–24 mmHg group, and 12.4% in the mPAP ≥ 25 mmHg group. At 5 years, 17.4% had died in the mPAP <21 mmHg group, 27.7% in the mPAP 21–24 mmHg group, and 47.3% in the mPAP ≥25 mmHg group (P < .000001; Table 5).
Figure 1.
Unadjusted effect of mean pulmonary artery pressure on survival.
Table 5.
1, 3, and 5-year mortality across mean pulmonary artery pressure groups
| mPAP <21 mmHg (n = 968) | mPAP 21–24 mmHg (n = 689) | mPAP ≥25 mmHg (n = 1272) | P-value | |
|---|---|---|---|---|
| 1-year mortality | ||||
| Alive | 927 (95.8%) | 644 (93.5%) | 1114 (87.6%) | |
| Deaths | 41 (4.2%)a,b | 45 (6.5%)c | 158 (12.4%) | <.000001 |
| 3-year mortality | ||||
| Alive | 866 (89.5%) | 586 (85.1%) | 865 (68.0%) | |
| Deaths | 102 (10.5%)a,b | 103 (14.9%)c | 407 (32.0%) | <.000001 |
| 5-year mortality | ||||
| Alive | 771 (82.6%) | 488 (72.3%) | 664 (52.7%) | |
| Deaths | 162 (17.4%)a,b | 187 (27.7%)c | 595 (47.3%) | <.000001 |
| Missingd | 35 (3.6%) | 14 (2.0%) | 13 (1.0%) |
a P < .05 between mPAP <21 mmHg and mPAP 21–24 mmHg.
b P < .05 between mPAP <21 mmHg and mPAP ≥25 mmHg.
c P < .05 between mPAP 21–24 mmHg and mPAP ≥25 mmHg.
dMissing as 5-year follow-up not complete.
On Cox-proportional hazard regression, excess mortality in the mPAP 21–24 mmHg and >25 mmHg groups remained significant (compared to the mPAP <21 mmHg group) after adjustment of lung disease, left heart disease, CTD, age, and gender (P < .002 in all cases; Table 6). However, there was a statistically significant relationship between the Schoenfeld residuals and time (P = .00016), suggesting a time-dependent coefficient that does not meet the proportional hazards assumption. Therefore, we also separately performed subgroup non-parametric analysis for patients with heart disease, lung disease, and CTD (see Supplementary data online, Figure S1A–C). In the heart disease and CTD patients, Kaplan–Meier curves demonstrate a worse survival outcome with each incremental mPAP group, and pairwise comparisons were significant across all mPAP groups (see Supplementary data online, Figure S1B and C, P < .03). In patients without heart and lung disease, there was also worsening mortality with each increment of mPAP group (see Supplementary data online, Figure S1D) with significant pairwise comparison across all groups (P = .013). However, in patients with lung disease alone, there was no significant difference in outcome between mPAP <21 mmHg and mPAP 21–24 mmHg groups (see Supplementary data online, Figure S1A P = .72), although the mPAP ≥25 mmHg group did have worse outcome (P < .000001). To further understand the impact of lung disease across the mPAP groups, Kaplan–Meier curves (see Supplementary data online, Figure S2A–C), for transfer factor for carbon monoxide (TLCO) were constructed which demonstrated that TLCO <45%, confers a poorer survival across all mPAP groups (P < .000001).
Table 6.
Adjusted mortality for confounders across mean pulmonary artery pressure groups
| Adjusted for lung diseasea n = 2873 | Adjusted for heart diseasea n = 2783 | Adjusted for CTDa n = 2929 | Adjusted for lung and heart diseasea n = 2736 | Adjusted for all comorbiditiesa n = 2736 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| mPAP 21–24 mmHg | 1.30 (1.10–1.53) | .00179 | 1.44 (1.22–1.70) | .0000191 | 1.41 (1.20–1.66) | .0000257 | 1.36 (1.14–1.61) | .000442 | 1.37 (1.15–1.62) | .000330 |
| mPAP ≥25 mmHg | 2.29 (1.99–2.63) | <.000001 | 2.48 (2.15–2.86) | <.000001 | 2.58 (2.26–2.95) | <.000001 | 2.24 (1.94–2.60) | <.000001 | 2.30 (1.98–2.66) | <.000001 |
aAdjusted for age/sex.
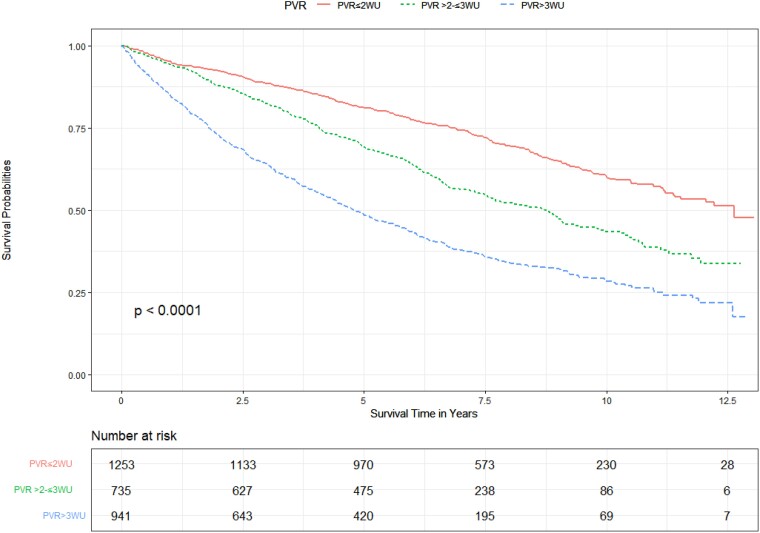
Association between PVR and survival outcomes
In the whole population, 1253 (42.8%) had a PVR ≤2 WU, 735 (25.1%) had a PVR >2–≤3 WU, and 941 (32.1%) had a PVR >3 WU. Kaplan–Meier survival curves (Figure 2) reveal a stepwise worsening of unadjusted survival with each increase in PVR group with pairwise comparison being statistically significant across all groups (P < .000001). Excess mortality (compared to PVR ≤2 WU) remained significant for PVR >2–≤3 WU, and PVR >3 WU, when individually adjusted for lung disease, left heart disease and CTD, age, and gender (P < .0014 in all cases; Table 7). However, the proportional hazard assumption was not met with an association between the Schoenfeld residuals and time (P = .00016). Consequently, we also performed subgroup analysis for patients with heart disease, lung disease, and CTD. Kaplan–Meier curves in lung disease alone, heart disease alone, and CTD alone, demonstrate a worse survival outcome with each incremental PVR group. Pairwise log-rank comparison is significant across all groups (P < .047; Supplementary data online, Figure S3A–C). Kaplan–Meier curve analysis of PVR groups among patients without evidence of heart or lung disease (see Supplementary data online, Figure S3D), reveals worsening survival with each increase in PVR group, statistically significant with pairwise comparison across all groups (P = .0038).
Figure 2.
Unadjusted effect of pulmonary vascular resistance on survival.
Table 7.
Adjusted mortality for confounders across pulmonary vascular resistance groups
| Adjusted for lung diseasea n = 2873 | Adjusted for heart diseasea n = 2783 | Adjusted for CTDa n = 2929 | Adjusted for lung and heart diseasea n = 2736 | Adjusted for all comorbiditiesa n = 2736 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| PVR >2–≤3 WU | 1.28 (1.10–1.48) | .00108 | 1.44 (1.24–1.66) | .00000144 | 1.43 (1.24–1.65) | .00000129 | 1.28 (1.10–1.49) | .00119 | 1.28 (1.10–1.49) | .00147 |
| PVR >3 WU | 2.15 (1.89–2.45) | <.000001 | 2.54 (2.23–2.90) | <.000001 | 2.47 (2.18–2.81) | <.000001 | 2.22 (1.93–2.54) | <.000001 | 2.23 (1.95–2.55) | <.000001 |
aAdjusted for age/sex.
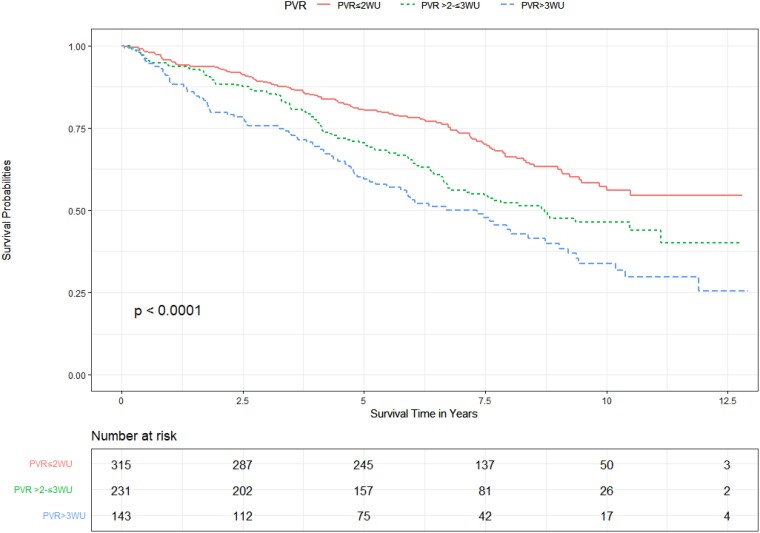
Stratification in the mPAP 21–24 mmHg population
Of the 689 patients with a mPAP 21–24 mmHg, 315 patients (45.7%) had a PVR ≤2WU, 231 patients (33.5%) had a PVR >2–≤3 WU, and 143 patients (20.8%) had a PVR >3 WU. Stratifying this group by PVR, Kaplan–Meier curves reveal that a PVR >2–≤3 WU confers higher mortality compared to a PVR of ≤2 WU, but lower than among patients with a PVR >3 WU (Figure 3) with pairwise comparisons being statistically significant across all groups (P < .03).
Figure 3.
Unadjusted effect of pulmonary vascular resistance on survival in patients with a mean pulmonary artery pressure 21–24 mmHg.
There is no significant difference in survival with increases in mPAP defined by integer increases in mPAP, an mPAP of 24 mmHg did not confer worse survival compared to patients with a mPAP 21 mmHg (P = .91; Supplementary data online, Figure S4) or stratified by PAWP (PAWP <12 mmHg, 12–15 mmHg, > 15 mmHg) (P = .34; Supplementary data online, Figure S5).
In the mPAP 21–24 mmHg group, Cox-proportional hazard regression confirmed excess mortality in the PVR >2–≤3 WU and PVR >3 WU group was independent of lung disease, left heart disease, CTD, age, and gender (P < .05; Table 8). Proportional hazard assumption was met (P = .66). Kaplan–Meier survival curves in the absence of lung and heart disease reveal a significant difference for patients with a PVR >2 WU, separating at 4 years (see Supplementary data online, Figure S6).
Table 8.
Adjusted mortality for confounders across pulmonary vascular resistance groups in patients with a mean pulmonary artery pressure 21–24 mmHg
| Adjusted for lung diseasea n = 678 | Adjusted for heart diseasea n = 644 | Adjusted for CTDa n = 689 | Adjusted for lung and heart diseasea n = 634 | Adjusted for all comorbiditiesa n = 634 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| PVR >2–≤3 WU | 1.33 (1.01–1.75) | .042240 | 1.46 (1.11–1.93) | .007101 | 1.35 (1.03–1.77) | .0280 | 1.40 (1.06–1.86) | .019011 | 1.38 (1.04–1.83) | .024295 |
| PVR >3 WU | 1.62 (1.20–2.19) | .001497 | 1.72 (1.27–2.33) | .000508 | 1.64 (1.22–2.20) | .0011 | 1.58 (1.16–2.16) | .003846 | 1.51 (1.11–2.07) | .009225 |
aAdjusted for age/sex.
Within the subgroup with a mPAP 21–24 mmHg, 68.3% (n = 438) had heart and/or lung disease. Kaplan–Meier survival curves demonstrate that the presence of a lung disease, left heart disease, and/or CTD confers higher mortality (P < .004), compared to patients who have no lung, heart, or CTD (see Supplementary data online, Figure S7A).
Comparing this to patients with a mPAP <21 mmHg, Kaplan–Meier survival curves show no significant difference between patients with CTD, in the absence of co-existing lung and heart disease, compared to patients without CTD, and no lung and/or heart disease (P = .15; Supplementary data online, Figure S7B). For comparison, mPAP ≥ 25 mmHg, Kaplan–Meier survival curves reveal a significant difference in survival in the presence of all comorbidity and risk factors (P = .000028; Supplementary data online, Figure S7C).
Stratification in the PVR >2–≤3 WU population
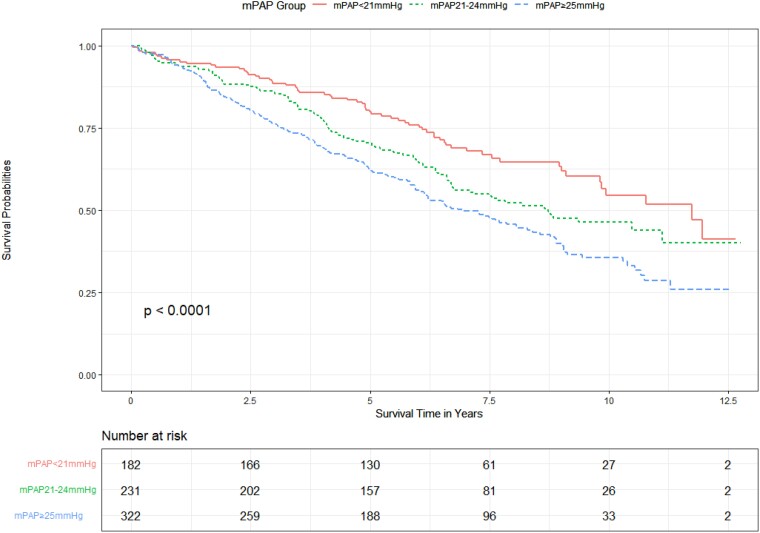
Of the 735 patients with a PVR >2–≤3 WU, 182 patients (24.8%) had a mPAP <21 mmHg, 231 patients (31.4%) had a mPAP 21–24 mmHg, and 322 patients (43.8%) had a mPAP ≥25 mmHg. Kaplan–Meier curves (Figure 4) show a worsening mortality with increasing pressure group, and pairwise comparisons were significant for all group comparisons (P = .02).
Figure 4.
Unadjusted effect of mean pulmonary artery pressure on survival in patients with a pulmonary vascular resistance >2–≤3 WU.
Within subgroup PVR >2–≤3 WU, 23.5% (n = 173) patients did not have evidence of lung and/or heart disease. Using Cox-proportional hazard model within the PVR >2–≤3 WU subgroup, mPAP 21–24 mmHg and mPAP ≥25 mmHg remained significant independent predictors of mortality, when adjusting for lung disease, left heart disease and CTD, age, and gender (P = .005; Table 9). Proportional hazard assumption was met (P = .61).
Table 9.
Adjusted mortality for confounders across mean pulmonary artery pressure groups in patients with a pulmonary vascular resistance >2–≤3 WU
| Adjusted for lung diseasea n = 718 | Adjusted for heart diseasea n = 694 | Adjusted for CTDa n = 735 | Adjusted for lung and heart diseasea n = 680 | Adjusted for all comorbiditiesa n = 680 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| mPAP 21–24 mmHg | 1.56 (1.13–2.15) | .007147 | 1.64 (1.18–2.26) | .00283 | 1.56 (1.14–2.13) | .00537 | 1.71 (1.23- 2.39) | .00151 | 1.75 (1.25–2.44) | .00107 |
| mPAP ≥25 mmHg | 2.12 (1.57–2.86) | .000000914 | 1.96 (1.44–2.67) | .0000210 | 2.14 (1.60–2.86) | .000000244 | 2.00 (1.45–2.75) | .0000236 | 2.05 (1.48–2.82) | .0000125 |
aAdjusted for age/sex.
Impact of the 2022 ESC/ERS guideline definition
Retrospectively redefining the PH groups by haemodynamic criteria based on the 2022 ESC guideline definition of PH, we find that of the patients with a mPAP 21–24 mmHg who were previously diagnosed at not having PH, 53.3% are now classified as having pre-capillary PH, 11.3% are classified as having isolated post-capillary PH, 1% are classified as having combined post-capillary PH, and 34.4% have unclassifiable PH (Table 10).
Table 10.
Pulmonary hypertension classification based on 2015 and 2022 ESC/ERS guideline definitions
| mPAP <21 mmHg (n = 968) | mPAP 21–24 mmHg (n = 689) | mPAP ≥25 mmHg (n = 1272) | Overall (n = 2929) | |
|---|---|---|---|---|
| 2015 ESC/ERS guideline definition | ||||
| Pre-capillary pulmonary hypertensiona | 0 (0%) | 0 (0%) | 757 (59.5%) | 757 (25.8%) |
| Isolated post-capillary pulmonary hypertensionb | 0 (0%) | 0 (0%) | 395 (31.1%) | 395 (13.5%) |
| Combined post-capillary pulmonary hypertensionc | 0 (0%) | 0 (0%) | 120 (9.4%) | 120 (4.1%) |
| No pulmonary hypertension | 968 (100%) | 689 (100%) | 0 (0%) | 1657 (56.6%) |
| 2022 ESC/ERS guideline definition | ||||
| Unclassified pulmonary hypertensiond | 0 (0%) | 237 (34.4%) | 43 (3.4%) | 280 (9.6%) |
| Pre-capillary pulmonary hypertensiona | 0 (0%) | 367 (53.3%) | 714 (56.1%) | 1081 (36.9%) |
| Isolated post-capillary pulmonary hypertensionb | 0 (0%) | 78 (11.3%) | 175 (13.8%) | 253 (8.6%) |
| Combined post-capillary pulmonary hypertensionc | 0 (0%) | 7 (1.0%) | 340 (26.7%) | 347 (11.8%) |
| No pulmonary hypertension | 968 (100%) | 0 (0%) | 0 (0%) | 968 (33.1%) |
a2015 ESC/ERS criteria; mPAP ≥25 mmHg, PAWP ≤15 mmHg. 2022 ESC/ERS criteria; mPAP >20 mmHg, PVR >2 WU, PAWP ≤15 mmHg.
b2015 ESC/ERS criteria; mPAP ≥25 mmHg, PAWP >15 mmHg, DPG, diastolic pulmonary gradient <7 mmHg and/or PVR ≤3 WU. 2022 ESC/ERS criteria; mPAP >20 mmHg, PVR ≤2 WU, PAWP >15 mmHg.
c2015 ESC/ERS criteria; mPAP ≥25 mmHg, PAWP >15 mmHg, DPG ≥7 mmHg and/or PVR >3 WU. 2022 ESC/ERS criteria; mPAP >20 mmHg, PVR >2 WU, PAWP >15 mmHg.
d2022 ESC/ERS criteria; mPAP >20 mmHg PVR ≤2 WU, PAWP ≤15 mmHg.
Discussion
To our knowledge, EVIDENCE-PAH UK is the first study aiming to understand the impact of the new ESC/ERS guidelines on a national cohort specific to PH. In this study, we confirm that among patients referred to PH centres the finding of a mPAP 21–24 mmHg or PVR >2–≤3 WU confers an adverse prognosis even in the absence of confounding lung and heart disease (Structured Graphical Abstract). This supports the changes made by the new ESC/ERS guidance in its definition of pre-capillary PH. Most patients referred to PH centres with a mPAP 21–24 mmHg and PVR >2–≤3 WU have comorbid lung or heart disease and may therefore represent Groups 2 or 3 patients rather than a primary vasculopathy.
This study provides a UK national perspective on the newly included cohort of PH patients based on updated ESC/ERS guidelines in a real-life population referred to PH services. The population presented here represents the typical PH referral population in contrast to the landmark Veteran study which evaluated a predominantly heart failure population, with an overwhelming male bias (96.6%).11 PAH registry data show female predominance in patients diagnosed with PAH,24–27 as found in the population presented here.
There was no difference in patient’s subjective assessment of symptoms score; WHO, CAMPHOR symptoms, Borg score during exercise tests, between patients who had a mPAP <21 mmHg and mPAP 21–24 mmHg. These observations suggest symptomatic status does not discriminate between haemodynamic groups, despite differing prognosis. All patients referred to PH services have a high symptom burden, which is associated with an increased mortality even among those in whom haemodynamic findings were normal.
With the new 2022 ESC/ERS Guidelines, lowering the thresholds for diagnosis of pre-capillary PH to a mPAP >20 mmHg and PVR >2 WU (and a PAWP ≤15 mmHg), it is important to understand this population in greater detail. This study focuses on these mild elevations of haemodynamics; mPAP 21–24 mmHg and PVR >2–≤3 WU and the interplay with comorbidities. Observed survival across all mPAP groups is consistent with data published in the literature.10–14 We show a mPAP of 21–24 mmHg confers poorer survival outcomes compared to patients with normal pulmonary artery pressure (mPAP <21 mmHg).
We used standard investigations used in common clinical practice to further phenotype this population, to identify conditions that may act as contributors to mortality in this population. mPAP 21–24 mmHg remained an independent predictor of mortality when adjusting for significant lung, left heart disease, age, and gender in the presence of mildly elevated PVR (PVR >2–≤3 WU). This excess mortality within the mPAP group 21–24 mmHg, in certain subgroups might be influenced by associations, for instance with chronic thrombo-embolic disease, and is associated with malignancy,9 while patients with a multisystem disease such as CTD, may have a poor prognosis due to organ involvement other than pulmonary vasculopathy. Understanding causality requires further evaluation.
Analysis of the population free of evidence of lung or heart disease reveals an inverse association between mPAP and survival. The Kaplan–Meier curves suggest similar survival between those with normal haemodynamics and those with a mPAP between 21–24 mmHg for the first 4 years after catheterization, thereafter diverging. It is tempting to consider this as evidence of a progressive vasculopathy in this population and certainly justifies close follow-up of patients in this group.
With the new ESC/ERS guidelines, PVR will play a key role in stratification in patients with a mPAP 21–24 mmHg as well as those with a mPAP ≥25 mmHg. Pulmonary vascular resistance is a predictor of mortality in the whole study population and within those with a mPAP 21–24 mmHg. This supports previous published data that suggest even mild elevations in PVR are associated with a poorer survival outcome.15 The data presented here further support even mildly elevated PVR as an important predictor of survival amongst the newly defined subgroup of patients with a mPAP 21–24 mmHg.
The leading causes of death in CTD are PAH and lung disease.28–30 Our study shows that among CTD patients referred to PH centres, in the absence of lung and/or heart disease, survival is directly associated with mPAP. The presence of CTD without comorbidity does not confer a worse survival outcome in patients with a normal pulmonary artery pressure (mPAP <21 mmHg); however, a mPAP 21–24 mmHg in the presence of isolated pre-capillary PH in patients with CTD does impact survival, Kaplan–Meier curves separating after 5 years, prior to this the survival curves are similar to that of patients with no CTD, heart or lung disease but normal haemodynamics. This again suggests a progressive vasculopathy, and prospective surveillance of these patients should be recommended, since this group may benefit from early intervention.31
The presence of heart or lung disease, alone or in combination, is associated with poorer survival outcomes irrespective of PH severity. Excluding ‘comorbidity’ plays a vital role when making a diagnosis of PAH at lower thresholds. Large registry studies demonstrate the changing demographics in PAH, with older and more comorbidity present in a real-life population, resulting in diagnostic and treatment challenges.21,32 Our study shows within the subgroup mPAP 21–24 mmHg, only 31.8% of patients did not have underlying heart of lung disease. Given the updated guidelines, many more patients with ‘early’ PH are likely to be identified at PH centres. We have shown that this is a very heterogeneous population, potentially only a minority having a true vasculopathy, thus recruiting to interventional studies in this population may prove challenging. Including a minority of patients with a mPAP of 21–24 mmHg could simply reduce the signal-to-noise ratio without providing any real evidence of efficacy among the newly included subpopulation, for example, the UNISUS of high dose vs. usual dose macitentan (NCT04273945), while in studies dedicated to this population it may prove challenging to recruit only those without comorbidity (ESRA study, NCT05339087).
Pulmonary arterial hypertension is a progressive disease, associated with poor prognosis despite advances in therapy. Patients often have a delay between symptom onset and diagnosis of PAH, registry data suggesting close to 2 years.32,33 Diagnosis is often late in the disease process. At diagnosis, patients are highly symptomatic with a World Health Organization functional Class III/IV and exhibit high-risk criteria on risk stratification.25,33,34 The updated diagnostic criteria provide an opportunity to identify patients earlier thus encouraging close monitoring and inclusion in studies. There are risks to be navigated. Pulmonary hypertension clinicians must avoid treatment without evidence. We must avoid mis-labelling patients with heart and lung disease as having PAH and undertreating the primary pathology. Finally, we must determine how to manage the psychological and social impact of diagnosing but not treating patients with PAH given the terrible prognosis of untreated PAH.
Limitations
This study has several limitations. Firstly, the referral population is a large and heterogeneous population. By the time referral is made to PH services, patients are often a sicker population with multiple comorbidities that may affect survival across mPAP groups. Sampling of mPAP ≥25 mmHg was limited to allow for site feasibility during the coronavirus disease 2019 pandemic and introduces selection bias. By design, the mPAP ≥25 mmHg population was stratified to include patients with Groups 2 and 3 PH as well including patients labelled as PAH using the 2009 definition which did not require the PVR to be elevated, thus the comparator population included a large proportion that does not meet the standard PAH definition of patients included in the pivotal interventional trials. In a large retrospective cohort study, missing data and data errors are unavoidable, we made attempts to minimize this by training and verification checks and regular queries to individual sites. Exclusion of missing PVR values may exclude patients where right heart catheterizations were performed early in the eligibility period or patients with extremely severe PH where a complete procedure could not be performed. In the design of the study, we set a haemodynamic threshold (PVR 2–<3 WU) as the upper limit of normal. To understand the impact of the ESC guidelines, we analysed as per newly defined threshold, PVR >2–≤3 WU. Fluid and exercise challenge was irregularly utilized and documented with significant variation of use, lack of standardization, and interpretation. Patient participation in investigational trials may lack full documentation, especially amongst patients who did not previously meet the new criteria for pre-capillary PH. Although standardized investigations were used to assess clinical data, in non-quantitative measures, there is always risk of introducing bias despite best efforts to standardize care across the UK.
Conclusion
Patients referred to PH services who have a mPAP of 21–24 mmHg or a PVR of >2–≤3 WU have an increased mortality compared to those with normal haemodynamics, even in the absence of comorbidity. The population is very heterogeneous, most having heart or lung comorbidity, requiring comprehensive assessment to ensure that any underlying pathology is identified and treated. Interventional studies in this population must focus on the group with a ‘true’ vasculopathy; however, given the relatively late divergence in mortality we have found, demonstrating improvement in mortality and possibly morbidity may prove difficult.
Supplementary Material
Acknowledgements
I would like to give acknowledgement to clinical trials teams at each designated Pulmonary Hypertension tertiary centre for their contributions to the study. I would like to thank Dr Ryan KM Dhunnookchand, Martin Kwok, and Anita Saigal for their contributions to data extraction.
Contributor Information
Nina Karia, National Pulmonary Hypertension Service, Royal Free Hospital London NHS Foundation Trust, Pond Street, London NW3 2QG, UK; Institute of Cardiovascular Science, University College London, London WC1E 6BT, UK.
Luke Howard, National Pulmonary Hypertension Service, Hammersmith Hospital, London W12 0HS, UK.
Martin Johnson, Scottish Pulmonary Vascular Unit, Golden Jubilee National Hospital, Glasgow G81 4DY, UK.
David G Kiely, Sheffield Pulmonary Vascular Disease Unit, Royal Hallamshire Hospital, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield S10 2JF, UK.
James Lordan, Pulmonary Vascular Unit, Freeman Hospital, Newcastle upon Tyne NE7 7DN, UK.
Colm McCabe, National Pulmonary Hypertension Service, Royal Brompton Hospital, London SW3 6NP, UK; National Heart and Lung Institute, Imperial College, London SW3 6LY, UK.
Joanna Pepke-Zaba, Pulmonary Vascular Disease Unit, Royal Papworth Hospital NHS Foundation Trust, Cambridge CB2 0AY, UK.
Rose Ong, Actelion Pharmaceuticals Ltd, A Janssen Pharmaceutical Company of Johnson and Johnson, Global Epidemiology, Allschwil CH-4123, Switzerland.
Michael Preiss, Actelion Pharmaceuticals Ltd, Janssen Pharmaceutical Company of Johnson & Johnson, Global Medical Affairs, Allschwil CH-4123, Switzerland.
Daniel Knight, National Pulmonary Hypertension Service, Royal Free Hospital London NHS Foundation Trust, Pond Street, London NW3 2QG, UK; Institute of Cardiovascular Science, University College London, London WC1E 6BT, UK.
Vivek Muthurangu, Institute of Cardiovascular Science, University College London, London WC1E 6BT, UK.
J Gerry Coghlan, National Pulmonary Hypertension Service, Royal Free Hospital London NHS Foundation Trust, Pond Street, London NW3 2QG, UK; Institute of Cardiovascular Science, University College London, London WC1E 6BT, UK.
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
Conflict of interest has been submitted for all authors in separate conflict of interest forms. EVIDENCE-PAH was partly funded by Actelion, Janssen. To summarize, individual grants unrelated to the manuscript, include; Janssen Ltd, NIHR Sheffield Biomedical Research Centre, British Heart Foundation (BHF) Clinical Research Leave Fellowship (FS/CRLF/20/23004) and Ferrer. Individual consulting fees unrelated to the manuscript include Janssen Ltd, MSD, Ferrer, Altavant and United Therapeutics. Payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or education events, unrelated to the manuscript, include; Janssen Ltd, Inari Medical, MSD, Ferrer, Altavant, United Therapeutics and Tavneos. Support for attending meetings and/or travel include; Janssen Ltd, AOP, MSD, Ferrer, and Unit. Patents planned, unrelated to the manuscript include; United Therapeutics. Participation on a Data Safety Monitoring Board or Advisory Board, unrelated to the manuscript, include; Janssen, MSD, Gossamer, United Therapeutics, and MSD. Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid, unrelated to the manuscript, include; Member of Clinical Reference Group for Specialised Respiratory Medicine (NHS England) and Lead of UK National Audit of Pulmonary Hypertension. Stock or stock options, unrelated to the manuscript, include; ATXA Therapeutics and Janssen.
Data Availability
To allow independent interpretation of the clinical study results, all clinical authors had access to anonymised data, to fulfil their roles under the ICMJE criteria. To allow for full transparency, researchers can request access to study data after publication.
Funding
EVIDENCE-PAH was partly funded by Actelion, Janssen. Sponsors of the study were the Royal Free Hospital. All data gathering and analysis were conducted by the academic institution. There has been no sharing of patient level data. Actelion/Janssen provided support to ensure adherence to the protocol, study delivery, and appropriateness of statistical analysis performed. There was no input in the manuscript writing and no direct alteration by the Janssen authors. All authors reviewed and commented on the manuscript.
Ethical Approval
Ethical approval for this study was obtained by the national ethics committee, Health Research Authority, London-Harrow Research committee REC reference 20/LO/0344. IRAS project ID 275470. Patients who met the criteria for inclusion were centrally submitted to NHS Digital national opt-out service. Patients who had opted out from having their data looked at for research purposes were excluded from the study population. The study was registered at the ISRCTN registry (ISRCTN34481181).
Pre-registered Clinical Trial Number
The study was registered at the ISRCTN registry (ISRCTN34481181).
References
- 1. World Health Organization . Primary Pulmonary Hypertension. Geneva: Report on WHO Meeting; 1975. [Google Scholar]
- 2. Kovacs G, Olschewski A, Berghold A, Olschewski H. Pulmonary vascular resistances during exercise in normal subjects: a systematic review. Eur Respir J 2012;39:319–28. 10.1183/09031936.00008611 [DOI] [PubMed] [Google Scholar]
- 3. Galie N, McLaughlin VV, Rubin LJ, Simonneau G. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J 2019;53:1802148. 10.1183/13993003.02148-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67–119. 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 5. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53:1801913. 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022;43:3618–731. 10.1093/eurheartj/ehac237 [DOI] [PubMed] [Google Scholar]
- 7. Bae S, Saggar R, Bolster MB, Chung L, Csuka ME, Derk C, et al. Baseline characteristics and follow-up in patients with normal haemodynamics versus borderline mean pulmonary arterial pressure in systemic sclerosis: results from the PHAROS registry. Ann Rheum Dis 2012;71:1335–42. 10.1136/annrheumdis-2011-200546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Valerio CJ, Schreiber BE, Handler CE, Denton CP, Coghlan JG. Borderline mean pulmonary artery pressure in patients with systemic sclerosis: transpulmonary gradient predicts risk of developing pulmonary hypertension. Arthritis Rheum 2013;65:1074–84. 10.1002/art.37838 [DOI] [PubMed] [Google Scholar]
- 9. Taboada D, Pepke-Zaba J, Jenkins DP, Berman M, Treacy CM, Cannon JE, et al. Outcome of pulmonary endarterectomy in symptomatic chronic thromboembolic disease. Eur Respir J 2014;44:1635–45. 10.1183/09031936.00050114 [DOI] [PubMed] [Google Scholar]
- 10. Kolte D, Lakshmanan S, Jankowich MD, Brittain EL, Maron BA, Choudhary G. Mild pulmonary hypertension is associated with increased mortality: a systematic review and meta-analysis. J Am Heart Assoc 2018;7:e009729. 10.1161/JAHA.118.009729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maron BA, Hess E, Maddox TM, Opotowsky AR, Tedford RJ, Lahm T, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the veterans affairs clinical assessment, reporting, and tracking program. Circulation 2016;133:1240–8. 10.1161/CIRCULATIONAHA.115.020207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heresi GA, Minai OA, Tonelli AR, Hammel JP, Farha S, Parambil JG, et al. Clinical characterization and survival of patients with borderline elevation in pulmonary artery pressure. Pulm Circ 2013;3:916–25. 10.1086/674756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Douschan P, Kovacs G, Avian A, Foris V, Gruber F, Olschewski A, et al. Mild elevation of pulmonary arterial pressure as a predictor of mortality. Am J Respir Crit Care Med 2018;197:509–16. 10.1164/rccm.201706-1215OC [DOI] [PubMed] [Google Scholar]
- 14. Assad TR, Maron BA, Robbins IM, Xu M, Huang S, Harrell FE, et al. Prognostic effect and longitudinal hemodynamic assessment of borderline pulmonary hypertension. JAMA Cardiol 2017;2:1361–8. 10.1001/jamacardio.2017.3882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maron BA, Brittain EL, Hess E, Waldo SW, Barón AE, Huang S, et al. Pulmonary vascular resistance and clinical outcomes in patients with pulmonary hypertension: a retrospective cohort study. Lancet Respir Med 2020;8:873–84. 10.1016/S2213-2600(20)30317-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med 1998;158:1384–7. 10.1164/ajrccm.158.5.9710086 [DOI] [PubMed] [Google Scholar]
- 17. Probst VS, Hernandes NA, Teixeira DC, Felcar JM, Mesquita RB, Goncalves CG, et al. Reference values for the incremental shuttle walking test. Respir Med 2012;106:243–8. 10.1016/j.rmed.2011.07.023 [DOI] [PubMed] [Google Scholar]
- 18. Seeger W, Adir Y, Barbera JA, Champion H, Coghlan JG, Cottin V, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol 2013;62:D109–16. 10.1016/j.jacc.2013.10.036 [DOI] [PubMed] [Google Scholar]
- 19. Nathan SD, Barbera JA, Gaine SP, Harari S, Martinez FJ, Olschewski H, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J 2019;53:1801914. 10.1183/13993003.01914-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vachiery JL, Tedford RJ, Rosenkranz S, Palazzini M, Lang I, Guazzi M, et al. Pulmonary hypertension due to left heart disease. Eur Respir J 2019;53:1801897. 10.1183/13993003.01897-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoeper MM, Pausch C, Grunig E, Staehler G, Huscher D, Pittrow D, et al. Temporal trends in pulmonary arterial hypertension: results from the COMPERA registry. Eur Respir J 2022;59:2102024. 10.1183/13993003.02024-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Galie N, Barbera JA, Frost AE, Ghofrani HA, Hoeper MM, McLaughlin VV, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015;373:834–44. 10.1056/NEJMoa1413687 [DOI] [PubMed] [Google Scholar]
- 23. Van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 Classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 2013;65:2737–47. 10.1002/art.38098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rich S, Dantzker DR, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med 1987;107:216–23. 10.7326/0003-4819-107-2-216 [DOI] [PubMed] [Google Scholar]
- 25. Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006;173:1023–30. 10.1164/rccm.200510-1668OC [DOI] [PubMed] [Google Scholar]
- 26. Boucly A, Weatherald J, Savale L, Jais X, Cottin V, Prevot G, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J 2017;50:1700889. 10.1183/13993003.00889-2017 [DOI] [PubMed] [Google Scholar]
- 27. Hoeper MM, Kramer T, Pan Z, Eichstaedt CA, Spiesshoefer J, Benjamin N, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J 2017;50:1700740. 10.1183/13993003.00740-2017 [DOI] [PubMed] [Google Scholar]
- 28. Hoffmann-Vold AM, Molberg O, Midtvedt O, Garen T, Gran JT. Survival and causes of death in an unselected and complete cohort of Norwegian patients with systemic sclerosis. J Rheumatol 2013;40:1127–33. 10.3899/jrheum.121390 [DOI] [PubMed] [Google Scholar]
- 29. Garen T, Lerang K, Hoffmann-Vold AM, Andersson H, Midtvedt O, Brunborg C, et al. Mortality and causes of death across the systemic connective tissue diseases and the primary systemic vasculitides. Rheumatology (Oxford) 2019;58:313–20. 10.1093/rheumatology/key285 [DOI] [PubMed] [Google Scholar]
- 30. Tyndall AJ, Bannert B, Vonk M, Airo P, Cozzi F, Carreira PE, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010;69:1809–15. 10.1136/ard.2009.114264 [DOI] [PubMed] [Google Scholar]
- 31. Humbert M, Yaici A, de Groote P, Montani D, Sitbon O, Launay D, et al. Screening for pulmonary arterial hypertension in patients with systemic sclerosis: clinical characteristics at diagnosis and long-term survival. Arthritis Rheum 2011;63:3522–30. 10.1002/art.30541 [DOI] [PubMed] [Google Scholar]
- 32. Ling Y, Johnson MK, Kiely DG, Condliffe R, Elliot CA, Gibbs JS, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med 2012;186:790–6. 10.1164/rccm.201203-0383OC [DOI] [PubMed] [Google Scholar]
- 33. Brown LM, Chen H, Halpern S, Taichman D, McGoon MD, Farber HW, et al. Delay in recognition of pulmonary arterial hypertension: factors identified from the REVEAL Registry. Chest 2011;140:19–26. 10.1378/chest.10-1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prins KW, Thenappan T. World Health Organization Group I pulmonary hypertension: epidemiology and pathophysiology. Cardiol Clin 2016;34:363–74. 10.1016/j.ccl.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
To allow independent interpretation of the clinical study results, all clinical authors had access to anonymised data, to fulfil their roles under the ICMJE criteria. To allow for full transparency, researchers can request access to study data after publication.