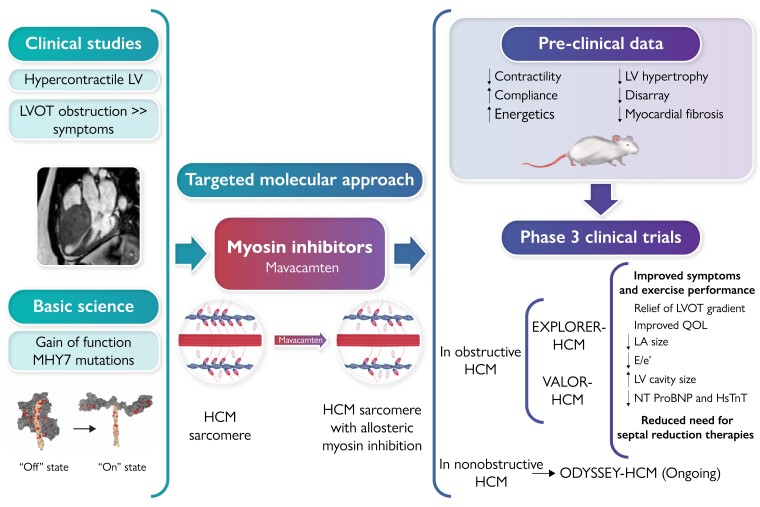
Graphical Abstract
Graphical Abstract.
The path to treatment of obstructive hypertrophic cardiomyopathy. (Top left) Haemodynamic observations demonstrated. Left ventricular (LV) obstruction and symptoms related to LV hypertrophy. (Bottom left) Discovery of genetic variants in ∼40% of patients. (Centre) Sarcomeres in obstructive hypertrophic cardiomyopathy (oHCM) show excess of myosin–actin cross-bridges that are normalized by mavacamten. (Top right) Pre-clinical observations in mouse and pig models of oHCM. (Bottom right) The two placebo-controlled clinical trials of mavacamten in oHCM. HCM, hypertrophic cardiomyopathy; hsTnT, high-sensitivity troponin T; LA, left atrial; LV, left ventricular; LVOT, left ventricular outflow tract; NT-proBNP, N-terminal pro-B-type natriuretic peptide; QOL, quality of life.
Keywords: Pressure gradient, Myectomy, Ejection fraction, Myosin
Abstract
Mavacamten is a first-in-class, targeted, cardiac-specific myosin inhibitor approved by the US Food and Drug Administration for the treatment of adults with symptomatic New York Heart Association Classes II and III obstructive hypertrophic cardiomyopathy (oHCM). Mavacamten was developed to target the hyper-contractile phenotype, which plays a critical role in the pathophysiology of the disease. In Phase 2 and 3 clinical trials, mavacamten was well tolerated, reduced left ventricular outflow tract gradients, improved exercise capacity and symptoms, and was associated with improvements in other clinically relevant parameters, such as patient-reported outcomes and circulating biomarkers. In addition, treatment with mavacamten was associated with evidence of favourable cardiac remodelling in multi-modality imaging studies. Mavacamten substantially reduced guideline eligibility for septal reduction therapy candidates with oHCM and drug-refractory symptoms. In this article, the available efficacy and safety data from completed and ongoing clinical studies of mavacamten in patients with symptomatic oHCM are reviewed. Longer term extension studies may help address questions related to the positioning of mavacamten in current oHCM management algorithms, interactions with background therapy, as well as the potential for disease modification beyond symptomatic relief of left ventricular outflow tract obstruction.
Hypertrophic cardiomyopathy (HCM) is a complex disorder that is caused by dysfunction of the cardiac sarcomere resulting in excessive cardiac myosin–actin cross-bridging and increased sensitivity to calcium.1,2 Core pathophysiologic features of HCM include left ventricular hypertrophy (LVH), most often involving the subaortic region of the inter-ventricular septum, microvascular ischaemia, myocardial fibrosis, and diastolic dysfunction.1–3 Sixty years ago, when the first detailed clinical reports of the disease were published, HCM was considered to be an uncommon condition with high mortality and limited treatment options.4,5 Today, it is estimated that 1:500 persons in the general population have a HCM phenotype.1,6–9
Hypertrophic cardiomyopathy is frequently inherited as an autosomal dominant trait with variable penetrance. Pathogenic variations most frequently occur in genes coding for the sarcomeric proteins beta myosin heavy chain 7 (MYH7) and myosin-binding protein C3 (MYBPC3).1–3,9 Pathogenic variants of myosin encoding genes alter the relaxed state of sarcomeric proteins causing increased cardiomyocyte contractility and energy requirements and impair left ventricular (LV) relaxation and filling.10 About 60% of all HCM patients have negative tests for sarcomeric variants, some of whom may present with a family history of the disease but may have a polygenic aetiology.11,12 Other patients are sporadic, without detectable genetic variants or a family history. The molecular basis for ventricular hypertrophy has not been established.
Approximately two-thirds of the patients with HCM have obstruction of the LV outflow tract (LVOT),5 a major determinant of symptoms and outcomes.5,13 The hyper-contractile phenotype, combined with septal hypertrophy and anatomical abnormalities of the mitral valve apparatus, leads to systolic anterior motion (SAM) of the mitral valve causing mitral-septal contact and subaortic obstruction,13 which is often dynamic and can be intensified with physiologic or pharmacologic interventions, such as exercise, the Valsalva manoeuvre, or a beta adrenergic agonist.13–15
Hypertrophic cardiomyopathy has a diverse clinical presentation and course. Some patients may be asymptomatic or mildly symptomatic, while others experience severe symptoms that impact functional capacity.1,16–18 The most frequent symptoms are exertional dyspnoea, palpitations, fatigue, pre-syncope, and angina, the latter caused by myocardial ischaemia due to coronary arteriolar thickening and/or increased myocardial energy consumption.5,19 Significant complications include syncope, recurrent atrial fibrillation, ventricular tachycardia, stroke, heart failure, and sudden death.20–23 Implantable cardioverter defibrillator placement can reduce the risk of the latter in high-risk patients.24
First-line treatment of obstructive HCM (oHCM) includes oral beta-blockers25 and/or non-dihydropyridine calcium channel blockers.1 Both drug classes slow heart rate, and their modest negative inotropic actions may provide some reduction of intra-cardiac obstruction. Disopyramide, an antiarrhythmic, may be added because of its additional negative inotropic action, but its anticholinergic side effects are frequent limitations. While these three drug classes have been the mainstay of pharmacologic treatment for decades, their use is largely supported by observational studies.1 None address the underlying molecular mechanisms of the disease.
Septal reduction therapy (SRT), either septal myectomy26 or alcohol septal ablation,27 is recommended for patients with symptomatic oHCM, who are refractory to medical treatment.1 Septal reduction therapy substantially improves symptoms and quality of life17,28,29 but may not be appropriate for patients with serious comorbidities or frailty and others who may prefer not to undergo an invasive procedure. To be effective and safe, these procedures require substantial operator experience, which is limited to a few centres of excellence and is not accessible to the majority of patients worldwide.1,26 Therefore, medical management of oHCM remains a major unmet need.
Mavacamten
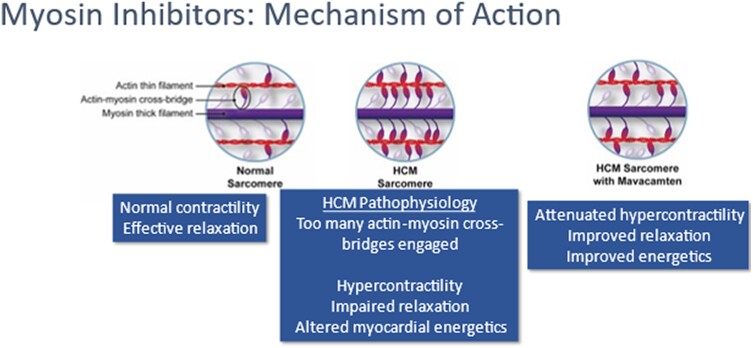
Mavacamten is a selective, allosteric, reversible small molecular cardiac myosin inhibitor, which represents the first disease-specific treatment for oHCM targeting the core pathophysiological mechanism of the disease (see Graphical Abstract, Supplementary data online, Prescribing Information).30 Preclinical studies have shown that mavacamten reduces the probability of myosin–actin cross-bridge formation by decreasing the number of myosin heads that can enter the ‘on actin’ (power-generating) state and shifts the myosin population towards an energy-sparing, super-relaxed ‘off actin’ state10,31–33 by reversibly binding to myosin ATPase (Figure 1). In vivo mouse models that express human myosin mutations and cause oHCM develop age-dependent LVH. Early treatment of these models with mavacamten prevents the development of LVH. Structural studies have shown that while HCM mutations disrupt normal interactions between sarcomere proteins, mavacamten normalizes these interactions and restores physiologic sarcomere function.
Figure 1.
Rationale for the use of mavacamten in hypertrophic cardiomyopathy. Molecular basis of hyper-contractility in hypertrophic cardiomyopathy and the effect of mavacamten. Hypertrophic cardiomyopathy-causing mutations may lead to a gain-of-function effect, increasing the proportion of myosin heads in the active state and leading to adverse energetic, structural, and clinical consequences. Mavacamten binds to the myosin molecules and reduces their likelihood of being in the active state, thus attenuating hyper-contractility and its adverse metabolic effects.31,34 Reprinted from Ho et al.35 (https://www.ahajournals.org/doi/10.1161/CIRCHEARTFAILURE.120.006853) with permission from Wolters Kluwer Health, Inc.
By normalizing the ratio of ‘on’ and ‘off’ myosin heads, mavacamten reduces sarcomeric hyperactivity and the resultant myocardial hypercontractility,31,34 reducing LVOT obstruction and reducing LV filling pressure.31,36,37 Mavacamten has also been shown to reduce maximal force, Ca2+ sensitivity,34 myocardial energy demands, and diastolic dysfunction. In a feline model of oHCM, mavacamten was shown to inhibit myosin ATPase and reduce outflow tract obstruction.38 Additional studies are needed to establish whether mavacamten has other disease-modifying potential of the structural abnormalities of oHCM.
Phase 1 trials of mavacamten were conducted to determine the pharmacokinetic properties and to assess its safety and tolerability. The drug is readily absorbed and is extensively metabolized, primarily through cytochrome (CYP) P450 enzymes, CYP2C19 as well as CYP3A4. The terminal half-life of mavacamten is dependent on CYP2C19 metabolic status and ranges from 6 to 23 days. Inducers and inhibitors of CYP2C19 and CYP3A4 may influence mavacamten systemic exposure.39,40 Detailed pharmacodynamics, pharmacokinetics, and drug interactions are shown in the Supplementary data online, Prescribing Information and Table S1.
PIONEER-HCM
PIONEER-HCM was a 12-week, proof-of-concept and safety, non-randomized, non–placebo-controlled, open-label, Phase 2 trial in 21 patients with symptomatic oHCM (Table 1).41 Two cohorts of patients were studied. In Cohort A, patients were started on mavacamten at 10 or 15 mg/day, with dose titration at 4 weeks based on a targeted reduction in resting LV ejection fraction (LVEF) by 15%–20% from baseline. In Cohort B, patients were started on mavacamten at 2 mg/day, with the potential to increase to 5 mg/day at 4 weeks if the resting LVOT gradient had not decreased by >50% from baseline.
Table 1.
Mavacamten trial characteristics and outcomes
| Title (reference) | PIONEER HCM41,42 | EXPLORER HCM36,37 | VALOR-ACH43 |
|---|---|---|---|
| Design | Open-label Non-randomized |
Double-blind randomized | Double-blind Randomized |
| N | 21 | 251 | 112 |
| Duration (weeks) | 12 | 30 | 16 |
| NYHA class | II/III | II/III | III/IV |
| Dose (mg/day) | 2–20 | 2.5–15 | 2.5–15 |
| Primary endpoint | Change in post-exercise LVOT gradient | Exercise capacity symptom burden | Continued eligibility for SRT |
| OUTCOMES | ↓ LVOT gradients | ↓ LVOT gradients | ↓ eligibility for SRT |
| Improved exercise capacity and ventilatory efficiency | Improved exercise capacity | ↓ LVOT gradients | |
| ↓ NYHA class | ↓ NYHA class | ↓ NYHA class | |
| ↓ NRS dyspnoea score | ↓ NT-proBNP and hs-cTnI | ↓ NT-proBNP and hs-cTnI | |
| Improved health status | Improved diastolic function | Improved health status |
hs-cTnI, high-sensitivity cardiac Troponin I; LVOT, left ventricular outflow tract; N, patient number; NYHA, New York Heart Association; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NRS, numerical rating scale; SRT, septal reduction therapy.
Both cohorts met the primary endpoint of reduction of the post-exercise LVOT gradient from baseline to Week 12, with significant mean changes of −89.5 and −25.0 mmHg in Cohorts A and B, respectively. Administration of mavacamten also resulted in improvements in secondary endpoints, including resting and Valsalva gradients, left ventricular outflow tract gradients, exercise capacity [measured as peak oxygen consumption (pVO2)], ventilatory efficiency [volume expired/carbon dioxide production slope (VE/VCO2 slope)], and numerical rating scale dyspnoea score.
Mavacamten reduced LVEF in a concentration-dependent manner, with substantial reductions in LVOT obstruction occurring at plasma concentrations between 350 and 695 ng/mL. In this range, all patients maintained an LVEF >50%. Plasma concentrations above 695 ng/mL were associated with reduction in LVEF to 34%–49%.41 Otherwise, mavacamten was generally well tolerated with most adverse events (AEs) considered mild or moderate and unrelated to the study drug.
PIONEER open-label extension
Patients who participated in PIONEER-HCM were invited to participate in an ongoing open-label extension study, PIONEER-OLE. After a washout period, the starting dose of mavacamten was 5 mg/day followed by titration at 6 weeks to doses of 5, 10, or 15 mg/day to achieve a plasma concentration of ∼250–500 ng/mL. An interim analysis after 48 weeks of treatment showed persistent and durable reductions in LVOT obstruction, in New York Heart Association (NYHA) functional class and serum concentrations of N-terminal pro-B-type natriuretic peptide (NT-proBNP). Patient-reported symptoms assessed using the Kansas City Cardiomyopathy Questionnaire (KCCQ) also improved. Importantly, LVEF remained above 50% in all patients. An additional follow-up at 3 years was associated with sustained improvement in cardiovascular haemodynamics, symptoms, and quality of life.42
In an analysis using artificial intelligence (AI)-enhanced electrocardiography (AI-ECG) to monitor disease status,44 mean HCM scores from two different AI-ECG algorithms decreased over time with mavacamten, suggesting improvement in ECG morphology. Artificial intelligence-enhanced electrocardiography scores also correlated with favourable measures of disease status, including reductions in Valsalva LVOT gradients and NT-proBNP levels.
EXPLORER-HCM
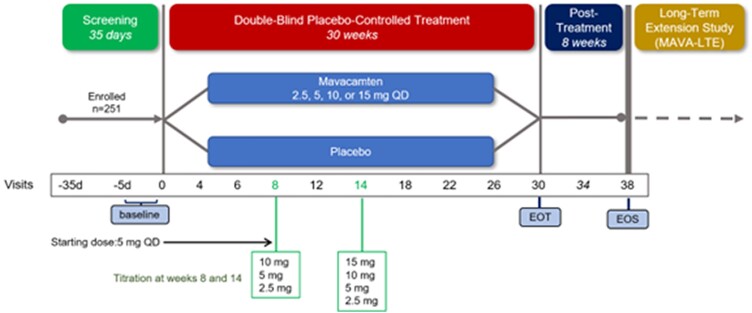
Based on the encouraging results of the PIONEER-HCM trial, mavacamten was advanced to EXPLORER-HCM, a pivotal Phase 3 trial, the largest, double-blind, placebo-controlled, randomized trial in oHCM with a myosin inhibitor conducted to date (Table 1, Figure 2, Supplementary data online, Prescribing Information and Table S2).36,45 The trial was carried out in 68 centres in 13 countries and randomized 251 patients with symptomatic (NYHA Class II/III) oHCM with an LVEF >55%. Almost all patients (92%) were on beta-blocker or calcium channel blocker therapy; treatment with disopyramide was not permitted due to concern over its additive negative inotropic effect in association with mavacamten. The mean age was 58.5 years, and the mean LVEF 74%. The LVOT gradients at rest had a mean of 51.5 mmHg and rose to 73 mmHg with Valsalva.
Figure 2.
EXPLORER-HCM study design. Patients with baseline left ventricular outflow tract pressure gradient >50 mmHg and New York Heart Association Classes II and III symptoms were randomized 1:1 to receive once-daily oral mavacamten (starting dose of 5 mg with a two-step dose titration) or placebo for 30 weeks. Adapted from Ho et al.35 with permission from Wolters Kluwer Health, Inc.
The starting dose of mavacamten was 5 mg/day with dose adjustments at Weeks 8 and 14 to achieve a Valsalva LVOT gradient <30 mmHg and a mavacamten plasma concentration of 350–700 ng/mL.36 There are a number of ways in which clinical outcomes in oHCM can be assessed. In EXPLORER-HCM, the primary endpoint was a composite of exercise capacity and symptom burden at Week 30 compared with baseline, defined as a ≥1.5 mL/kg/min increase in pVO2 and ≥1 NYHA class reduction or a ≥3.0 mL/kg/min improvement in pVO2 and no worsening of NYHA class. The protocol pre-specified temporary discontinuation of study drug if the LVEF fell below 50%.
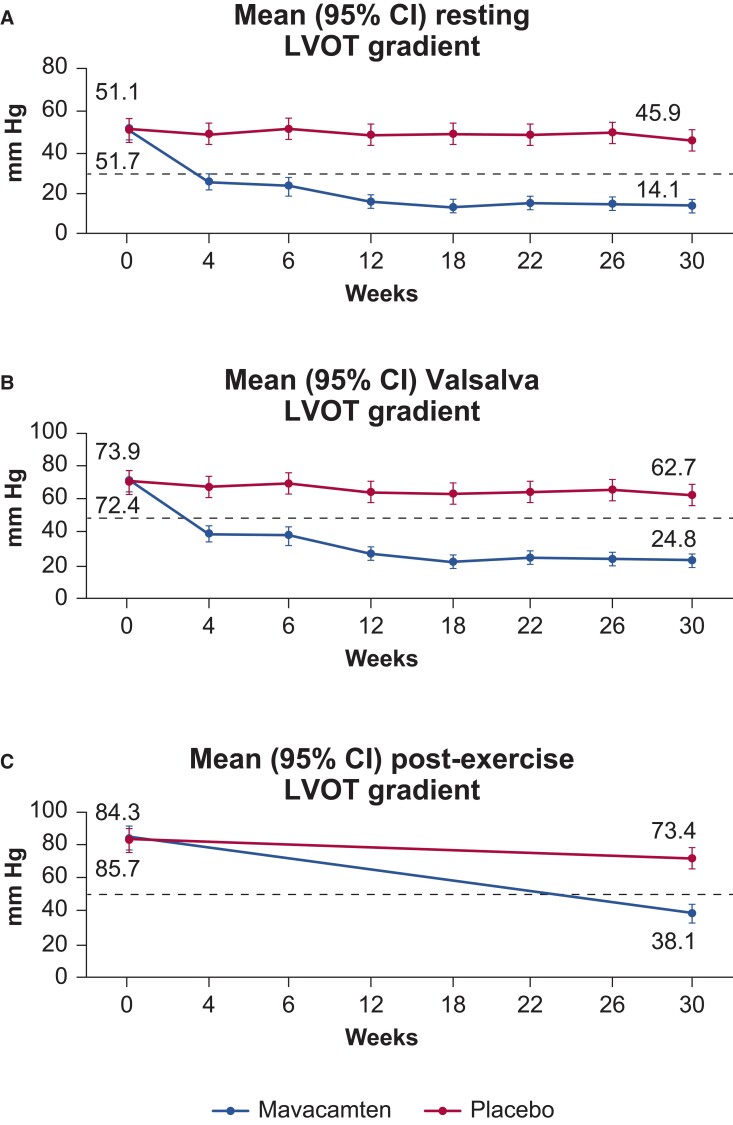
After 30 weeks, significantly more patients in the mavacamten than in the placebo group met the primary endpoint (37% vs. 17%; P = .0005). Mavacamten also demonstrated greater improvements in the secondary and exploratory endpoints; these included significant reductions in the Valsalva and LVOT gradients (Figure 3). There was marked improvement in NYHA class and patient-reported outcomes, including KCCQ clinical summary score (KCCQ-CSS) and an oHCM Symptom Questionnaire Shortness-of-Breath subscore (HCMSQ-SoB). Assignment to mavacamten was also associated with significantly greater reductions of NT-proBNP and high-sensitivity cardiac troponin I (hs-cTnI) than assignment to placebo.36 It is not clear how much the negative inotropy of mavacamten contributes to the observed results.
Figure 3.
Left ventricular outflow tract gradients at baseline and after 30 weeks of mavacamten. (A) Gradients at rest. (B) Valsalva gradient. (C) Post-exercise gradient. Adapted from Olivotto et al.36 with permission from Elsevier.
Almost all patients were compliant and maintained their background HCM therapy during the study; 97% completed the trial. Significant improvements in several peak exercise parameters were observed with mavacamten compared with placebo, including pVO2, ventilatory efficiency (VE/VCO2), circulatory power, exercise time, and end-tidal carbon dioxide (PETCO2). Treatment with mavacamten also significantly improved non-peak exercise parameters, including VE/VCO2 slope, ventilatory power, resting PETCO2, and O2 uptake/workload slope. Among patients treated with mavacamten, improvements in pVO2 and the VE/VCO2 slope correlated significantly with reductions in NT-proBNP (P = .002 and P = .003, respectively). No such correlations were observed in the placebo group.36,46 Thus, these findings demonstrate that mavacamten improved a broad range of both peak and submaximal cardiopulmonary exercise testing (CPET) parameters in patients with oHCM. Results were similar in pre-specified subgroups, including sex, age, duration of diagnosis, LV filling pressure, and presence or absence of hypertension.
Safety
Among the 251 patients in EXPLORER-HCM, 10 patients (8%) in the mavacamten group experienced 11 serious AEs (SAEs), while in the placebo group, 11 patients (9%) experienced 20 SAEs.36 Eight patients experienced cardiac SAEs, including four patients in the mavacamten group (two stress cardiomyopathy and two atrial fibrillation) and four patients in the placebo group (four atrial fibrillation, including one accompanied by heart failure). Temporary drug discontinuation due to LVEF <50% was reported in five patients (three in the mavacamten group and two in the placebo group). Four more patients (3.3%) on mavacamten had LVEF <50% at the end of treatment, three of whom returned to baseline values by the end of washout. Two patients in the mavacamten group permanently discontinued treatment due to AEs (atrial fibrillation, syncope), and one patient in the placebo group died suddenly.45
EXPLORER-HCM secondary analyses
Impact of beta-blocker therapy
A pre-specified subgroup analysis of EXPLORER-HCM showed that mavacamten’s effects on the primary composite endpoint of pVO2 and NYHA class were greater in patients who were not taking beta-blockers during the study compared with those who were (Table 2).36,47 This is explained largely by the blunting of heart rate response to exercise in treated patients. However, the benefits of mavacamten on LVOT obstruction, NYHA class, ventilatory efficiency (VE/VCO2 slope), KCCQ-CSS, and NT-proBNP levels were not altered by beta-blockade. These findings indicate that patients with oHCM can potentially benefit from mavacamten treatment irrespective of concomitant beta-blockade.
Table 2.
EXPLORER-HCM secondary analyses
| Analysis | Key results |
|---|---|
| Beta-blocker subgroup analysis EXPLORER-HCM36: N = 251 (BB: n = 189; no BB: n = 62) EXPLORER-LTE53 cohort of MAVA-LTE (NCT03723655) N = 231 (BB: n = 175; no BB: n = 56) |
|
| CMR subgroup study48 N = 35 |
|
| Echocardiographic parameters37 N = 251 |
|
| Health status analysis50,51 n = 180a |
|
BB, beta-blocker; CMR, cardiac magnetic resonance; E/eʹ, ratio between early mitral inflow velocity and mitral annular early diastolic velocity; EQ-5D, EuroQoL; KCCQ, Kansas City Cardiomyopathy Questionnaire; LAVI, left atrial volume index; LV, left ventricular; LVOT, left ventricular outflow tract; N, number of subjects; NYHA, New York Heart Association; NT-proBNP, N-terminal pro-B-type natriuretic peptide; pVO2, peak oxygen consumption; QoL, quality of life; SAM, systolic anterior motion; VE/VCO2, expired ventilation/carbon dioxide output.
a n = 180 from Spertus et al.50
Cardiac remodelling
Two imaging sub-studies were carried out in EXPLORER-HCM to determine the effect of mavacamten on cardiac structure and function. The cardiac magnetic resonance (CMR) sub-study included 35 patients, 17 of whom received mavacamten (Figure 4).48 After 30 weeks, a reduction in LV mass index, the primary endpoint of this sub-study, occurred with mavacamten but not with placebo (−17.4 vs. −1.6 g/m2; P < .0001). When compared with placebo, mavacamten also significantly reduced maximum LV wall thickness and left atrial volume index (LAVI) from baseline. Cardiac magnetic resonance repeated after 96 weeks of treatment showed persistent cardiac remodelling and normal contractile function.49
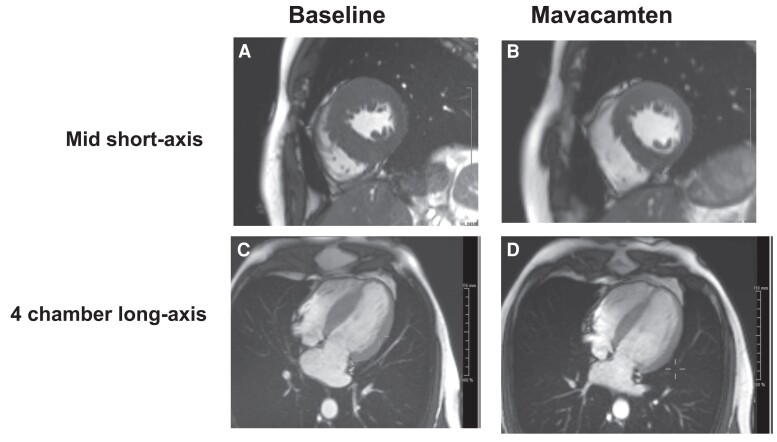
Figure 4.
Cine end-diastolic cardiac magnetic resonance images of a patient with obstructive hypertrophic cardiomyopathy: effects of mavacamten. Baseline before treatment (A and C) and after 30 weeks of mavacamten (B and D). Mid short axis (A and B). Four-chamber long axis (C and D). Compared with baseline, at Week 30, left ventricular mass and maximal wall thickness were reduced from 149 g/m2 and 26 mm to 117 g/m2 and 20 mm, respectively; maximal left atrial size fell from 77 to 59 mL/m2. Left atrial total emptying fraction increased from 27% to 50%. Data are from the EXPLORER-HCM trial.36,48 Images courtesy of Dr Raymond Kwong, Brigham and Women’s Hospital.
The echocardiographic substudy37 (Figure 5) showed that among patients with SAM of the mitral valve at baseline, those assigned to mavacamten showed a significantly higher percentage with complete resolution of SAM than those assigned to placebo. In patients with mitral regurgitation (MR) at baseline, 9% in the mavacamten group vs. no patients in the placebo group exhibited complete resolution of MR at 30 weeks (P < .001). Patients in the mavacamten group had significant associations between serum NT-proBNP level reduction and echocardiographic parameters such as the LAVI, LV thickness, the ratio between mitral inflow velocity and annular early diastolic velocity (E/eʹ), eʹ, and LVOT gradients (rest, Valsalva, and post-exercise; Table 2, Figure 6).
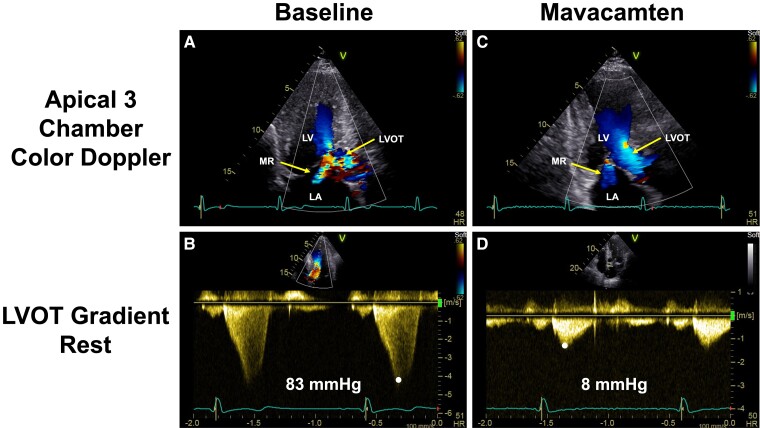
Figure 5.
Echocardiographic images of a patient with obstructive hypertrophic cardiomyopathy: effects of mavacamten. (A) At baseline, colour Doppler shows flow acceleration in the left ventricular outflow tract and significant mitral regurgitation. (B) At baseline, continuous wave Doppler through the left ventricular outflow tract shows a late peak consistent with dynamic left ventricular outflow tract obstruction and a peak gradient of 83 mmHg. (C) Continuous wave Doppler flow through the left ventricular outflow tract demonstrates an early peak and reduction of the peak gradient to 8 mmHg. (D) Following 30 weeks of mavacamten, the colour Doppler flow in the left ventricular outflow tract and in the mitral regurgitation jet are consistent with resolution of the left ventricular outflow tract obstruction and reduction in mitral regurgitation, respectively. Data are from the EXPLORER HCM trial.36,37 Images courtesy of Dr Sheila Hegde, Brigham and Women’s Hospital.
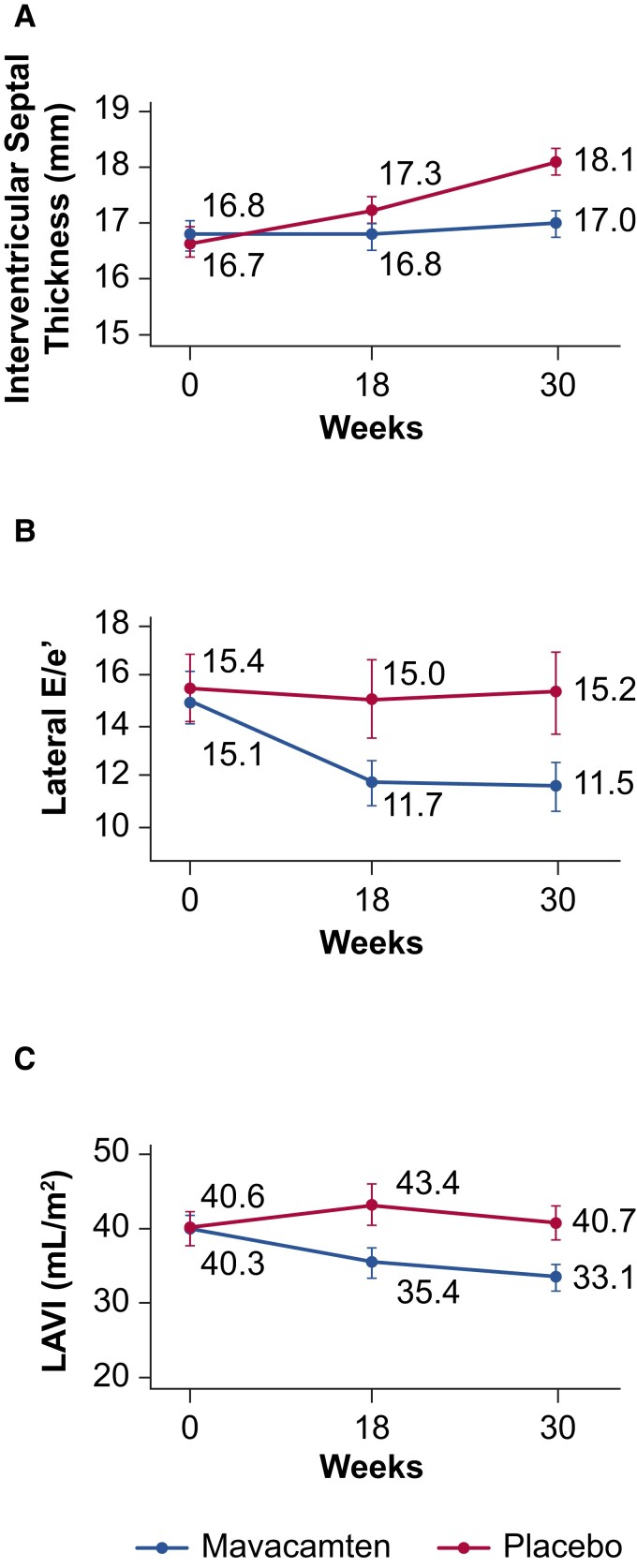
Figure 6.
Echocardiographic findings in EXPLORER-HCM. Line graphs show mean (95% confidence interval) echocardiographic parameters over time. (A) Inter-ventricular septal thickness. (B) Lateral E/eʹ. (C) Left atrial volume index. E/eʹ, ratio between early mitral inflow velocity and mitral annular early diastolic velocity; LAVI, left atrial volume index. Adapted from Hegde et al.37 with permission from Elsevier.
Thus, significant reductions in LAVI, LV mass index, and LV wall thickness with mavacamten were observed in both the CMR and echocardiographic sub-studies. Taken together, these observations indicate that mavacamten improves cardiac structure and function in patients with oHCM.
Health status and quality of life
Patients treated with mavacamten showed greater improvement in overall health status as measured by the KCCQ-CSS, a pre-specified secondary outcome of the trial, which was validated in 196 patients in the EXPLORER-HCM trial.50 Patients treated with mavacamten also experienced greater improvements in the KCCQ Overall Summary Score (KCCQ-OSS), which combines scores from the total symptom, physical limitation, social limitation, and quality of life sub-scales.51 The mean increase from baseline to Week 30 in the KCCQ-OSS was 14.9 in the mavacamten group compared with 5.4 in the placebo group (P < .0001). Similar benefits were observed with mavacamten compared with placebo across all sub-scales. After treatment ended, these benefits of mavacamten on KCCQ scores did not persist; the scores returned to baseline levels after the 8-week washout period.
A separate analysis assessed the effects of mavacamten on health-related quality of life using the EuroQoL 5-dimension 5-level (EQ-5D-5L) index score and the EuroQoL visual analogue scale (EQ-VAS).52 At Week 30, patients randomized to mavacamten reported significantly greater improvements in both EQ-5D-5L and EQ-VAS scores compared with placebo. Taken together, these patient-reported outcome analyses from EXPLORER-HCM indicate that in patients with oHCM, the physiological benefits of mavacamten translate into improved health status and quality of life.
Summary
The EXPLORER-HCM trial demonstrated efficacy of mavacamten in oHCM. In this trial, statistical significance was achieved for the primary and all secondary endpoints.36 Almost 75% of patients exhibited a reduction below guideline-defined thresholds for invasive SRT (post-exercise LVOT peak gradient <50 mmHg), and 56% showed even greater relief of obstruction. Mavacamten improved NYHA class, exercise performance, key aspects of health status, and reduced serum NT-proBNP and troponin I levels and was, overall, safe and well tolerated.
In its review of EXPLORER-HCM, the US Food and Drug Administration (FDA) determined that the number of patients needed to treat to achieve a primary endpoint was 5.2, while the number needed to harm (heart failure or LVEF <30%) was 141.45 The FDA granted mavacamten ‘breakthrough therapy’ designation and in April 2022 approved mavacamten for adults with symptomatic oHCM30; it has also been approved in Australia, Brazil, Canada, Macao, and Switzerland. Applications to other regulatory bodies around the world are under review.
MAVA-LTE: EXPLORER-LTE cohort
While EXPLORER-HCM was under FDA review, the patients who completed the trial were invited to enrol in the EXPLORER-LTE cohort of MAVA-LTE, an ongoing, open-label, dose-blinded, long-term extension study (NCT03723655). Of the 244 patients who completed EXPLORER-HCM, 231 (95%) enrolled in EXPLORER-LTE. After washout of the original treatment (mavacamten or placebo), patients from both arms were started on mavacamten 5 mg/day, with dose adjustments at 4, 8, 12, and 24 weeks based on site-read echocardiographic measures of the Valsalva LVOT gradient and LVEF. This differed from the parent study in which dose adjustment was based on serum mavacamten concentration and central-read echocardiographic parameters. After 24 weeks, dose increases were permitted if the site-read Valsalva LVOT gradient was >30 mmHg or if the post-exercise gradient was >50 mmHg and the LVEF was ≥50%.
An interim analysis performed at a median follow-up of 62.3 weeks showed that mavacamten was associated with clinically important and sustained improvements of LVOT gradients, NYHA class, and NT-proBNP levels that were consistent with those observed in the parent trial. Treatment with mavacamten was generally well tolerated over 315 patient-years of exposure. Of the 231 patients in EXPLORER-LTE, 34 (15%) experienced SAEs at the time of data cut-off, including 5 patients (2.2%) with events related to the drug (3 heart failure and 2 decreased LVEF). Cardiovascular-related AEs that resulted in permanent treatment discontinuation included decreased LVEF (two patients), heart failure, cardiac arrest, and acute myocardial infarction (one patient each). Temporary treatment discontinuation was required in 26 (11%) patients, which included 12 (5.2%) who temporarily discontinued due to LVEF <50%. Left ventricular ejection fraction recovered to >50% in all of them. Seven of these participants resumed treatment, while five withdrew from the study.
These interim results from EXPLORER-LTE support the longer term use of mavacamten in patients with symptomatic oHCM as well as a dose titration and monitoring strategy guided exclusively by site-measured clinical parameters, including LVOT gradients and LVEF, as specified in the current prescribing information (see Supplementary data online, Prescribing Information).
VALOR-HCM
VALOR-HCM was a randomized, double-blind, placebo-controlled, Phase 3 trial that enrolled 112 patients (Table 1).43,54,55 All had been referred for SRT on maximally tolerated background medical therapy, including disopyramide in some. Myectomy had been recommended in 86.6% of the patients and alcohol septal ablation in the remainder. This trial was designed to test the hypothesis that 16 weeks of treatment with mavacamten added to maximally tolerated medical therapy reduces guideline eligibility for SRT43; it did not compare mavacamten with SRT.
The average age of the patients was 60.4 years; 92.9% were in NYHA Class III or IV, with the remainder experiencing Class II symptoms and exertional syncope or near syncope while at rest. The mean resting, Valsalva, and post-exercise LVOT gradients were 49, 76, and 84 mmHg, respectively, and the mean LVEF was 68%. The starting dose of mavacamten was 5 mg/day with titration at 8 and 12 weeks based on core laboratory-measured LVEF and resting and Valsalva LVOT gradients. After 16 weeks of treatment, 76.8% in the placebo group compared with 17.9% of patients in the mavacamten group met the primary composite endpoint of continued eligibility for SRT or patient decision to proceed with SRT (P < .001).43 When compared with placebo, patients in the mavacamten group also showed significant improvements in all secondary outcomes, including resting, Valsalva, and post-exercise LVOT gradients, NYHA class, KCCQ-CSS, and NT-proBNP and cTnI concentrations.
At the end of treatment, 95% of all patients, including most in the placebo group, elected to participate in the active, long-term, extension phase of the trial instead of undergoing SRT. Follow-up at 32 weeks of treatment showed that the clinical benefits observed at 16 weeks were sustained, with 33.0 and 43.0 mmHg mean reductions of resting and Valsalva gradients, respectively.54 Mavacamten was generally well tolerated. Two patients developed atrial fibrillation, one of whom developed an LVEF <30% and underwent permanent drug discontinuation. Seven of 56 patients (12.5%) in the mavacamten group underwent drug discontinuation for LVEF <50% and resumed treatment at a lower dose.
These findings from VALOR-HCM expand upon the results from EXPLORER-HCM to a population with more severe symptoms and demonstrate that mavacamten has the potential to reduce SRT eligibility, although longer term data are needed to confirm these findings.
Discussion
The three completed clinical trials reviewed above have demonstrated the efficacy and relative safety of mavacamten in patients with oHCM who were on standard-of-care background therapies. Mavacamten not only reduced LVOT obstruction and enhanced left ventricular filling but also improved exercise capacity, quality of life, and symptom burden and provided global, multi-dimensional improvement in clinically relevant CPET, CMR, and echocardiographic parameters, patient-reported outcome measures, and biomarkers. Mavacamten also reduced the need for SRT after 16–32 weeks of treatment of severely symptomatic patients with oHCM on maximally tolerated medical therapy. Two of the trials (PIONEER-HCM and EXPLORER-HCM) used pharmacokinetic monitoring.
In 2022, at the time of FDA approval, Bristol Myers Squibb announced that the wholesale acquisition cost per capsule was ∼$245.20 with a monthly list price of $7,356.16.56
Safety
Because mavacamten may cause decreases in LVEF, regular monitoring for clinical symptoms of heart failure (e.g. dyspnoea, fatigue, palpitations, worsening or new arrhythmia, leg oedema) and for systolic dysfunction is recommended, including echocardiographic assessments at 4, 8, and 12 weeks after initiating mavacamten treatment and every 12 weeks thereafter (see Supplementary data online, Prescribing Information). For patients with LVEF <50% at any time during mavacamten treatment, temporary or permanent treatment discontinuation is warranted (see Supplementary data online, Figure S1). Open-label, follow-up studies evaluating the long-term efficacy and safety of mavacamten in the three cited trials will provide more information on the durability of improvements and the safety profile of the drug.
To minimize risk, mavacamten is currently available in the USA through a Risk Evaluation and Mitigation Strategy (REMS) programme, designed to monitor patients periodically with echocardiograms for early detection of systolic dysfunction and to screen for drug interactions prior to each prescription fill.30 Risk Evaluation and Mitigation Strategy requires the healthcare provider and pharmacist to undergo educational programmes, including counselling patients on the risk of heart failure, assessing the patient’s cardiovascular status, and obtaining echocardiograms at specific times after starting the drug. It also provides a guide for patients who should be screened for potential drug–drug interactions and undergo an echocardiogram prior to enrolling in the REMS programme (see Supplementary data online, REMS).
In commenting on the EXPLORER-HCM trial, the FDA review stated:
Although it is unclear whether the magnitude of improvement in pVO2 in this trial (mean treatment effect for pVO2 of 1.4 mL/kg/min) will lead to improved mortality, the consistency of effect between variables (i.e. improvement of pVO2, reduction or no worsening of NYHA class, improvements in patient reported outcomes and reduction of the LVOT gradient), provided compelling evidence of the utility of mavacamten for improving how patients with oHCM feel and function.45
In commenting on the safety findings of EXPLORER-HCM, the FDA review stated:
The overall safety profile of mavacamten was similar to placebo in the setting of careful safety monitoring … The main concern is mavacamten-mediated reversible induction of systolic dysfunction in the real-world setting where rigorous safety monitoring may not occur, thus potentially magnifying the differential risk of heart failure and/or systolic dysfunction observed in the Phase 3 trial.45
The precise role of myosin inhibition in the management of oHCM is not clear at this time. However, the National Institute for Health and Care Excellence in the UK recommends mavacamten ‘as an option for treating obstructive hypertrophic cardiomyopathy in adults who have a NYHA class of II or III, if it is an add-on to individually optimized standard care that includes beta-blockers, non-dihydropyridine calcium-channel blockers or disopyramide, unless these are contraindicated.’57
It would appear that maintaining patients on the standard drugs should not be considered mandatory in order to proceed to mavacamten since important limiting side effects or contraindications to these agents may exist. The DISCOVER-HCM registry for mavacamten (NCT05489705) is expected to enrol ∼1500 patients with oHCM and will assess the real-world safety and effectiveness of mavacamten in the USA. ClinicalTrials.gov lists trials on adult patients with oHCM in China (NCT05174416) and Japan (NCT05414175). The EMBARK-HFpEF trial (NCT04766892) will examine its role in heart failure with preserved ejection fraction and the ODYSSEY-HCM trial (NCT05582395) non-oHCM. As the results from these trials become available, it will be possible to develop more precise clinical guidelines for the role of mavacamten.
The future
More information is needed to understand the characteristics and predictors of responders vs. non-responders, the safety and efficacy of mavacamten begun in childhood, the responses in different genotypes, and the role of mavacamten in non-oHCM; a pilot study of the latter has been performed,58 and a Phase 3 ODYSSEY trial of such patients has begun (NCT05582395).59 It is not known whether patients with heart failure and preserved ejection fraction without HCM can be improved by mavacamten; a proof of concept trial to test this is ongoing in the EMBARK HFpEF trial (NCT04766892).60 More follow-up is also needed to determine whether mavacamten can reduce the need for SRT in the longer term. Optimal duration of therapy and the potential disease-modifying effects of mavacamten in HCM should be defined.
Aficamten, a synthesized next-generation, small-molecular, selective cardiac myosin inhibitor, has a shorter human half-life (3.4 days) than mavacamten (7–9 days), allowing a shorter time interval to reach a steady state plasma concentration and more rapid reversibility after dose reduction.61 In a feline translational model of oHCM, aficamten demonstrated a dose-dependent reduction of the LVOT pressure gradients.62 A Phase 1 dose escalation study in normal subjects showed aficamten to be well tolerated and to reduce LVEF in a concentration dependent manner.63
In REDWOOD-HCM (NCT 04212896), a Phase 2, placebo-controlled trial of 41 patients with oHCM with peak LVOT gradients >50 mmHg and LVEF >60%, aficamten appeared to be safe, well tolerated, and reduced the systolic pressure gradient at rest and after Valsalva; it also reduced both LVEF and symptoms.64 In FOREST-HCM (NCT04848508), the open-label extension of the REDWOOD-HCM trial, aficamten maintained efficacy and was well tolerated for up to 48 weeks. Of the 19 patients meeting standard criteria for SRT at baseline, none still met these criteria at 48 weeks.65 The Phase 3 SEQUOIA-HCM study (NCT05186818) is currently ongoing.66 ClinicalTrials.gov also lists two other aficamten trials, MAPLE-HCM (NCT05767346) comparing it with metoprolol and FOREST HCM (NCT04848506), an open-label study to collect safety data.
Conclusions
Mavacamten is the first cardiac myosin inhibitor approved for the treatment of adults with symptomatic oHCM. It provides a novel pharmacologic treatment option for patients, which targets the underlying pathophysiology of the disease, and it is well tolerated in a large majority of patients. Results from ongoing long-term extension studies and real-world experience in clinical practice will expand upon the efficacy and safety findings obtained in the clinical trials summarized in this review.
Based on the available data, mavacamten is beneficial, at least in the short to medium term, in patients with oHCM who remain symptomatic despite single or multi-drug dose treatment with beta-blockers and calcium channel blockers and may postpone or avoid the need for SRT.
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
E.B. has received research support through Brigham and Women’s Hospital from AstraZeneca, Daiichi-Sankyo, Merck, and Novartis and payments as a consultant from MyoKardia Inc., a wholly owned subsidiary of Bristol Myers Squibb and from Amgen, Boehringer-Ingelheim/Lilly, Cardurion, NovoNordisk, and Verve. S.S. has received personal fees from MyoKardia Inc., a wholly owned subsidiary of Bristol Myers Squibb and payments as a consultant for Cytokinetics. T.P.A. has no competing interests to disclose. P.M.E. has received payments as a consultant and personal fees from MyoKardia Inc., a wholly owned subsidiary of Bristol Myers Squibb, Sanofi Genzyme, AstraZeneca, Pfizer, and DinaQor and reports a patent GB1815111.8 issued to his institution. I.O. has received advisory board and research grants from MyoKardia Inc., a wholly owned subsidiary of Bristol Myers Squibb, Cytokinetics, Boston Scientific, Sanofi-Genzyme, Shire Takeda, Amicus Therapeutics, Menarini International, Bayer, and Tenaya Therapeutics; personal fees from Sanofi-Genzyme, Shire Takeda, and Bayer; and payments as a consultant from MyoKardia Inc., a wholly owned subsidiary of Bristol Myers Squibb.
Supplementary Material
Contributor Information
Eugene Braunwald, Division of Cardiovascular Medicine, TIMI Study Group, Brigham and Women’s Hospital, 60 Fenwood Road, Boston, MA 02115, USA; Department Medicine, Harvard Medical School, Boston, MA, USA.
Sara Saberi, Department of Internal Medicine, Division of Cardiovascular Medicine, University of Michigan Medical School, Ann Arbor, MI, USA.
Theodore P Abraham, UCSF HCM Center of Excellence, University of California San Francisco, San Francisco, CA, USA.
Perry M Elliott, Institute of Cardiovascular Science, University College London, London, UK.
Iacopo Olivotto, Meyer Children’s Hospital, University of Florence, Florence, Italy.
Data Availability
No data were generated or analysed for or in support of this paper.
Funding
Medical writing and editorial support were provided by Kim Fuller, PhD, of Lumanity Communications Inc., funded by MyoKardia Inc., a wholly owned subsidiary of Bristol Myers Squibb. The authors received no compensation for the preparation of this review.
References
- 1. Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation 2020;142:e558–631. 10.1161/CIR.0000000000000937 [DOI] [PubMed] [Google Scholar]
- 2. Marian AJ, Braunwald E. Hypertrophic cardiomyopathy: genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ Res 2017;121:749–70. 10.1161/CIRCRESAHA.117.311059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lehman SJ, Crocini C, Leinwand LA. Targeting the sarcomere in inherited cardiomyopathies. Nat Rev Cardiol 2022;19:353–63. 10.1038/s41569-022-00682-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morrow AG, Braunwald E. Functional aortic stenosis; a malformation characterized by resistance to left ventricular outflow without anatomic obstruction. Circulation 1959;20:181–9. 10.1161/01.cir.20.2.181 [DOI] [PubMed] [Google Scholar]
- 5. Braunwald E, Lambrew CT, Rockoff SD, Ross J Jr, Morrow AG. Idiopathic hypertrophic subaortic stenosis. I. A description of the disease based upon an analysis of 64 patients. Circulation 1964;30 Suppl 4:3–119. 10.1161/01.cir.29.5s4.iv-3 [DOI] [PubMed] [Google Scholar]
- 6. Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Circulation 1995;92:785–9. 10.1161/01.cir.92.4.785 [DOI] [PubMed] [Google Scholar]
- 7. Maron BJ, Ommen SR, Semsarian C, Spirito P, Olivotto I, Maron MS. Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol 2014;64:83–99. 10.1016/j.jacc.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 8. Maron MS, Hellawell JL, Lucove JC, Farzaneh-Far R, Olivotto I. Occurrence of clinically diagnosed hypertrophic cardiomyopathy in the United States. Am J Cardiol 2016;117:1651–4. 10.1016/j.amjcard.2016.02.044 [DOI] [PubMed] [Google Scholar]
- 9. Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol 2015;65:1249–54. 10.1016/j.jacc.2015.01.019 [DOI] [PubMed] [Google Scholar]
- 10. Toepfer CN, Garfinkel AC, Venturini G, Wakimoto H, Repetti G, Alamo L. Myosin sequestration regulates sarcomere function, cardiomyocyte energetics, and metabolism, informing the pathogenesis of hypertrophic cardiomyopathy. Circulation 2020;141:828–42. 10.1161/CIRCULATIONAHA.119.042339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mazzarotto F, Olivotto I, Boschi B, Girolami F, Poggesi C, Barton PJR et al. Contemporary insights into the genetics of hypertrophic cardiomyopathy: toward a new era in clinical testing? J Am Heart Assoc 2020;9:e015473. 10.1161/JAHA.119.015473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Watkins H. Time to think differently about sarcomere-negative hypertrophic cardiomyopathy. Circulation 2021;143:2415–7. 10.1161/CIRCULATIONAHA.121.053527 [DOI] [PubMed] [Google Scholar]
- 13. Maron MS, Olivotto I, Zenovich AG, Link MS, Pandian NG, Kuvin JT et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation 2006;114:2232–9. 10.1161/CIRCULATIONAHA.106.644682 [DOI] [PubMed] [Google Scholar]
- 14. Braunwald E, Ebert PA. Hemodynamic alterations in idiopathic hypertrophic subaortic stenosis induced by sympathomimetic drugs. Am J Cardiol 1962;10:489–95. 10.1016/0002-9149(62)90373-9 [DOI] [PubMed] [Google Scholar]
- 15. Braunwald E, Oldham HN Jr, Ross J Jr, Linhart JW, Mason DT, Fort L Jr. The circulatory response of patients with idiopathic hypertrophic subaortic stenosis to nitroglycerin and to the Valsalva maneuver. Circulation 1964;29:422–31. 10.1161/01.cir.29.3.422 [DOI] [PubMed] [Google Scholar]
- 16. Stewart S, Mason DT, Braunwald E. Impaired rate of left ventricular filling in idiopathic hypertrophic subaortic stenosis and valvular aortic stenosis. Circulation 1968;37:8–14. 10.1161/01.cir.37.1.8 [DOI] [PubMed] [Google Scholar]
- 17. Maron BJ. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med 2018;379:655–68. 10.1056/NEJMra1710575 [DOI] [PubMed] [Google Scholar]
- 18. Capota R, Militaru S, Ionescu AA, Rosca M, Baicus C, Popescu BA et al. Quality of life status determinants in hypertrophic cardiomyopathy as evaluated by the Kansas City Cardiomyopathy Questionnaire. Health Qual Life Outcomes 2020;18:351. 10.1186/s12955-020-01604-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zaiser E, Sehnert AJ, Duenas A, Saberi S, Brookes E, Reaney M. Patient experiences with hypertrophic cardiomyopathy: a conceptual model of symptoms and impacts on quality of life. J Patient Rep Outcomes 2020;4:102. 10.1186/s41687-020-00269-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guttmann OP, Rahman MS, O’Mahony C, Anastasakis A, Elliott PM. Atrial fibrillation and thromboembolism in patients with hypertrophic cardiomyopathy: systematic review. Heart 2014;100:465–72. 10.1136/heartjnl-2013-304276 [DOI] [PubMed] [Google Scholar]
- 21. Ho CY, Day SM, Ashley EA, Michels M, Pereira AC, Jacoby D et al. Genotype and lifetime burden of disease in hypertrophic cardiomyopathy: insights from the sarcomeric human cardiomyopathy registry (SHaRe). Circulation 2018;138:1387–98. 10.1161/CIRCULATIONAHA.117.033200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marstrand P, Han L, Day SM, Olivotto I, Ashley EA, Michels M et al. Hypertrophic cardiomyopathy with left ventricular systolic dysfunction: insights from the SHaRe registry. Circulation 2020;141:1371–83. 10.1161/CIRCULATIONAHA.119.044366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spirito P, Autore C, Rapezzi C, Bernabo P, Badagliacca R, Maron MS et al. Syncope and risk of sudden death in hypertrophic cardiomyopathy. Circulation 2009;119:1703–10. 10.1161/CIRCULATIONAHA.108.798314 [DOI] [PubMed] [Google Scholar]
- 24. Maron BJ, Shen WK, Link MS, Epstein AE, Almquist AK, JP et al. Efficacy of implantable cardioverter defibrillators for the prevention of sudden death in patients with hypertrophic cardiomyopathy. N Engl J Med 2000;342:365–73. 10.1056/NEJM200002103420601 [DOI] [PubMed] [Google Scholar]
- 25. Cohen LS, Braunwald E. Chronic beta adrenergic receptor blockade in the treatment of idiopathic hypertrophic subaortic stenosis. Prog Cardiovasc Dis 1968;11:211–21. 10.1016/0033-0620(68)90011-x [DOI] [PubMed] [Google Scholar]
- 26. Maron BJ, Dearani JA, Smedira NG, Schaff HV, Wang S, Rastegar H et al. Ventricular septal myectomy for obstructive hypertrophic cardiomyopathy (Analysis Spanning 60 Years Of Practice): AJC expert panel. Am J Cardiol 2022;180:124–39. 10.1016/j.amjcard.2022.06.007 [DOI] [PubMed] [Google Scholar]
- 27. Batzner A, Pfeiffer B, Neugebauer A, Aicha D, Blank C, Seggewiss H. Survival after alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol 2018;72:3087–94. 10.1016/j.jacc.2018.09.064 [DOI] [PubMed] [Google Scholar]
- 28. Rastegar H, Boll G, Rowin EJ, Dolan N, Carroll C, Udelson JE et al. Results of surgical septal myectomy for obstructive hypertrophic cardiomyopathy: the Tufts experience. Ann Cardiothorac Surg 2017;6:353–63. 10.21037/acs.2017.07.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Desai MY, Tower-Rader A, Szpakowski N, Mentias A, Popovic ZB, Smedira NG. Association of septal myectomy with quality of life in patients with left ventricular outflow tract obstruction from hypertrophic cardiomyopathy. JAMA Netw Open 2022;5:e227293. 10.1001/jamanetworkopen.2022.7293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bristol Myers Squibb Company . CAMZYOS™ (Mavacamten) Prescribing Information. Princeton, NJ: Bristol Myers Squibb Company, 2022. [Google Scholar]
- 31. Green EM, Wakimoto H, Anderson RL, Evanchik MJ, Gorham JM, Harrison BC et al. A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science 2016;351:617–21. 10.1126/science.aad3456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kawas RF, Anderson RL, Ingle SRB, Song Y, Sran AS, Rodriguez HM. A small-molecule modulator of cardiac myosin acts on multiple stages of the myosin chemomechanical cycle. J Biol Chem 2017;292:16571–7. 10.1074/jbc.M117.776815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Anderson RL, Trivedi DV, Sarkar SS, Henze M, Ma W, Gong H et al. Deciphering the super relaxed state of human beta-cardiac myosin and the mode of action of mavacamten from myosin molecules to muscle fibers. Proc Natl Acad Sci U S A 2018;115:E8143–52. 10.1073/pnas.1809540115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Awinda PO, Watanabe M, Bishaw YM, Huckabee AM, Agonias KB, Kazmierczak K et al. Mavacamten decreases maximal force and Ca(2+)-sensitivity in the N47K-myosin regulatory light chain mouse model of hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol 2021;320:H881–90. 10.1152/ajpheart.00345.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ho CY, Olivotto I, Jacoby D, Lester SJ, Roe M, Wang A et al. Study design and rationale of EXPLORER-HCM: evaluation of mavacamten in adults with symptomatic obstructive hypertrophic cardiomyopathy. Circ Heart Fail 2020;13:e006853. 10.1161/CIRCHEARTFAILURE.120.006853 [DOI] [PubMed] [Google Scholar]
- 36. Olivotto I, Oreziak A, Barriales-Villa R, Abraham TP, Masri A, Garcia-Pavia P et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2020;396:759–69. 10.1016/S0140-6736(20)31792-X [DOI] [PubMed] [Google Scholar]
- 37. Hegde SM, Lester SJ, Solomon SD, Michels M, Elliott PM, Nagueh SF et al. Effect of mavacamten on echocardiographic features in symptomatic patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2021;78:2518–32. 10.1016/j.jacc.2021.09.1381 [DOI] [PubMed] [Google Scholar]
- 38. Stern JA, Markova S, Ueda Y, Kim JB, Pascoe PJ, Evanchik MJ et al. A small molecule inhibitor of sarcomere contractility acutely relieves left ventricular outflow tract obstruction in feline hypertrophic cardiomyopathy. PLoS One 2016;11:e0168407. 10.1371/journal.pone.0168407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Perera V, Gretler D, Seroogy JD, Chiang M, Palmisano M, Florea V. Pharmacokinetic drug-drug interaction study of mavacamten with verapamil in healthy subjects. Clin Pharm Drug Dev 2022;11:24–5. [DOI] [PubMed] [Google Scholar]
- 40. Grillo MP, Erve JC, Dick R, Driscoll JP, Haste N, Markova S et al. In vitro and in vivo pharmacokinetic characterization of mavacamten, a first-in-class small molecule allosteric modulator of beta cardiac myosin. Xenobiotica 2019;49:718–33. 10.1080/00498254.2018.1495856 [DOI] [PubMed] [Google Scholar]
- 41. Heitner SB, Jacoby D, Lester SJ, Owens A, Wang A, Zhang D et al. Mavacamten treatment for obstructive hypertrophic cardiomyopathy: a clinical trial. Ann Intern Med 2019;170:741–8. 10.7326/M18-3016 [DOI] [PubMed] [Google Scholar]
- 42. Heitner SB, Lester S, Wang A, Hegde SM, Fang L, Balaratnam G et al. Precision pharmacological treatment for obstructive hypertrophic cardiomyopathy with mavacamten: one-year results from PIONEER-OLE. Circulation 2019;140:A13962. [Google Scholar]
- 43. Desai MY, Owens A, Geske JB, Wolski K, Naidu SS, Smedira NG et al. Myosin inhibition in patients with obstructive hypertrophic cardiomyopathy referred for septal reduction therapy. J Am Coll Cardiol 2022;80:95–108. 10.1016/j.jacc.2022.04.04 [DOI] [PubMed] [Google Scholar]
- 44. Tison GH, Siontis KC, Abreau S, Attia Z, Agarwal P, Balasubramanyam A et al. Assessment of disease status and treatment response with artificial intelligence-enhanced electrocardiography in obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2022;79:1032–4. 10.1016/j.jacc.2022.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. FDA Center for Drug Evaluation and Research . Mavacamten (Camzyos). Clinical and Statistical Reviews Application 214998Orig1s000. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/214998Orig1s000Med_StatR.pdf (23 March 2023, date last accessed).
- 46. Wheeler MT, Olivotto I, Elliott PM, Saberi S, Owens AT, Maurer MS et al. Effects of mavacamten on measures of cardiopulmonary exercise testing beyond peak oxygen consumption: a secondary analysis of the EXPLORER-HCM randomized trial. JAMA Cardiol 2023;8:240–7. 10.1001/jamacardio.2022.5099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wheeler MT, Jacoby D, Elliott PM, Saberi S, Hegde SM, Lakdawala NK et al. Effect of beta-blocker therapy on the response to mavacamten in patients with symptomatic obstructive hypertrophic cardiomyopathy. Eur J Heart Fail 2023;25:260–70. 10.1002/ejhf.2737 [DOI] [PubMed] [Google Scholar]
- 48. Saberi S, Cardim N, Yamani MH, Schulz-Menger J, Li W, Florea V et al. Mavacamten favorably impacts cardiac structure in obstructive hypertrophic cardiomyopathy: EXPLORER-HCM CMR substudy analysis. Circulation 2021;143:606–8. 10.1161/CIRCULATIONAHA.120.052359 [DOI] [PubMed] [Google Scholar]
- 49. Saberi S, Kramer CM, Oreziak A, Masri A, Villa RB, Abraham TP et al. 96 week cardiac magnetic resonance (CMR) results of treatment with mavacamten from the EXPLORER cohort of the Mava Long term Extension (LTE) study in patients with obstructive hypertrophic cardiomyopathy HCM). J Am Coll Cardiol 2023;81:326. 10.1016/S0735-1097(23)00770-2 [DOI] [Google Scholar]
- 50. Spertus JA, Fine JT, Elliott P, Ho CY, Olivotto I, Saberi S et al. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): health status analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2021;397:2467–75. 10.1016/S0140-6736(21)00763-7 [DOI] [PubMed] [Google Scholar]
- 51. Nassif M, Fine JT, Dolan C, Reaney M, Addepalli P, Allen VD et al. Validation of the Kansas City Cardiomyopathy Questionnaire in symptomatic obstructive hypertrophic cardiomyopathy. JACC Heart Fail 2022;10:531–9. 10.1016/j.jchf.2022.03.002 [DOI] [PubMed] [Google Scholar]
- 52. Xie J, Wang Y, Xu Y, Fine JT, Lam J, Garrison LP. Assessing health-related quality-of-life in patients with symptomatic obstructive hypertrophic cardiomyopathy: EQ-5D-based utilities in the EXPLORER-HCM trial. J Med Econ 2022;25:51–8. 10.1080/13696998.2021.2011301 [DOI] [PubMed] [Google Scholar]
- 53. Rader F, Choudhury L, Saberi S et al. Updated cumulative results of treatment with mavacamten from the EXPLORER-LTE cohort of the MAVA-LTE study in patients with obstructive hypertrophic cardiomyopathy. In: Presented at the American College of Cardiology Annual Scientific Session; 2–4 April 2022; Washington, DC. [Google Scholar]
- 54. Desai MY, Owens AT, Geske JB, Wolski K, Saberi S, Wang A et al. Dose-blinded myosin inhibition in patients with obstructive HCM referred for septal reduction therapy: outcomes through 32-weeks. Circulation 2023;147:850–63. 10.1161/CIRCULATIONAHA.122.062534 [DOI] [PubMed] [Google Scholar]
- 55. Cremer PC, Geske JB, Owens A, Jaber WA, Harb SC, Saberi S et al. Myosin inhibition and left ventricular diastolic function in patients with obstructive hypertrophic cardiomyopathy referred for septal reduction therapy: insights from the VALOR-HCM study. Circ Cardiovasc Imaging 2022;15:e014986. 10.1161/CIRCIMAGING.122.014986 [DOI] [PubMed] [Google Scholar]
- 56. Reuters . FDA approves Bristol Myers’ oral heart disease drug. https://www.reuters.com/business/healthcare-pharmaceuticals/fda-approves-bristol-myers-heart-disease-drug-2022-04-29/ (9 August 2023, date last accessed.
- 57. The National Institute for Health and Care Excellence (NICE) . Mavacamten for treating symptomatic obstructive hypertrophic cardiomyopathy. https://www.nice.org.uk/guidance/gid-ta10824/documents/674 (13 June 2023, date last accessed).
- 58. Ho CY, Mealiffe ME, Bach RG, Bhattacharya M, Choudhury L, Edelberg JM et al. Evaluation of mavacamten in symptomatic patients with nonobstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2020;75:2649–60. 10.1016/j.jacc.2020.03.064 [DOI] [PubMed] [Google Scholar]
- 59. ClinicalTrials.gov . A study of mavacamten in non-obstructive hypertrophic cardiomyopathy (ODYSSEY-HCM). ClinicalTrials.gov Identifier: NCT05582395. https://www.clinicaltrials.gov/ct2/show/NCT05582395 (13 June 2023, date last accessed).
- 60. ClinicalTrials.gov . A study of mavacamten in participants with HFpEF and elevation of NT-proBNP with or without elevation of cTnT (EMBARK-HFpEF). ClinicalTrials.gov Identifier: NCT04766892. https://www.clinicaltrials.gov/ct2/show/NCT04766892 (13 June 2023, date last accessed).
- 61. Chuang C, Collibee S, Ashcraft L, Wang W, Vander Wal M, Wang X et al. Discovery of aficamten (CK-274), a next-generation cardiac myosin inhibitor for the treatment of hypertrophic cardiomyopathy. J Med Chem 2021;64:14142–52. 10.1021/acs.jmedchem.1c01290 [DOI] [PubMed] [Google Scholar]
- 62. Sharpe AN, Olach MS, Rivas VN, Kaplan JL, Walker AL, Kovacs SL et al. Effects of aficamten on cardiac contractility in a feline translational model of hypertrophic cardiomyopathy. Sci Rep 2023;13:32. 10.1038/s41598-022-26630-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Malik FI, Robertson LA, Armas DR, Robbie EP, Osmukhina A, Xu D et al. A phase 1 dose-escalation study of the cardiac myosin inhibitor aficamten in healthy participants. JACC Basic Transl Sci 2022;7:763–75. 10.1016/j.jacbts.2022.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Maron MS, Masri A, Choudhury L, Olivotto I, Saberi S, Wang A et al. Phase 2 study of aficamten in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2023;81:34–45. 10.1016/j.jacc.2022.10.020 [DOI] [PubMed] [Google Scholar]
- 65. Saberi S, Abraham TP, Choudhury L, Owens AT, Tower-Rader A, Rader F et al. Long-term efficacy and safety of aficamten in patients with symptomatic obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2023;81:324. 10.1016/S0735-1097(23)00768-4 [DOI] [Google Scholar]
- 66. Evaluation of the effects of treatment with aficamten over a 24-week period on cardiopulmonary exercise capacity and health status in patients with symptomatic oHCM (SEQUOIA-HCM). ClinicalTrials.gov Identifier: NCT05186818. https://clinicaltrials.gov/ct2/show/NCT05186818 (13 June 2023, date last accessed).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data were generated or analysed for or in support of this paper.