Abstract
Cancer-related cognitive decline (CRCD) is a clinically important problem and negatively affects daily functioning and quality of life. We conducted a pilot longitudinal study from pre- to post-chemotherapy in patients with breast cancer to assess changes in inflammation and cognition over time, as well as the impact of baseline cytokine level on post-chemotherapy cognitive scores. We found that concentrations of IL-6, MCP-1, sTNFRI, and sTNFRII significantly increased in patients, while IL-1β significantly decreased (p < 0.05). After controlling for covariates, increases in IL-6 and MCP-1 were associated with worse executive function and verbal fluency in patients from pre- to post-chemotherapy (p < 0.05). Higher baseline IL-6 was associated with better performance on executive function and verbal fluency post chemotherapy (p < 0.05). Overall, these results suggest that chemotherapy-associated increases in cytokines/receptors is associated with worse cognitive function. Larger studies are needed to confirm these findings.
Keywords: Cognition, Cytokines, cancer, Chemotherapy, Cancer-related cognitive decline (CRCD)
1. Introduction
Cancer-related cognitive decline (CRCD) involves impairments in the cognitive domains of memory, attention, executive functioning, verbal fluency, and processing speed (Wefel et al., 2015). CRCD in patients receiving chemotherapy or who previously received chemotherapy has a significant negative impact on quality of life and overall daily functioning (Janelsins et al., 2014; Von Ah et al., 2013; Wefel et al., 2015). Several prospective longitudinal studies evaluating cognitive function prior to and following chemotherapy have reported decreases in cognitive performance on tests of memory, executive function, processing speed, and attention; 75% of patients experience these cognitive problems during treatment, and up to 35% of patients continue to be afflicted several years post-treatment (Cerulla et al., 2017; Ganz et al., 2013; Janelsins et al., 2014; Rodriguez Martin et al., 2020; Von Ah et al., 2013; Wefel et al., 2004). Chemotherapy, either adjuvant or neoadjuvant, is frequently a component of multimodal breast cancer treatment (Korde et al., 2021); the success of chemotherapy has led to a large and increasing number of patients surviving for decades following initial chemotherapy who are experiencing CRCD. There is a pressing need to investigate the mechanisms and etiology of CRCD so that treatments and preventive measures can be developed to alleviate this burdensome side effect.
While the etiology of CRCD is unknown, some clues point to inflammation as a possible contributing mechanism through neural-immune interactions. Elevation of circulating pro-inflammatory factors in patients with cancer may relate to CRCD. For example, we and others have also shown that elevated levels of inflammatory cytokines and chemokines in the peripheral blood are a consequence of chemotherapy and related to cognitive problems (Cheung et al., 2015; Ganz et al., 2011; Janelsins et al., 2012; S. Kesler et al., 2013; Mills et al., 2008). However, most of these studies have not assessed changes from pre- to post-chemotherapy, assessed how pre-treatment levels of cytokines relate to post-chemotherapy cognitive function, and did not include a cancer-free control group for comparisons.
To address these gaps, and in order to prepare for a larger study assessing changes in cytokines and cognition, we conducted a pilot study investigating these markers in a convenience subset of patients from a longitudinal cohort of female breast cancer patients receiving chemotherapy compared to age-matched controls assessed at equivalent time points (Janelsins et al., 2018). In this study, patients with breast cancer performed significantly less well on cognitive assessments from pre- to post-chemotherapy compared to controls assessed at the same times. To explore inflammatory mechanisms underlying cognitive decline, we assessed changes in circulating cytokines and cytokine receptors from pre- to post-chemotherapy, and how these changes related to changes in cognitive function. We focused our analyses on cytokines and cytokine receptors that were previously reported in the literature to play a role in CRCD pre-chemotherapy, during chemotherapy, or post-chemotherapy—IL-1β, IL-6, IL-8, MCP-1, TNFRI, and TNFRII (Cheung et al., 2015; Ganz et al., 2013; Ganz et al., 2011; Hayslip et al., 2015; Janelsins et al., 2012; S. Kesler et al., 2013; Lyon et al., 2016; Mills et al., 2008; Patel et al., 2015; Pusztai et al., 2004; Villani et al., 2008). Of note, the majority of studies have been performed in post-treatment survivors and only a few longitudinal studies have been conducted. To our knowledge, this is one of the first longitudinal studies that compares cognitive function in patients during this critical period against non-cancer controls. We hypothesized that pro-inflammatory cytokines would increase from pre- to post-chemotherapy in patients and not controls at equivalent time points, and that increases in these markers would be be associated with cognitive decline in multiple cognitive domains in patients with breast cancer.
2. Methods
2.1. Participants and study design
This pilot study included a convenience sample of patients from our previously published nationwide, multicenter, prospective longitudinal study of female patients with breast cancer and age- and gender-matched non-cancer controls, investigating the trajectory of cognitive changes in patients with cancer receiving chemotherapy (Janelsins et al., 2018). Briefly, participants were recruited from National Cancer Institute (NCI) Community Oncology Research Program (NCORP) locations (clinicaltrials.gov NCT03137095); study assessments were conducted at baseline (pre-chemotherapy) and within one month of the last chemotherapy session (post-chemotherapy), or time-equivalents for participants who served as non-cancer controls.
The eligibility criteria for the breast cancer patients included: chemotherapy naïve, a diagnosis of invasive non-metastatic breast cancer (stage I-IIIC), scheduled to begin a course of chemotherapy, and not scheduled to receive concurrent radiation treatment while receiving chemotherapy. Controls were the same age as patients (within five years) and met applicable eligibility criteria other than the cancer-specific criteria. Detailed eligibility criteria for patients and controls were previously reported (Janelsins et al., 2018). The institutional review boards (IRBs) at the University of Rochester Cancer Center (URCC) Research Base and each of the NCORP sites reviewed and approved this study before participant recruitment. All participants provided informed consent.
2.2. Cognitive assessments
All cognitive assessments were conducted under the supervision of the study team by trained study personnel who completed standardized training and evaluation. A standardized manual was used for all testing. All paper-based and computerized testing was conducted at the clinic/NCORP location by a trained administrator. Following the paper-based and computerized testing, the phone-based testing was scheduled and administered by study personnel at URCC. On average, in-person testing was approximately 60–90 min and the phone-based testing was approximately 30 min or less.
2.2.1. Computerized neuropsychological assessments
The Cambridge Neuropsychological Test Automated Battery (CANTAB) is a semi-automated computerized assessment (Fray and Robbins, 1996; Robbins et al., 1994). The Delayed Matching to Sample (DMS) test was used to evaluate visual working memory. The subject was shown a multi-colored pattern and then, simultaneously or after a delay, identified the original pattern from four pattern choices. Next, the Verbal Recall Memory (VRM) test was used to assess immediate recall and delayed recognition memory. This test involved free recall of words, as well as later recognition of words from a list. Subsequently, the Rapid Visual Information Processing (RVP) test was used to evaluate visual sustained attention and processing speed through recognition of a number series. Lastly, executive function was examined using the One Touch Stockings of Cambridge (OTS) test, which assessed spatial planning.
2.2.2. Paper-based neuropsychological assessments
Short-term memory was assessed by the Hopkins Verbal Learning and Memory Test-Revised (HVLT-R), a word list test including immediate and delayed recall (Brandt and Benedict, 2001; Rasmusson et al., 1995; Shapiro et al., 1999). Attention, speed/sequencing, and executive function were assessed by the Trail Making Test (Comprehensive Trail Making Test Trails 1 and 5, similar to TMT A and B, respectively), in which the participant had to connect numbers or alternating numbers and letters, respectively (Reynolds, 2002; Gaudino et al., 1995; Goul and Brown, 1970). Verbal fluency/executive function was assessed by the Controlled Oral Word Association Test (COWA) and included the recall of words beginning with C, F, and L (Benton and Sivan, 1978; M. Lezak, 2004).
2.2.3. Phone-based cognitive assessments
The Brief Test of Adult Cognition by Telephone (BTACT), developed at the Lifespan Developmental Psychology Laboratory (http://www.brandeis.edu/projects/lifespan), was administered via telephone pre- and post-chemotherapy and included the Rey Auditory Verbal Learning Test (RAVLT) (M. D. Lezak, 1979), digits backward (of number series with varying lengths), category fluency (Drachman and Leavitt, 1972) (number of animals correctly listed within 60 s), and backward counting (from 100) (Tun and Lachman, 2006).
2.2.4. Cytokine and chemokine/receptor quantification
We measured cytokines, chemokines and cytokine receptors from serum. Serum samples were collected and processed at NCORP sites according to standard operating procedures developed by the Cancer Control and Psychoneuroimmunology Lab (CCPL) and the URCC NCORP Research Base. Serum was temporarily stored at − 20 °C or − 80 °C prior to being shipped to the CCPL for long-term storage at −80 °C. All samples were analyzed on a Luminex Magpix (Luminex Corp., Austin, TX). The median of 50 beaded reactions per well was used to determine concentration per participant. Customized Milliplex xMAP human cytokine and cytokine receptor immunoassay kits (catalog numbers: HSCRMAG-32 K (sTNFRI, sTNFRII), HCYTOMAG-60 K (MCP-1, IL-6, IL-8); Luminex Corp.) were used for the study. All samples were run within the same batch.
2.2.5. Covariate measures
Clinical information for patients was obtained from their medical record. The Wide Range Achievement Test (Version 4) reading test (Wilkinson and Robertson, 2006) and STAI anxiety measure {Spielberger, 1999 #1990} were administered at the study visit. Information on race and education was self-reported by the participant.
2.3. Statistical analyses
2.3.1. Cytokine/receptor data preparation
Some concentrations of cytokines were out of the instruments' limit of detection (minDC): IL-1β (minDC range = 0.03–0.08) values ≤0.04 pg/mL were assigned 0.04 pg/mL (46%); IL-6 (minDC range = 0.04–0.83) values ≤0.06 pg/mL were assigned 0.06 pg/mL (14%); MCP-1 (minDC range = 1.0–16) values <16 pg/mL were assigned 16 pg/mL (1%), and MCP-1 values >1066 pg/mL were assigned 1066 pg/mL. Upon initial assessment of the data, data points that exhibited baseline cytokine concentration or change score that was more than three standard deviations of the mean were excluded from the analysis. This affected between 1 and 3 samples per biomarker. The Shapiro-Wilk test and Kolmogorov-Smirnov test were used to test the normality of change in each individual cytokine. The results showed that changes in IL-1β, IL-8, and IL-6 were not normally distributed and logarithmic transformations were applied. We added 0.1 to all the cytokine concentrations before performing the natural logarithm transformation to avoid the influence of extremely small cytokine values. Changes in MCP-1, TNFRI, and TNFRII were normally distributed and were not transformed. In the analysis below, change in cytokines refers to the change in the log transformed values of IL-1β, IL-6, and IL-8, and change in the raw values of MCP-1, TNFRI, and TNFRII.
2.3.2. Associations of cytokines and cognition in patients receiving chemotherapy
For the analysis of associations of cytokines and cognition, we concentrated on patients only as we did not expect pre-post changes in the measure values in controls. The relationship between cytokines and cognitive tests were initially evaluated with Pearson correlation to selected (p-values ≤0.1) for subsequent analyses. This resulted in five cytokines/receptors—IL-1β, IL-6, IL-8, TNFRI, and TNFRII—and six cognitive tests— ‘mean choices to correct’ on the OTS of the CANTAB, which was the mean number of unique choices the subject made before choosing the correct solution; ‘problems solved on first try’ on the OTS of the CANTAB; the phone-based category fluency test; the phone-based ‘word recall;’ and ‘free recall of total correct words,’ which was the total number of correct words recalled immediately from a list as part of the VRM task in CANTAB; and Trail Making Test B (CTMT 5; Supplemental Table 1). Then we evaluated the effect of the potentially confounding covariates (i.e., age, race, education, baseline reading score, and baseline anxiety) on the association between cytokines (independent variables) and cognitive test scores (dependent variables) in multivariable linear regression model controlling for the covariates.
Multivariable analysis assessed the association between change in cytokines and change in cognitive tests controlling for the same covariates. In order to identify potential predictors for CRCD, we also tested the association of baseline cytokines and post-chemotherapy cognition tests controlling for the same covariates and also baseline cognition score.
From all regression analyses, p ≤ 0.05 was used to assess statistical significance. We acknowledge that we performed multiple comparisons and there is risk of type 1 error. However, this is an exploratory analysis that is hypothesis-generating by nature with the goal of not increasing potential for type 2 error of missing true association due to a low statistical power. Our results should be tested in future studies and we plan further confirmatory studies. SAS, version 9.4 (SAS Institute, Cary, NC) was used for all analyses.
From all regression analyses, p ≤ 0.05 was used to assess statistical significance.
3. Results
3.1. Baseline characteristics
The mean age for patients (n = 78) was 53.1 and 54.4 for controls (n = 78) (p = 0.486; Table 1). There were no differences between groups in regard to education. Baseline reading score, as assessed using the Wide Range Achievement Test (WRAT), was slightly higher for the controls than the patients (p = 0.061). There was a higher proportion of White participants in the controls (p = 0.034), and the patients had higher level of anxiety (p < 0.001). In total, 78 female patients with breast cancer and 78 age-matched controls had evaluable cytokine data (Table 2).
Table 1.
Baseline characteristics.
| Patients with Breast Cancer/ Chemotherapy (N = 78) |
Age-Matched Individuals without Cancer/Controls (N = 78) |
P-value | |
|---|---|---|---|
| Age (Years) | |||
| Mean | 53.1 | 54.4 | 0.486 |
| Range | 30–73 | 28–81 | |
| Race | |||
| White | 70 (89.7%) | 77 (98.7%) | 0.034 |
| Other | 8 (10.3%) | 1 (1.3%) | |
| Education | |||
| <High School, High School or GED | 18 (23.1%) | 16 (20.5%) | 0.649 |
| Some College or Above | 60 (76.9%) | 62 (79.5%) | |
| Cancer Stage | |||
| I | 13 (16.7%) | – | |
| II | 22 (28.2%) | – | |
| III | 13 (16.7%) | – | |
| Unknown | 30 (38.5%) | – | |
| Chemo Type | |||
| Anthracycline | 42 (53.8%) | – | |
| Non-anthracycline | 36 (46.2%) | – | |
| WRAT Score | |||
| Mean | 63.4 | 64.8 | 0.061 |
| Range | 43–69 | 47–70 | |
| Anxiety Score (STAI) | |||
| Mean | 34.6 | 27.4 | <0.001 |
| Range | 20–66 | 20–52 | |
| Cognitive Test Score Mean (Std Dev) | |||
| CANTAB: OTS Mean | |||
| Choice to Correct (N = 76) | 1.45 (0.33) | 1.46 (0.24) | 0.798 |
| CANTAB: OTS | |||
| Problems Solved on First Try (N = 76) | 17.41 (3.38) | 17.03 (2.73) | 0.443 |
| CANTAB: VRM Total | |||
| Correct Immediate Recall (N = 77) | 23.49 (0.88) | 23.32 (1.03) | 0.579 |
| Paper-based: Trail | |||
| Making Test B (CTMT-5) (N = 77) | 60.62 (26.99) | 69.05 (38.26) | 0.116 |
| Phone-based: RAVLT | |||
| Word Recall - Delayed Recall (N = 56) | 5.04 (2.67) | 4.5 (2.57) | 0.269 |
| Phone-based: | |||
| Category Fluency (N = 56) | 7.3 (2.76) | 6.71 (2.52) | 0.224 |
Data are presented as No. (%) unless otherwise noted. To compare baseline characteristics between patients with breast cancer and age-matched controls, Fisher's Exact test was used for race, Chi-square test was used for education, and Student's t-test was used for continuous variables including age, WRAT reading score, and anxiety score (STAI).
GED: General Educational Development; WRAT: Wide Range Achievement Test, 4th edition; STAI: State-Trait Anxiety Inventory; OTS: One Touch Stockings of Cambridge; VRM: Verbal recognition memory; TMT: Trail Making Test; RAVLT: Rey Auditory Verbal Learning Test.
Table 2.
Changes in cytokines from pre- to post-chemotherapy or time equivalent in controls.
| Variables |
|
Patients with Breast Cancer (n = 78) |
|
Age-matched Controls (n = 78) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-chemo | Post-chemo | Change | p- valuea |
Pre-chemo time equivalent |
Post-chemo time equivalent |
Change | p- valuea |
|||
|
|
|
|
|
|
|
|
|
|
|
|
| N Analyzed |
Mean (SD) | Mean (SD) | Mean (SD) | N Analyzed |
Mean (SD) | Mean (SD) | Mean (SD) | |||
| Cytokine (pg/mL) | ||||||||||
| IL-1β (log) | 77 | −0.78 (1.51) | −1.03 (1.40) | −0.25 (0.71) | 0.003 | 77 | −0.13 (1.87) | −0.09 (1.95) | 0.04 (0.50) | 0.450 |
| IL-6 (log) | 77 | 1.42 (2.28) | 1.97 (1.96) | 0.55 (2.21) | 0.032 | 77 | 1.95 (2.67) | 2.24 (2.55) | 0.29 (1.47) | 0.089 |
| IL-8 (log) | 77 | 2.39 (0.84) | 2.36 (0.80) | −0.03 (0.81) | 0.811 | 77 | 2.62 (0.84) | 2.68 (0.80) | 0.06 (0.47) | 0.469 |
| MCP-1 | 76 | 895.75 (353.39) | 1099.45 (489.76) | 203.70 (357.42) | <0.001 | 74 | 906.35 (377.87) | 991.29 (445.24) | 84.94 (184.67) | <0.001 |
| sTNFRI | 76 | 178.97 (94.96) | 214.35 (104.29) | 35.38 (79.09) | <0.001 | 77 | 197.56 (74.74) | 195.61 (69.92) | −1.94 (81.75) | 0.836 |
| sTNFRII | 75 | 1093.81 (499.06) | 1329.01 (515.68) | 235.20 (412.93) | <0.001 | 76 | 1123.94 (355.53) | 1134.65 (361.50) | 10.71 (421.74) | 0.825 |
When baseline or change score values of cytokines/receptors were greater than three standard deviations from the mean, we dropped the data from that participant for that particular measure. This effected between 1 and 3 of 78 data points per marker.
t-tests are performed to examine the difference in cytokines from pre- to post-chemotherapy, or time-equivalent for controls.
p-values tests the null hypothesis of no pre- to post- difference via paired t-test. IL= interleukin, MCP-1 = monocyte chemoattractant protein-1, sTNFR= soluble tumor necrosis factor receptor
3.2. Changes in cytokines and cytokine receptors from pre- to post-chemotherapy
In patients, IL-6, MCP-1, sTNFRI, and sTNFRII concentrations increased from pre- to post-chemotherapy (p < 0.05 for all); IL-1β concentrations decreased over the course of chemotherapy (p = 0.003); and IL-8 concentrations did not change (Table 2). In contrast, among the controls, MCP-1 increased (p < 0.001), but to a lesser degree than the patients, while the concentrations of the other cytokines and receptors did not change. We saw minimal changes in cytokines/receptors in controls.
3.3. Longitudinal changes in cytokines and receptors and changes in cognitive function
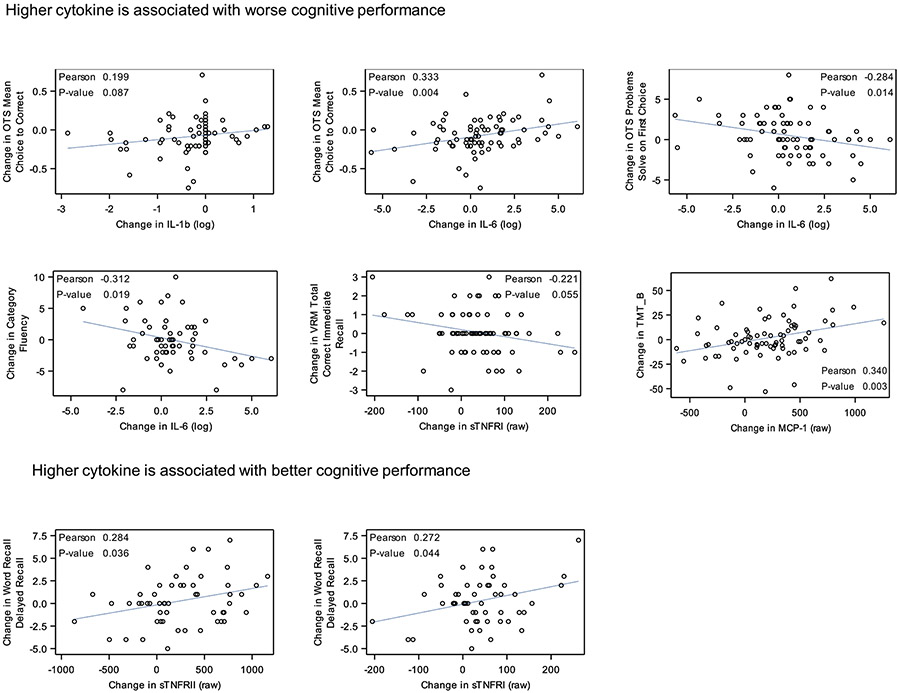
The change in each cytokine/receptor was plotted against the change in each cognitive outcome for the patients with breast cancer, and eight correlations met our cutoff for being statistically meaningful (p ≤ 0.10; Fig. 1): The relationship between cytokines and cognitive tests were initially evaluated with Pearson correlation to selected (p-values ≤0.1); for subsequent analyses we further evaluated IL-1β, IL-6, IL-8, TNFRI, and TNFRII—and six cognitive outcomes (Supplemental Table 1). Baseline level of cytokine/receptor was also correlated with post-chemotherapy cognitive scores and five correlations were revealed (p ≤ 0.10; Fig. 2).
Fig. 1.
Scatter plot of the relationship between change in cytokines and change in cognitive test scores.
Fig. 2.
Scatter plot of post-chemotherapy cognitive test scores and baseline cytokine concentration.
In order to better understand how changes in inflammation relate to changes in cognitive function from pre- to post-chemotherapy, we assessed changes in both over time (Table 3). In multivariate linear analyses, controlling for age, race, education, baseline reading score, and baseline anxiety, increases in IL-6 were associated with worse change from pre- to post-chemotherapy in performance on the executive function OTS Mean Choice to Correct test (β = −0.033; SE = 0.011, p = 0.004), Problems Solved on First Try test (β = −0.298; SE = 0.127, p = 0.022), as well as a verbal fluency test (β = −0.548; SE = 0.255, p = 0.036). Additionally, increases in MCP-1 were associated with worse change in executive function from pre- to post-chemotherapy (β = 0.017; SE = 0.006, p = 0.006). In order to identify any potential cytokine-based predictors for CRCD, we assessed the association between baseline concentrations of cytokines/receptors and post-chemotherapy cognitive measures controlling for age, race, education, baseline reading score, baseline anxiety, and baseline cognition score. Higher pre-chemotherapy IL-6 was associated with better post-chemotherapy executive function score on the OTS test and better score on the category fluency test (all p < 0.05).
Table 3.
Multivariate analyses: Association between change of cytokine and change of cognition and between baseline cytokine and post-chemotherapy cognition measures.
| Cytokine | Cognitive Test | Type of Test | N | Change of Cytokine and Change of Cognitive Measure |
Baseline Cytokine and Post-chemotherapy Cognition Measure |
||
|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
||
| Coef. (SE) | P-value | Coef. (SE) | P-value | ||||
| IL-1β (log) | OTS Mean Choice to Correct | Executive function | 75 | 0.057 (0.035)a | 0.105 | −0.011 (0.014)b | 0.441 |
| IL-6 (log) | OTS Mean Choice to Correct | Executive function | 75 | 0.033 (0.011) a | 0.004 | −0.026 (0.009) b | 0.004 |
| IL-6 (log) | OTS Problems Solved on First Try | Executive function | 75 | −0.298 (0.127) a | 0.022 | 0.328 (0.111) b | 0.004 |
| IL-6 (log) | Category Fluency | Verbal fluency | 56 | −0.548 (0.255) a | 0.036 | 0.515 (0.201) b | 0.014 |
| sTNFRI (raw) | VRM Total Correct Immediate Recall | Verbal memory | 75 | −0.003 (0.002)a | 0.075 | 0.000 (0.001) | 0.864 |
| sTNFRI (raw) | Word Recall Delayed Recall | Verbal memory | 54 | 0.009 (0.005)b | 0.070 | 0.002 (0.004)b | 0.535 |
| sTNFRII (raw) | Word Recall Delayed Recall | Verbal memory | 54 | 0.002 (0.001)b | 0.070 | 0.000 (0.001) | 0.802 |
| MCP-1 (raw) | TMT–B (CTMT5) | Executive Function | 75 | 0.017 (0.006) a | 0.006 | 0.011 (0.007)a | 0.126 |
Each regression model assessing the relationship of the change of cytokine with change of cognitive measure was adjusted for age, race, education, baseline reading score, and baseline anxiety. Each regression model assessing the relationship of baseline cytokine concentration with post-chemotherapy cognition measure was adjusted for the same covariates and baseline cognitive score.
OTS: One Touch Stockings of Cambridge.
VRM: Verbal recognition memory.
TMT: Trail Making Test.
An increase in cytokines over time or a higher baseline cytokine concentration is associated with worse cognitive function.
An increase in cytokines over time or a higher baseline cytokine concentration is associated with better cognitive function.
4. Discussion
In this preliminary study sampling from a nationwide, longitudinal cohort of female breast cancer patients and age-matched non-cancer controls, we found that IL-6, MCP-1, sTNFRI, and sTNFRII, but not IL-1β or IL-8, increased over the course of chemotherapy, and that changes in these cytokines were associated with changes in cognitive function, especially worse executive function, verbal memory and verbal fluency. This is the first study we are aware of to describe a relationship between MCP-1 expression and lower objectively assessed cognitive function scores in patients with breast cancer. These results contribute valuable insight into the relationship between cancer- and chemotherapy-induced systemic inflammation and CRCD.
There is strong consensus that chemotherapy induces a systemic inflammatory response. In the literature, IL-6, which can play pro- and anti-inflammatory roles, appears to show the most consistent increases over the course of cancer treatment (Castel et al., 2017). We found that IL-6 increased over the course of chemotherapy and changes in IL-6 were associated with worse change in performance in domains of executive function and verbal fluency (Fig. 1 and Table 3). These results are consistent with our prior research investigating the relationship between IL-6 and self-reported cognitive difficulties (Janelsins et al., 2011). Similarly, we observed increases in chemokines MCP-1 as well as soluble cytokine receptors sTNFRI and sTNFRII (cytokine inhibitors) over the course of chemotherapy (Table 3), though we saw decreases in IL-1β which is not consistent with other reports (Castel et al., 2017; Cheung et al., 2015). Interestingly, IL-6 can also limit the inflammatory response by reducing expression of IL-1β and antagonizing IL-1β signaling pathways (Maggio et al., 2006). The pleotropic effects of IL-6 add a layer of complexity to interpretations of correlative studies, and necessitate future exploration into how the cellular source of chronic IL-6 production and the signaling context play a role in cognitive decline.
Lyon et al. followed 75 women with breast cancer starting chemotherapy for a period of two years; they also observed an increase in IL-6 and MCP-1 over the course of chemotherapy, which then decreased thereafter (Lyon et al., 2016). Several within time-point correlations between cytokine concentrations and cognitive abilities were also reported. During treatment, and following the completion of chemotherapy, they found that cytokines, such as IL-17, were positively associated with cognitive function, highlighting that fluctuating inflammation can have disparate effects on cognitive performance. Kesler et al. demonstrated a relationship between IL-6 (and TNF-α) concentrations and decreased lower left hippocampal volume and verbal memory in breast cancer survivors (S. Kesler et al., 2013). Henneghan et al. found that different cytokine profiles predicted scores on different cognitive tests, including IL-6 with tests of verbal fluency (Henneghan et al., 2018). In a recent nationwide study in Singapore, plasma IL-1β was correlated with slower response speed. (Cheung et al., 2015) Jenkins et al. observed chemotherapy-induced increases in IL-6, as well as IL-10, sTNFRII, and vascular endothelial growth factor. This study also found that increases in sTNFRII were associated with decreases in gray matter volume of the right posterior insula in both groups (Jenkins et al., 2016).
MCP-1 can mediate inflammatory responses by altering trafficking of immune cells, and variable MCP-1 levels in the blood have been related to memory decline during aging or Alzheimer's disease (AD) pathogenies (Bettcher et al., 2019; Galimberti et al., 2006). It is possible that MCP-1 levels are related to the severity of cognitive difficulties and speed of cognitive decline, as evidenced by one study that AD patients had higher MCP-1 levels compared to patients with mild cognitive impairment (MCI) (Lee et al., 2018). The relationships between cytokine concentrations and cognitive abilities are very complex, and it was recently revealed that these relationships are not necessarily linear (Henneghan et al., 2018); more complex models with further time points are needed to better describe the relationships between changes in cytokines and changes in cognitive abilities over the course of cancer treatment and into survivorship.
Our results showing a relationship between sTNFRI and a trend with worse verbal memory (immediate recall) is similar to our prior findings showing a relationship between increased sTNFR1 and worse performance on the DMS visual memory test in patients with breast cancer actively receiving chemotherapy (Williams et al., 2018). In the current study, we did not observe significant correlations between DMS and sTNFR1. We also observed inconclusive results on the relationships between sTNFR1 and sTNFRII and cognitive function (Table 3). Of note, Ganz et al. demonstrated a correlation between sTNFRII and memory complaints in breast cancer survivors post-chemotherapy as well as 12 months after the cessation of treatment (Ganz et al., 2013).
The data herein fit into a growing body of literature demonstrating the interplay between inflammation and CRCD (Cheung et al., 2015; Janelsins et al., 2011; Oppegaard et al., 2021; Rodriguez Martin et al., 2020). Very few studies have assessed baseline, pre-chemotherapy cytokine levels with post-chemotherapy cognitive scores. Because we included baseline cognitive scores as covariates, this is mathematically the same as assessing changes in cognition over time adjusted for the baseline level of cognition. Our results suggest that high IL-6 values at baseline were associated with better cognitive scores post-chemotherapy, and thus, may be protective for CRCD. However, it is possible that inflammation does indeed modulate cognitive function, and patients who had high inflammation at baseline were already experiencing cognitive impairments, which may have improved over time.
Cytokines can cause an impairment of cognitive function directly or indirectly. Directly, cytokines are involved in the metabolism of dopamine and serotonin (Miller et al., 2013), neurotransmitters that are involved in sensory perceptions and motor action-reaction mechanisms. Recently, reduced serotonin neurotransmission has been implicated in cognitive dysfunction associated with Alzheimer's disease and schizophrenia (Svob Strac et al., 2016). Moreover, cytokines are involved in neuronal repair and modulation of glial cells (Wang et al., 2015). Cytokines can cross the blood brain barrier (BBB) and lead to inflammation in the brain and neurotoxicity (reviewed in (Ren et al., 2017; Wardill et al., 2016)). It is also plausible that neurotoxicity, neuroinflammation, and CRCD could occur as a consequence of BBB disruption from elevated signaling molecules (Wardill et al., 2016) and migration of macrophages and other cells into the central nervous system.
Cancer and chemotherapy lead to broad, systemic, wide-reaching changes that have complex, direct and indirect effects on brain structure (S. Kesler et al., 2011), connectivity (S. R. Kesler and Blayney, 2016), and cognition (Janelsins et al., 2014; Von Ah et al., 2013; Wefel et al., 2015). Andreotti et al. eloquently proposed a mechanism by which a cancer diagnosis, cancer treatment, and other peripheral life stressors overtax the systems that allow healthy adaptation to stress, thereby causing pathophysiology (Andreotti et al., 2015). By this mechanism, stressors can cause acute cognitive impairment before cancer treatment (Ahles et al., 2008; Janelsins et al., 2018) that morph and/or become exaggerated over time. Studies have shown that compared to non-anxious controls, clinical anxiety was associated with elevated levels of IL-6 and reduced morning cortisol (O'Donovan et al., 2010). Indeed, a meta-analysis has revealed that increased circulating immunomodulatory cytokines, including IL-6 and IL-1β, have been associated with acute stress in humans (Steptoe et al., 2007). Investigating the interplay between stress-induced inflammation and elevated inflammatory markers following chemotherapy could provide significant insight into the mechanism driving CRCD, and presents unique challenge for psychoneuroimmunological research moving forward.
Our results should be interpreted in the light of the study's strengths and limitations. This is among the first studies to use a longitudinal design to assess pre- and post-chemotherapy cytokine/receptor concentrations with a large battery of cognitive assessments in cancer patients and non-cancer controls, allowing insight into how changes in these metrics are related. However, it is possible that the study was not powered high enough to detect relationships between inflammatory markers and cognitive assessments that exhibited weaker correlation. Additionally, because of the exploratory nature of these analyses, these results should be viewed as hypothesis-generating and should be tested for validation in a larger sample set.
In summary, we show that cytokines IL-6 and MCP-1 and cytokine receptors sTNFRI and sTNFRII increased and IL-1β decreased from pre- to post-chemotherapy in patients but not age-matched non-cancer controls at equivalent time points, and the changes in cytokine concentrations were related to changes in cognitive function, especially in the executive function and verbal fluency domains. These results further implicate systemic inflammation as part of the etiology of CRCD but this needs to be tested and confirmed in larger studies. Our results also indicicate that there is a complex interplay between cytokines and cognitive function. Additionally, reducing inflammation to prevent CRCD could be explored in future intervention studies. Studies specifically looking at anti-inflammatory agents and other interventions known to reduce inflammation and improve cognitive function (e.g. exercise, yoga) should be investigated for their effects on CRCD and as well as their mechanism.
Supplementary Material
Funding
This work was supported by the National Institutes of Health, National Cancer Institute (U10CA037420 Supplement, UG1CA189961, R01CA231014, T32 CA102618, K07CA168886, K07CA187546).
Footnotes
Declaration of Competing Interest
The authors do not have any financial disclosures or conflicts of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jneuroim.2021.577769.
References
- Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, Hanscom BS, Kaufman PA, 2008. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res. Treat 110 (1), 143–152. 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreotti C, Root JC, Ahles TA, McEwen BS, Compas BE, 2015. Cancer, coping, and cognition: a model for the role of stress reactivity in cancer-related cognitive decline. Psychooncology 24 (6), 617–623. 10.1002/pon.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton AL, Sivan AB, 1978. Controlled Oral Word Association Multilingual Aphasia Examination Professional Manual. Psychological Assessment Resources, Lutz, FL. [Google Scholar]
- Bettcher BM, Neuhaus J, Wynn MJ, Elahi FM, Casaletto KB, Saloner R, Kramer JH, 2019. Increases in a pro-inflammatory chemokine, MCP-1, are related to decreases in memory over time. Front. Aging Neurosci 11, 25. 10.3389/fnagi.2019.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, Benedict Ralph H.B., 2001. Hopkins Verbal LearningTest-Revised Professional Manual. Psychological Assessment Resources, Lutz, FL. [Google Scholar]
- Castel H, Denouel A, Lange M, Tonon MC, Dubois M, Joly F, 2017. Biomarkers associated with cognitive impairment in treated cancer patients: potential predisposition and risk factors. Front. Pharmacol 8, 138. 10.3389/fphar.2017.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerulla N, Arcusa A, Navarro JB, Garolera M, Enero C, Chico G, Fernandez-Morales L, 2017. Role of taxanes in chemotherapy-related cognitive impairment: a prospective longitudinal study. Breast Cancer Res. Treat 164 (1), 179–187. 10.1007/s10549-017-4240-6. [DOI] [PubMed] [Google Scholar]
- Cheung YT, Ng T, Shwe M, Ho HK, Foo KM, Cham MT, Chan A, 2015. Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort study. Ann. Oncol 26 (7), 1446–1451. 10.1093/annonc/mdv206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman DA, Leavitt J, 1972. Memory impairment in the aged: storage versus retrieval deficit. J. Exp. Psychol 93 (2), 302–308. 10.1037/h0032489. [DOI] [PubMed] [Google Scholar]
- Fray PJ, Robbins TW, 1996. CANTAB battery: proposed utility in neurotoxicology. Neurotoxicol. Teratol 18 (4), 499–504 doi:0892-0362(96)00027-X [pii]. [DOI] [PubMed] [Google Scholar]
- Galimberti D, Fenoglio C, Lovati C, Venturelli E, Guidi I, Corra B, Scarpini E, 2006. Serum MCP-1 levels are increased in mild cognitive impairment and mild Alzheimer's disease. Neurobiol. Aging 27 (12), 1763–1768. 10.1016/j.neurobiolaging.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Castellon SA, Silverman DHS, Kwan L, Bower JE, Irwin MR, Cole SW, 2011. Does circulating tumor necrosis factor (TNF) play a role in post-chemotherapy cerebral dysfunction in breast cancer survivors (BCS)? J. Clin. Oncol 29 (15_suppl), 9008. 10.1200/jco.2011.29.15_suppl.9008. [DOI] [Google Scholar]
- Ganz PA, Bower JE, Kwan L, Castellon SA, Silverman DH, Geist C, Cole SW, 2013. Does tumor necrosis factor-alpha (TNF-alpha) play a role in post-chemotherapy cerebral dysfunction? Brain Behav. Immun 30 (Suppl), S99–108. 10.1016/j.bbi.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudino EA, Geisler MW, Squires NK, 1995. Construct validity in the trail making test: what makes part B harder? J. Clin. Exp. Neuropsychol 17 (4), 529–535. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7593473. [DOI] [PubMed] [Google Scholar]
- Goul WR, Brown M, 1970. Effects of age and intelligence on trail making test performance and validity. Percept. Mot. Skills 30 (1), 319–326. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=5476120. [DOI] [PubMed] [Google Scholar]
- Hayslip J, Dressler EV, Weiss H, Taylor TJ, Chambers M, Noel T, Moscow JA, 2015. Plasma TNF-alpha and soluble TNF receptor levels after doxorubicin with or without co-administration of Mesna-A randomized, cross-over clinical study. PLoS One 10 (4), e0124988. 10.1371/journal.pone.0124988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneghan AM, Palesh O, Harrison M, Kesler SR, 2018. Identifying cytokine predictors of cognitive functioning in breast cancer survivors up to 10 years post chemotherapy using machine learning. J. Neuroimmunol 320, 38–47. 10.1016/j.jneuroim.2018.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins MC, Mustian KM, Palesh OG, Mohile SG, Peppone LJ, Sprod LK, Morrow GR, 2011. Differential expression of cytokines in breast cancer patients receiving different chemotherapies: implications for cognitive impairment research. Support Care Cancer. 10.1007/s00520-011-1158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins MC, Mustian KM, Palesh OG, Mohile SG, Peppone LJ, Sprod LK, Morrow GR, 2012. Differential expression of cytokines in breast cancer patients receiving different chemotherapies: implications for cognitive impairment research. Support Care Cancer 20 (4), 831–839. 10.1007/s00520-011-1158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins MC, Kesler SR, Ahles TA, Morrow GR, 2014. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int. Rev. Psychiatry 26 (1), 102–113. 10.3109/09540261.2013.864260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelsins MC, Heckler CE, Peppone LJ, Ahles TA, Mohile SG, Mustian KM, Morrow GR, 2018. Longitudinal trajectory and characterization of cancer-related cognitive impairment in a nationwide cohort study. J. Clin. Oncol, JCO2018786624 10.1200/JCO.2018.78.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins V, Thwaites R, Cercignani M, Sacre S, Harrison N, Whiteley-Jones H, Harder H, 2016. A feasibility study exploring the role of pre-operative assessment when examining the mechanism of 'chemo-brain' in breast cancer patients. Springerplus 5, 390. 10.1186/s40064-016-2030-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler S, Kent JS, O’Hara R, 2011. Prefrontal cortex and executive function impairments in primary breast cancer. Arch. Neurol 8 (11), 1447–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler S, Janelsins M, Koovakkattu D, Palesh O, Mustian K, Morrow G, Dhabhar FS, 2013. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav. Immun 30 (Suppl), S109–S116. 10.1016/j.bbi.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Blayney DW, 2016. Neurotoxic effects of Anthracycline- vs Nonanthracycline-based chemotherapy on cognition in breast cancer survivors. JAMA Oncol. 2 (2), 185–192. 10.1001/jamaoncol.2015.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korde LA, Somerfield MR, Carey LA, Crews JR, Denduluri N, Hwang ES, Hershman DL, 2021. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J. Clin. Oncol 39 (13), 1485–1505. 10.1200/JCO.20.03399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WJ, Liao YC, Wang YF, Lin IF, Wang SJ, Fuh JL, 2018. Plasma MCP-1 and cognitive decline in patients with Alzheimer’s disease and mild cognitive impairment: a two-year follow-up study. Sci. Rep 8 (1), 1280. 10.1038/s41598-018-19807-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak M (Ed.), 2004. Neuropsychological Assessment, 4 ed. [Google Scholar]
- Lezak MD, 1979. Recovery of memory and learning functions following traumatic brain injury. Cortex 15 (1), 63–72. 10.1016/s0010-9452(79)80007-6. [DOI] [PubMed] [Google Scholar]
- Lyon DE, Cohen R, Chen H, Kelly DL, McCain NL, Starkweather A, Jackson-Cook CK, 2016. Relationship of systemic cytokine concentrations to cognitive function over two years in women with early stage breast cancer. J. Neuroimmunol 301, 74–82. 10.1016/j.jneuroim.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio M, Guralnik JM, Longo DL, Ferrucci L, 2006. Interleukin-6 in aging and chronic disease: a magnificent pathway. J. Gerontol. A Biol. Sci. Med. Sci 61 (6), 575–584. Retrieved from. https://www.ncbi.nlm.nih.gov/pubmed/16799139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Haroon E, Raison CL, Felger JC, 2013. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress. Anxiety 30 (4), 297–306. 10.1002/da.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills PJ, Ancoli-Israel S, Parker B, Natarajan L, Hong S, Jain S, von Kanel R, 2008. Predictors of inflammation in response to anthracycline-based chemotherapy for breast cancer. Brain Behav. Immun 22 (1), 98–104. 10.1016/j.bbi.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Hughes BM, Slavich GM, Lynch L, Cronin MT, O’Farrelly C, Malone KM, 2010. Clinical anxiety, cortisol and interleukin-6: evidence for specificity in emotion-biology relationships. Brain Behav. Immun 24 (7), 1074–1077. 10.1016/j.bbi.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppegaard K, Harris CS, Shin J, Paul SM, Cooper BA, Chan A, Kober KM, 2021. Cancer-related cognitive impairment is associated with perturbations in inflammatory pathways. Cytokine 148, 155653. 10.1016/j.cyto.2021.155653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SK, Wong AL, Wong FL, Breen EC, Hurria A, Smith M, Bhatia S, 2015. Inflammatory biomarkers, comorbidity, and neurocognition in women with newly diagnosed breast cancer. J. Natl. Cancer Inst 107 (8) 10.1093/jnci/djv131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusztai L, Mendoza TR, Reuben JM, Martinez MM, Willey JS, Lara J, Hortobagyi GN, 2004. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine 25 (3), 94–102. 10.1016/j.cyto.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Rasmusson DX, Bylsma FW, Brandt J, 1995. Stability of performance on the Hopkins verbal learning test. Arch. Clin. Neuropsychol 10 (1), 21–26 doi:0887-6177(94) E0001-6 [pii]. [PubMed] [Google Scholar]
- Ren X, St Clair DK, Butterfield DA, 2017. Dysregulation of cytokine mediated chemotherapy induced cognitive impairment. Pharmacol. Res 117, 267–273. 10.1016/j.phrs.2017.01.001. [DOI] [PubMed] [Google Scholar]
- Reynolds Cecil R., 2002. Comprehensive Trail-Making Test Professional Manual. Pro-ed, Austin, TX. [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P, 1994. Cambridge neuropsychological test automated battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia 5 (5), 266–281. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7951684. [DOI] [PubMed] [Google Scholar]
- Rodriguez Martin B, Fernandez Rodriguez EJ, Rihuete Galve MI, Cruz Hernandez JJ, 2020. Study of chemotherapy-induced cognitive impairment in women with breast Cancer. Int. J. Environ. Res. Public Health 17 (23). 10.3390/ijerph17238896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AM, Benedict RH, Schretlen D, Brandt J, 1999. Construct and concurrent validity of the Hopkins verbal learning test-revised. Clin. Neuropsychol 13 (3), 348–358. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10726605. [DOI] [PubMed] [Google Scholar]
- Speilberger CD, Syderman SJ, Owen AE, Marsh BJ, 1999. Measuring anxiety and anger with the State-Trait Anxiety Inventory (STAI) and the State-Trait Anger Expression Inventory (STAXI). In: Maruish ME (Ed.), The use of psychological testing for treatment planning and outcomes assessment. Lawrence Erlbaum Associates Publishers, Mahwah, NJ, pp. 993–1021. [Google Scholar]
- Steptoe A, Hamer M, Chida Y, 2007. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav. Immun 21 (7), 901–912. 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Svob Strac D, Pivac N, Muck-Seler D, 2016. The serotonergic system and cognitive function. Transl. Neurosci 7 (1), 35–49. 10.1515/tnsci-2016-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun PA, Lachman ME, 2006. Telephone assessment of cognitive function in adulthood: the brief test of adult cognition by telephone. Age Ageing 35 (6), 629–632 afl095 [pii]. 10.1093/ageing/afl095. [DOI] [PubMed] [Google Scholar]
- Villani F, Busia A, Villani M, Vismara C, Viviani S, Bonfante V, 2008. Serum cytokine in response to chemo-radiotherapy for Hodgkin's disease. Tumori 94 (6), 803–808. Retrieved from. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19267096. [DOI] [PubMed] [Google Scholar]
- Von Ah D, Habermann B, Carpenter JS, Schneider BL, 2013. Impact of perceived cognitive impairment in breast cancer survivors. Eur. J. Oncol. Nurs 17 (2), 236–241. 10.1016/j.ejon.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Wang XM, Walitt B, Saligan L, Tiwari AF, Cheung CW, Zhang ZJ, 2015. Chemobrain: a critical review and causal hypothesis of link between cytokines and epigenetic reprogramming associated with chemotherapy. Cytokine 72 (1), 86–96. 10.1016/j.cyto.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardill HR, Mander KA, Van Sebille YZ, Gibson RJ, Logan RM, Bowen JM, Sonis ST, 2016. Cytokine-mediated blood brain barrier disruption as a conduit for cancer/chemotherapy-associated neurotoxicity and cognitive dysfunction. Int. J. Cancer 139 (12), 2635–2645. 10.1002/ijc.30252. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA, 2004. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer 100 (11), 2292–2299. 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- Wefel JS, Kesler SR, Noll KR, Schagen SB, 2015. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J. Clin 65 (2), 123–138. 10.3322/caac.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS, Robertson Gary J., 2006. Wide Range Achievement Test 4 (WRAT4). Psychological Assessment Resources. [Google Scholar]
- Williams AM, Shah R, Shayne M, Huston AJ, Krebs M, Murray N, Janelsins MC, 2018. Associations between inflammatory markers and cognitive function in breast cancer patients receiving chemotherapy. J. Neuroimmunol 314, 17–23. 10.1016/j.jneuroim.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.