Abstract
Objective
To report the outcomes of intra- and extra-peritoneal robot-assisted radical prostatectomy (RARP) and robot-assisted radical cystectomy (RARC) with Hugo™ robot-assisted surgery (RAS) system (Medtronic, Minneapolis, MN, USA).
Methods
Data of twenty patients who underwent RARP and one RARC at our institution between February 2022 and January 2023 were reported. The primary endpoint of the study was to report the surgical setting of Hugo™ RAS system to perform RARP and RARC. The secondary endpoint was to assess the feasibility of RARP and RARC with this novel robotic platform and report the outcomes.
Results
Seventeen patients underwent RARP with a transperitoneal approach, and three with an extraperitoneal approach; and one patient underwent RARC with intracorporeal ileal conduit. No intraoperative complications occurred. Median docking and console time were 12 (interquartile range [IQR] 7–16) min and 185 (IQR 177–192) min for transperitoneal RARP, 15 (IQR 12–17) min and 170 (IQR 162–185) min for extraperitoneal RARP. No intraoperative complications occurred. One patient submitted to extraperitoneal RARP had a urinary tract infection in the postoperative period that required an antibiotic treatment (Clavien-Dindo Grade 2). In case of transperitoneal RARP, two minor complications occurred (one pelvic hematoma and one urinary tract infection; both Clavien-Dindo Grade 2).
Conclusion
Hugo™ RAS system is a novel promising robotic platform that allows to perform major oncological pelvic surgery. We showed the feasibility of RARP both intra- and extra-peritoneally and RARC with intracorporeal ileal conduit with this novel platform.
Keywords: Bladder cancer, Prostate cancer, Radical cystectomy, Radical prostatectomy, Robotic surgery
1. Introduction
Robot-assisted surgery (RAS) is a widespread approach in urology, particularly in case oncologic pelvic surgery. The main indication of robot-assisted pelvic surgery is represented by robot-assisted radical prostatectomy (RARP). This technique showed a decreased rate of readmission and blood loss, even if no superiority was demonstrated in terms of functional or oncological outcomes compared to the open approach [1,2]. Furthermore, in the last decade, robot-assisted radical cystectomy (RARC) has become a safe and feasible technique which is generally accepted in case of less than or equal to pathological tumor stage 2 (pT2) bladder cancer [3].
After many years of monopoly of the medical robot systems market, recently some relevant patents of robotic platforms expired and have led to the rise of several robotic platforms. The arrival of new robotic platforms on the market may contribute to a decrease of the cost of robotic surgery and detailed cost analysis and comparison between all the platforms are needed [4]. The performances of these new robotic platforms have been evaluated in some single-center series, especially in the area of pelvic surgery [[5], [6], [7], [8], [9]]. We reported our experience with Hugo™ RAS system (Medtronic, Minneapolis, MN, USA) in pelvic oncological surgery describing the surgical setting of RARP and RARC performed with this novel robotic platform.
2. Patients and methods
2.1. Population
Data of twenty patients who underwent RARP and one RARC at our institution (Fundació Puigvert, Barcelona) between February 2022 and January 2023 were reported. Notably, the surgical activity with this new platform was paused for 4 months due to a structural defect of the robotic scissors that caused a premature deterioration. The possibility to perform RARP or RARC with Hugo™ RAS system was subjected to the availability of the surgeon, the trained team, and the robotic platform that is currently employed for other robotic surgeries (ureteral reimplantation, partial and radical nephrectomy).
This study was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki; all patients signed an informed consent; and the study was approved by the internal ethic committee of the hospital (approval number: C2021/33).
2.2. Endpoints and variables
The primary endpoint of the study was to report the surgical setting of Hugo™ RAS system to perform RARP and RARC. The secondary endpoints were to assess the feasibility of RARP and RARC with this novel robotic platform and report the outcomes. All patient candidates to RARP were investigated with a multiparametric magnetic resonance imaging (MRI) prior to prostate biopsy, if not contraindicated (e.g., in case of end stage renal disease). An abdominal and chest contrast enhanced computed tomography scan was performed in case of bladder cancer. Pre-, intra-, and post-operative data were collected. Post-operative complications were graded according to Clavien-Dindo classification [10]. We reported categorical variables as frequencies. Continuous variables (i.e., patient age) were reported as medians and interquartile ranges (IQRs). All statistical analyses were performed using R software Version 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria).
2.3. Patient and trocars positioning
All patients were positioned in supine position with a 30-degree (°) Trendelenburg position with the legs 30° abducted.
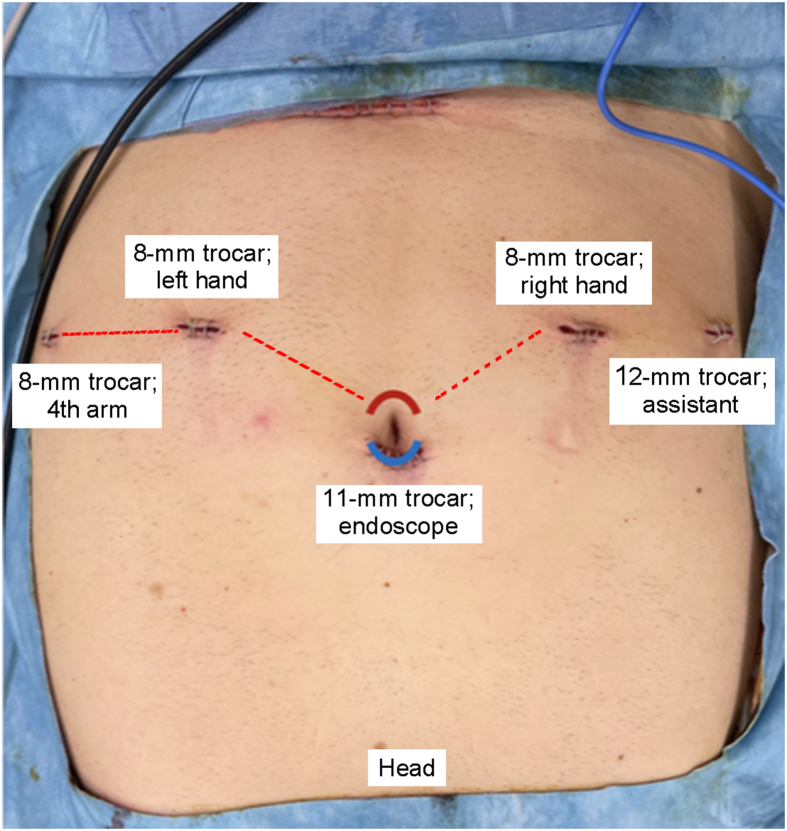
In case of a transperitoneal RARP, a 11-mm trocar for the endoscope was placed supra-umbilically and then two 8-mm robotic trocars were placed 5 cm below the umbilicus, maintaining 7 cm from the endoscope trocar. An 8-mm trocar for the fourth arm was placed on the left side of the abdomen laterally to the left-hand trocar. A laparoscopic 12-mm trocar for the assistant was placed in the right abdomen laterally to the right-hand arm (Fig. 1). A 2-cm distance among trocars and bony prominences was maintained.
Figure 1.
Trocar placement in RARP. In case of extraperitoneal RARP, the endoscope trocar was placed sub-umbilically (red line); in case of transperitoneal RARP, the endoscope trocar was placed supra-umbilically (blue line). RARP, robot-assisted radical prostatectomy.
In case of extraperitoneal RARP, the same trocars placement scheme of transperitoneal RARP was adopted, while the endoscope trocar was placed sub-umbilically (Fig. 1).
In case of RARC, the same scheme of transperitoneal RARP was adopted with the only difference that an additional 5-mm trocar for the assistant was placed between the endoscope and the right-arm trocar (Fig. 2).
Figure 2.
Trocar placement in robot-assisted radical cystectomy.
2.4. Carts positioning
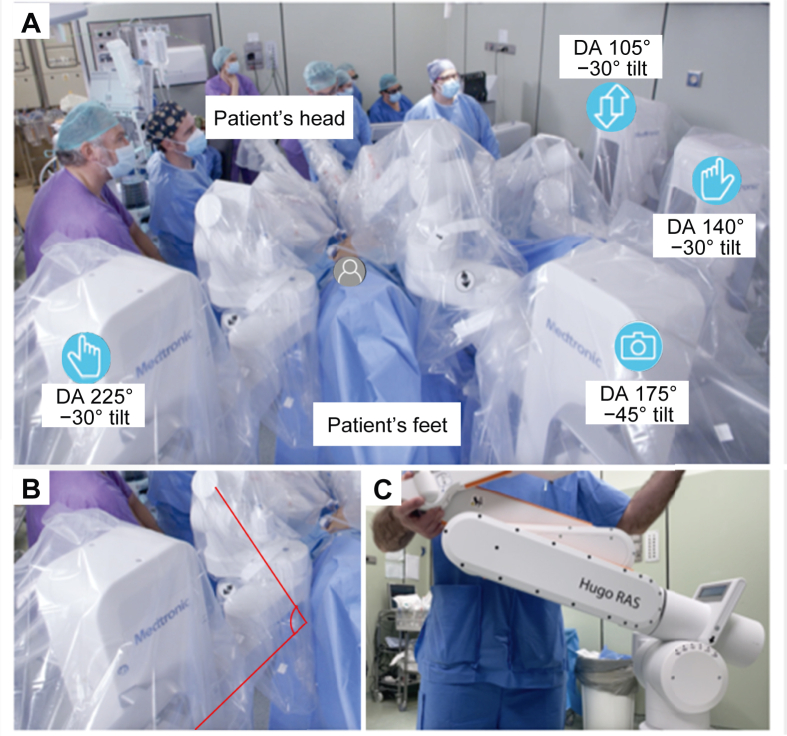
The operative room setting was the same for all the procedures (Fig. 3). The endoscope cart was placed between the patient's legs with an angulation between the cart and the operative table (docking angle) of 175° and a 45° tilted down position; the surgeon's left-hand cart was placed to the left of patient's legs at a 140° docking angle and a 30° tilted down position; the surgeon's right-hand cart was placed to the right of patient's legs at a 225° docking angle and a 30° tilted down position; finally, the fourth arm cart was placed upper to the left-hand cart at a 105° docking angle and a 30° tilted up position. All the carts were placed at a distance of 45–60 cm from the operative table.
Figure 3.
Carts positioning and DAs in case of pelvic surgery with Hugo™ robot-assisted surgery system. (A) Operating room setting with carts disposition, DAs, and tilt inclination of the robotic arms; (B) DA defined as the angle between the cart and the bed; (C) Tilt defined as the angle between the cart and the first part of the robotic arm. DA, docking angle.
2.5. Surgical technique
All procedures were performed by experienced robotic surgeons (performed more than 150 cases; Bravo A, Palou J, and Gaya JM). All members of the surgical team received the official training by Medtronic (Minneapolis, MN, USA). No changes in anesthesiologic procedures were necessary compared to those performed with standard laparoscopic or robotic surgery. In RARP, extended pelvic lymphadenectomy was performed in case of preoperative risk for nodal involvement more than 5% [11] or 7% [12].
The extra- or intra-peritoneal approach was selected according to the prior history of major abdominal surgery, the necessity of performing lymphadenectomy, and the patient tolerance to pneumoperitoneum. In case of an extraperitoneal RARP, the working space was created with a balloon-trocar under direct vision (Supplementary Video 1).
Supplementary video related to this article can be found at https://doi.org/10.1016/j.ajur.2023.05.003
The following is/are the supplementary data related to this article:
Working space creation and trocar positioning in case of extraperitoneal robot-assisted radical prostatectomy with HugoTM robot-assisted surgery system
The case of RARC was performed transperitoneally, with an ileal-conduit urinary derivation and a Wallace type 1 uretero-enteric anastomosis performed intracorporeally.
All the surgeries were performed using a Cadiere forceps in the fourth arm, a fenestrated or a Maryland bipolar forceps in the left-hand arm, and a monopolar scissor in the right-hand arm. For suturing, two large needle drivers were employed (Fig. 4). The specimens were extracted through a Pfannenstiel incision.
Figure 4.
Intraoperative view of Hugo™ robot-assisted surgery system. (A) Closure of dorsal venous complex during an extraperitoneal RARP; (B) Dissection of the prostate apex during a transperitoneal RARP; (C) Wallace type 1 urinary derivation during robot-assisted radical cystectomy. RARP, robot-assisted radical prostatectomy.
3. Results
3.1. Transperitoneal RARP
Seventeen patients underwent RARP with a transperitoneal approach at our institution. Demographic data are listed in Table 1. Median age was 64 (IQR 59–69) years, with a median prostate-specific antigen of 6.4 (IQR 5.1–9.4) ng/mL. At preoperative MRI median prostate volume was 35 (IQR 30–56) mL. In two cases, extracapsular extension was suspected at MRI. Median docking and console time were 12 (IQR 7–16) min and 185 (IQR 177–192) min for transperitoneal RARP, 15 (IQR 12–17) min and 170 (IQR 162–185) min for extraperitoneal RARP, respectively. Median estimated blood loss was 200 (IQR 150–250) mL. No intraoperative complications occurred. Median length of hospital stay was 3 (IQR 2–4) days.
Table 1.
Preoperative, intraoperative, and postoperative characteristic of patients treated with transperitoneal robot-assisted radical prostatectomy.
| Variable | Result |
|---|---|
| Patient, n | 17 |
| Agea, year | 64 (59–69) |
| BMIa, kg/m2 | 27 (24–27) |
| PSAa, ng/mL | 6.4 (5.1–9.4) |
| ISUP grade group at biopsya | 2 (2–3) |
| Prostate volume at MRIa, mL | 35 (30–56) |
| ECE at MRIb | 2 (11.8) |
| Positive DREb | 7 (41.2) |
| Docking timea, min | 12 (7–16) |
| Console timea, min | 185 (177–192) |
| Postoperative complicationb | |
| Pelvic hematoma (CD Grade 2) | 1 (5.9) |
| UTI that required antibiotic treatment (CD Grade 2) | 1 (5.9) |
| Pelvic bleeding that required a TAE (CD Grade 3a) | 1 (5.9) |
| Estimated blood lossa, mL | 200 (150–250) |
| Length of hospital staya, day | 3 (2–4) |
| ISUP grade group at final pathologyb | |
| ISUP 1 | 1 (5.9) |
| ISUP 2 | 9 (52.9) |
| ISUP 3 | 5 (29.4) |
| ISUP 4 | 0 (0) |
| ISUP 5 | 2 (11.8) |
| T stage at final pathologyb | |
| pT2 | 14 (82.4) |
| pT3a | 3 (17.6) |
| Positive surgical marginb | 5 (29.4) |
| Postoperative PSA at 3 monthsa, ng/mL | 0.009 (0.006–0.045) |
BMI, body mass index; CD, Clavien-Dindo; DRE, digital rectal examination; ECE, extracapsular extension; ISUP, International Society of Urological Pathology; MRI, magnetic resonance imaging; PSA, prostate-specific antigen; TAE, trans-arterial embolization; UTI, urinary tract infection.
Values are presented as median (interquartile range).
Values are presented as n (%).
3.2. Extraperitoneal RARP
Three patients were treated with an extraperitoneal approach. The individual features of each patients are listed in Table 2. No intraoperative complications occurred. One patient had a urinary tract infection in the postoperative period that required an antibiotic treatment (Clavien-Dindo Grade 2).
Table 2.
Preoperative, intraoperative, and postoperative characteristic of patients treated with extraperitoneal robot-assisted radical prostatectomy.
| Characteristic | Patient |
||
|---|---|---|---|
| No. 1 | No. 2 | No. 3 | |
| Age, year | 65 | 62 | 56 |
| BMI, kg/m2 | 27 | 24 | 26 |
| PSA, ng/mL | 4.4 | 30.0 | 5.4 |
| ISUP grade group at biopsy | 2 | 2 | 1 |
| Prostate volume at MRI, mL | 46 | 55 | 34 |
| ECE at MRI | 0 | 0 | 0 |
| DRE | Negative | Negative | Positive |
| Docking time, min | 20 | 15 | 10 |
| Console time, min | 200 | 170 | 155 |
| Postoperative complications | 0 | UTI (CD Grade 2) | 0 |
| Estimated blood loss, mL | 150 | 250 | 100 |
| Length of hospital stay, day | 3 | 3 | 4 |
| ISUP grade group at final pathology | 3 | 3 | 2 |
| T stage at final pathology | 2 | 3a | 2 |
| Positive surgical margin | 0 | 1 | 0 |
| Postoperative PSA at 3 months, ng/mL | 0.002 | 0.180 | 0.003 |
BMI, body mass index; CD, Clavien-Dindo; DRE, digital rectal examination; ECE, extracapsular extension; ISUP, International Society of Urological Pathology; MRI, magnetic resonance imaging; PSA, prostate-specific antigen; UTI, urinary tract infection.
3.3. RARC
A 71-year-old men with a history of very-high risk non-muscular invasive bladder cancer (Bacillus Calmette-Guerin unresponsive) was proposed for RARC with intracorporeal ileal conduit. Operative time was 360 min; estimated blood loss was 200 mL; and patient was discharged after 6 days. No intra- or post-operative complications were recorded, neither failure of the robotic system.
4. Discussion
In this single-center series, the settings and feasibility of extra- or intra-peritoneal RARP and RARC with Hugo™ RAS system have been shown. Some characteristics of this robotic platform should be underlined to facilitate the adaptation of robotic surgeons to the technology. In particular, the design of the open console with specific hand-controllers and the modularity of the robotic arms are the main novelties that should be carefully analyzed.
The open console allows a direct communication between the robotic surgeon and the operating room staff, and eases the consultation of external devices such as rapid consultations of radiological images, ultrasound images, and three-dimensional reconstructions. The surgeon needs to wear dedicated glasses in order to achieve a three-dimension vision. The endoscopic vision is provided by Karl-Storz through a 3D Tipcam S™ laparoscopic camera (Karl-Storz SE&Co. KG, Tuttlingen, Germany). The hand-controllers have a “pistol-like” ergonomic design with intuitive controls. The rotation of the wrist may be enhanced achieving an angle of wrist-rotation up to 529° to facilitate the movements of the needle-driver and improve the ergonomics especially during the urethro-vesical anastomosis.
The robotic arms are mounted on separated carts, de facto forming distinct modules. The modularity of this system allows different settings for surgery such as a three- or four-arm configuration. On the other hand, the four separate carts occupy more space and require more storage space than a single cart platform. Arms and carts tend to be cumbersome and may limit the space of the bed-side assistant.
The performances of Hugo™ RAS system have already been assessed in some urological procedures, showing a short learning curve in experienced robotic surgeons [[13], [14], [15], [16]]. The feasibility of RARP was explored on cadavers allowing the setting of the robotic platform to be tested [17]. The first performances of Hugo™ RAS system were assessed in a case-series published by Ragavan et al. [18] demonstrating the setting of this robotic platform in prostate (simple prostatectomy [n=1] and radical prostatectomy [n=3]) and renal surgery (robot-assisted nephrectomy [n=3]) and showed good results in terms of feasibility and safety. Recently, Bravi et al. [19] published the first large series of more than one hundred RARPs performed with Hugo™ RAS system, assessing the safety and versatility of this robotic platform, showing a median operative time of 180 (IQR 145–200) min and a rate of complications (7% for all complications and 2% for major complications) comparable to series of RARP performed with other surgical platforms.
On the other hand, this is the first report that provides evidence on the performances of Hugo™ RAS system in the setting of RARC and RARP performed extraperitoneally. The aim was to provide a standardized operative room and docking setting to approach the major urologic pelvic surgeries with Hugo™ RAS system. We modified the trocar position scheme proposed in the literature by placing the 4th arm trocar distally and positioning it in line with other arms (Figure 1, Figure 2). This configuration was studied to permit the 4th arm to reach without difficulties all the anatomical structures during the surgery. Especially, it demonstrated its usefulness during the nerve-sparing step when dissecting the left neurovascular bundle. Depending on the prostate conformation, we sometimes change the position of instruments between the left and the 4th arm, de facto limiting any potential clashing between robotic arms.
Reports from different centers are necessary to confirm the maneuverability of this robotic platform and the feasibility to perform major oncological surgeries with this novel platform. We strongly recommend ex-vivo training in dry or wet laboratories to tailor the cart positioning and docking setting to the characteristics of the operating room and the usual setting of each center. Studies to investigate the learning curve and the outcomes of Hugo™ RAS system in novice robotic surgeons or in robotic naïve centers are needed. These considerations extend to all new robotic platforms in order to prevent erroneous surgical settings before translating to patients. On the other hand, the adoption of this novel platform in centers with an established robotic experience requires a very steep learning curve after a short training course. The transferability of surgical skills has been already demonstrated to be quick among platforms [20].
The study is not devoid of limitations such as its nature of case-series and the relatively small number of patients may limit the generalizability of the findings even if the aim of the study was to report the setting and the feasibility of RARP and RARC with this novel robotic platform; the relatively low number of patients could also suggest a bias in patients’ selection. In our center, more than 50% of the prostatectomies are performed laparoscopically and only selected patients are selected for robotic cystectomy (e.g., early cystectomies in patients with non-muscle invasive bladder cancer or selected patients with T2 bladder cancer). Also, the cases are distributed between Hugo™ RAS and Da Vinci platforms.
Our results support the recent literature about the feasibility of RARP with Hugo™ RAS system and add additional important insights about this novel platform to perform extraperitoneal RARP and RARC with intracorporeal urinary diversion. Further studies with larger series of patients are needed to confirm these preliminary findings.
5. Conclusion
Hugo™ RAS system is a novel promising robotic platform that allows to perform major oncological pelvic surgery. We showed the feasibility of RARP performed both intra- and extra-peritoneally and RARC with intracorporeal ileal conduit with this novel platform.
Author contributions
Study concept and design: Alberto Breda, Joan Palou, Alessandro Uleri, Angelo Territo, Josep Maria Gaya.
Data acquisition: Alessandro Uleri, Alessandro Tedde, Alba Farré, Alejandra Bravo.
Data analysis: Paolo Verri, Giuseppe Basile.
Drafting of manuscript: Andrea Gallioli, Alessandro Uleri, Angelo Territo.
Critical revision of the manuscript: Joan Palou, Alberto Breda, Óscar Rodríguez Faba.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Tongji University.
References
- 1.Yaxley J.W., Coughlin G.D., Chambers S.K., Occhipinti S., Samaratunga H., Zajdlewicz L., et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet. 2016;388:1057–1066. doi: 10.1016/S0140-6736(16)30592-X. [DOI] [PubMed] [Google Scholar]
- 2.Coughlin G.D., Yaxley J.W., Chambers S.K., Occhipinti S., Samaratunga H., Zajdlewicz L., et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: 24-month outcomes from a randomised controlled study. Lancet Oncol. 2018;19:1051–1060. doi: 10.1016/S1470-2045(18)30357-7. [DOI] [PubMed] [Google Scholar]
- 3.Fontanet S., Basile G., Baboudjian M., Gallioli A., Huguet J., Territo A., et al. Robot-assisted vs. open radical cystectomy: systematic review and meta-analysis of randomized controlled trials. Actas Urol Esp (Engl Ed) 2023;47:261–270. doi: 10.1016/j.acuroe.2023.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Liatsikos E., Tsaturyan A., Kyriazis I., Kallidonis P., Manolopoulos D., Magoutas A. Market potentials of robotic systems in medical science: analysis of the Avatera robotic system. World J Urol. 2022;40:283–289. doi: 10.1007/s00345-021-03809-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alip S., Koukourikis P., Han W.K., Rha K.H., Na J.C. Comparing Revo-i and da Vinci in Retzius-sparing robot-assisted radical prostatectomy: a preliminary propensity score analysis of outcomes. J Endourol. 2022;36:104–110. doi: 10.1089/end.2021.0421. [DOI] [PubMed] [Google Scholar]
- 6.Hinata N., Yamaguchi R., Kusuhara Y., Kanayama H., Kohjimoto Y., Hara I., et al. Hinotori surgical robot system, a novel robot-assisted surgical platform: preclinical and clinical evaluation. Int J Urol. 2022;29:1213–1220. doi: 10.1111/iju.14973. [DOI] [PubMed] [Google Scholar]
- 7.Chang K.D., Abdel Raheem A., Choi Y.D., Chung B.H., Rha K.H. Retzius-sparing robot-assisted radical prostatectomy using the Revo-i robotic surgical system: surgical technique and results of the first human trial. BJU Int. 2018;122:441–448. doi: 10.1111/bju.14245. [DOI] [PubMed] [Google Scholar]
- 8.Totaro A., Campetella M., Bientinesi R., Gandi C., Palermo G., Russo A., et al. The new surgical robotic platform Hugo™ RAS: system description and docking settings for robot-assisted radical prostatectomy. Urologia. 2022;89:603–609. doi: 10.1177/03915603221107855. [DOI] [PubMed] [Google Scholar]
- 9.Venckus R., Jasenas M., Telksnys T., Venckus M., Janusonis V., Dulskas A., et al. Robotic-assisted radical prostatectomy with the Senhance® robotic platform: single center experience. World J Urol. 2021;39:4305–4310. doi: 10.1007/s00345-021-03792-5. [DOI] [PubMed] [Google Scholar]
- 10.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briganti A., Larcher A., Abdollah F., Capitanio U., Gallina A., Suardi N., et al. Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: the essential importance of percentage of positive cores. Eur Urol. 2012;61:480–487. doi: 10.1016/j.eururo.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 12.Gandaglia G., Fossati N., Zaffuto E., Bandini M., Dell'Oglio P., Bravi C.A., et al. Development and internal validation of a novel model to identify the candidates for extended pelvic lymph node dissection in prostate cancer. Eur Urol. 2017;72:632–640. doi: 10.1016/j.eururo.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 13.Mottaran A., Paciotti M., Bravi C.A., Sarchi L., Nocera L., Piro A., et al. Robot-assisted simple prostatectomy with the novel Hugo™ RAS system: feasibility, setting, and perioperative outcomes. Minerva Urol Nephrol. 2023;75:235–239. doi: 10.23736/S2724-6051.22.05031-5. [DOI] [PubMed] [Google Scholar]
- 14.Gallioli A., Uleri A., Gaya J.M., Territo A., Aumatell J., Verri P., et al. Initial experience of robot-assisted partial nephrectomy with Hugo™ RAS system: implications for surgical setting. World J Urol. 2023;41:1085–1091. doi: 10.1007/s00345-023-04336-9. [DOI] [PubMed] [Google Scholar]
- 15.Mottaran A., Bravi C.A., Sarchi L., Paciotti M., Nocera L., Piro A., et al. Robot-assisted sacropexy with the novel Hugo robot-assisted surgery system: initial experience and surgical setup at a tertiary referral robotic center. J Endourol. 2023;37:35–41. doi: 10.1089/end.2022.0495. [DOI] [PubMed] [Google Scholar]
- 16.Elorrieta V., Villena J., Kompatzki Á, Velasco A., Salvadó J.A. Robot assisted laparoscopic surgeries for nononcological urologic disease: initial experience with Hugo RAS system. Urology. 2023;174:118–125. doi: 10.1016/j.urology.2023.01.042. [DOI] [PubMed] [Google Scholar]
- 17.Sarchi L., Mottaran A., Bravi C.A., Paciotti M., Farinha R., Piazza P., et al. Robot-assisted radical prostatectomy feasibility and setting with the Hugo™ robot-assisted surgery system. BJU Int. 2022;130:671–675. doi: 10.1111/bju.15819. [DOI] [PubMed] [Google Scholar]
- 18.Ragavan N., Bharathkumar S., Chirravur P., Sankaran S., Mottrie A. Evaluation of Hugo RAS system in major urologic surgery: our initial experience. J Endourol. 2022;36:1029–1035. doi: 10.1089/end.2022.0015. [DOI] [PubMed] [Google Scholar]
- 19.Bravi C.A., Paciotti M., Balestrazzi E., Piro A., Piramide F., Peraire M., et al. Outcomes of robot-assisted radical prostatectomy with the Hugo RAS surgical system: initial experience at a high-volume robotic center. Eur Urol Focus. 2023;9:642–644. doi: 10.1016/j.euf.2023.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Larkins K.M., Mohan H.M., Gray M., Costello D.M., Costello A.J., Heriot A.G., et al. Transferability of robotic console skills by early robotic surgeons: a multi-platform crossover trial of simulation training. J Robot Surg. 2023;17:859–867. doi: 10.1007/s11701-022-01475-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Working space creation and trocar positioning in case of extraperitoneal robot-assisted radical prostatectomy with HugoTM robot-assisted surgery system