Abstract
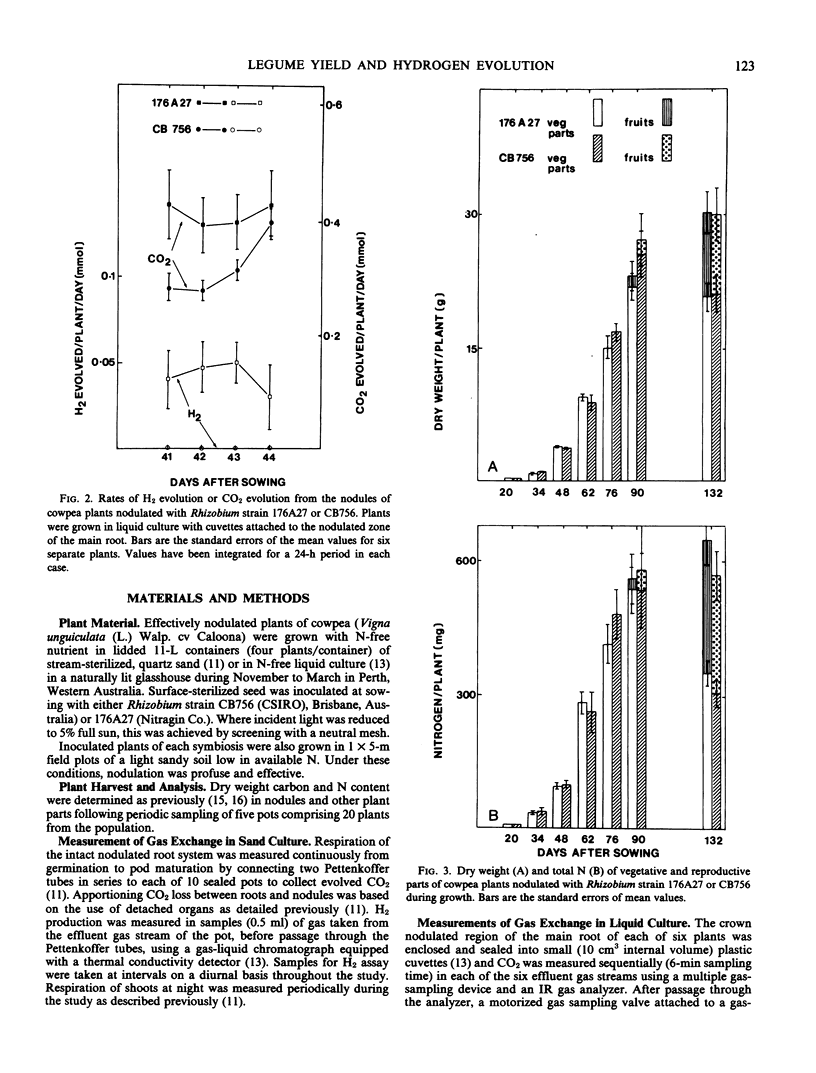
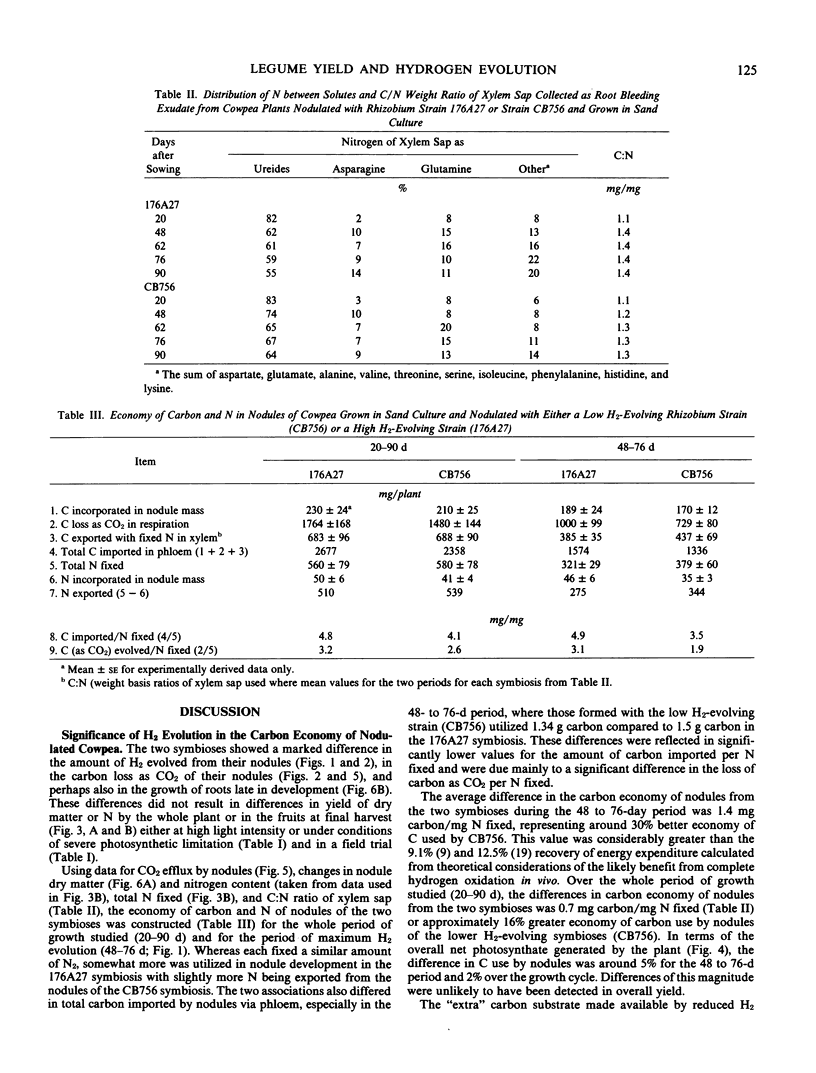
The carbon and nitrogen economies of a single cultivar of cowpea (Vigna unguiculata (L.) Walp.cv Caloona) nodulated with either a high H2-evolving strain (176A27) or a low H2-evolving strain (CB756) of Rhizobium were compared. The two symbioses did not differ in total dry matter production, seed yield, nitrogen fixed, the spectrum of nitrogenous solutes produced by nodules for export, or the partitioning of net photosynthate within the plant throughout the growth cycle. Detailed examination of the carbon and nitrogen economy of the nodules, however, showed a significant difference between the symbioses. Nodules formed with CB756 lost less CO2 in respiration compared to the higher H2-evolving symbioses and this could have been largely responsible for a 36% better economy of carbon use in CB756 nodules during the period of maximum H2 evolution (48-76 days) and over the whole growth period (20-90 days), a 16% economy. In terms of overall net photosynthate generated by the plant, these economies were equivalent to 5% and 2% of the carbon utilized in the two periods, respectively. From the differences in H2 evolution and CO2 production by nodules of the two symbioses, the cost of H2 evolution was found to be 3.83±0.6 millimoles CO2/millimoles H2 for plants grown in sand culture and 1.69 ± 0.48 millimoles CO2/millimoles H2 for those in water culture. In both symbioses, the ratio of H2 evolution to N2 fixed varied markedly during ontogeny, indicating a significant variation in the relative efficiency and thus metabolic cost of N2 fixation at different stages during development.
Full text
PDF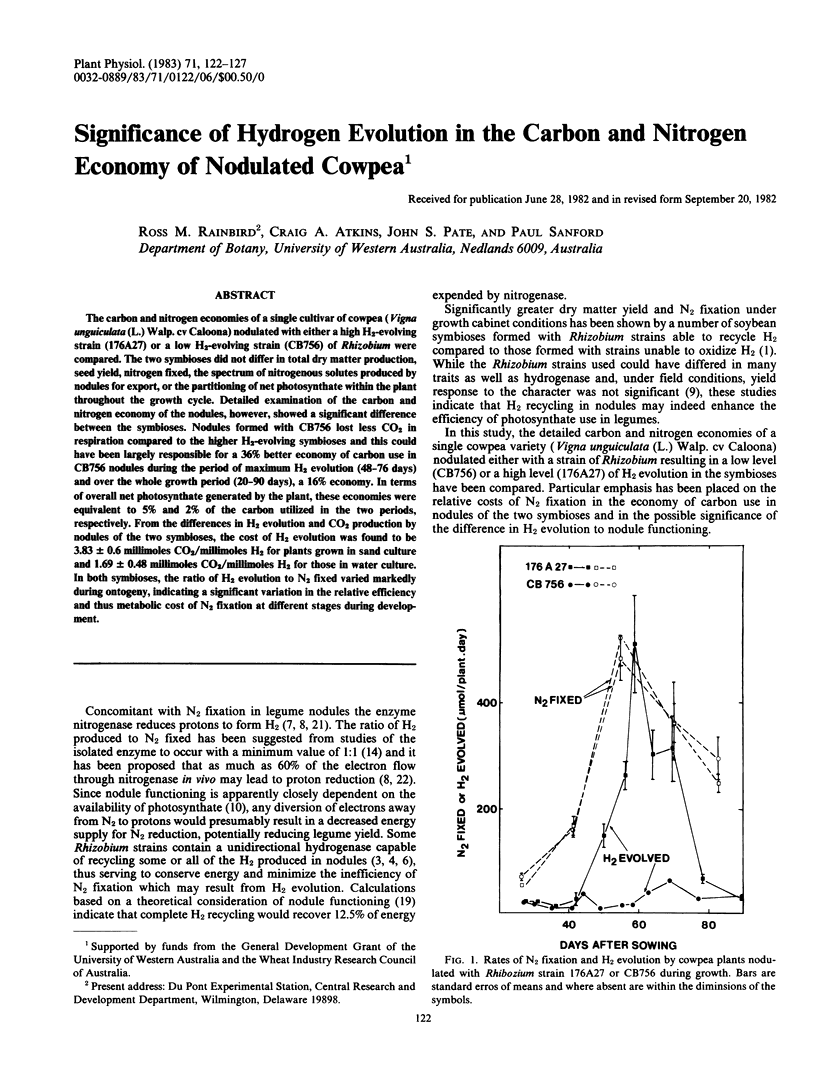



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht S. L., Maier R. J., Hanus F. J., Russell S. A., Emerich D. W., Evans H. J. Hydrogenase in Rhizobium japonicum Increases Nitrogen Fixation by Nodulated Soybeans. Science. 1979 Mar 23;203(4386):1255–1257. doi: 10.1126/science.203.4386.1255. [DOI] [PubMed] [Google Scholar]
- Atkins C. A., Pate J. S., Sharkey P. J. Asparagine metabolism-key to the nitrogen nutrition of developing legume seeds. Plant Physiol. 1975 Dec;56(6):807–812. doi: 10.1104/pp.56.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethlenfalvay G. J., Phillips D. A. Effect of Light Intensity on Efficiency of Carbon Dioxide and Nitrogen Reduction in Pisum sativum L. Plant Physiol. 1977 Dec;60(6):868–871. doi: 10.1104/pp.60.6.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethlenfalvay G. J., Phillips D. A. Ontogenetic Interactions between Photosynthesis and Symbiotic Nitrogen Fixation in Legumes. Plant Physiol. 1977 Sep;60(3):419–421. doi: 10.1104/pp.60.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethlenfalvay G. J., Phillips D. A. Variation in nitrogenase and hydrogenase activity of alaska pea root nodules. Plant Physiol. 1979 May;63(5):816–820. doi: 10.1104/pp.63.5.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. O. Nitrogenase--hydrogenase interrelationships in Rhizobia. Biochimie. 1978;60(3):233–236. doi: 10.1016/s0300-9084(78)80819-0. [DOI] [PubMed] [Google Scholar]
- Herridge D. F., Atkins C. A., Pate J. S., Rainbird R. M. Allantoin and Allantoic Acid in the Nitrogen Economy of the Cowpea (Vigna unguiculata [L.] Walp.). Plant Physiol. 1978 Oct;62(4):495–498. doi: 10.1104/pp.62.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herridge D. F., Pate J. S. Utilization of net photosynthate for nitrogen fixation and protein production in an annual legume. Plant Physiol. 1977 Nov;60(5):759–764. doi: 10.1104/pp.60.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layzell D. B., Rainbird R. M., Atkins C. A., Pate J. S. Economy of Photosynthate Use in Nitrogen-fixing Legume Nodules: Observations on Two Contrasting Symbioses. Plant Physiol. 1979 Nov;64(5):888–891. doi: 10.1104/pp.64.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortenson L. E. Regulation of nitrogen fixation. Curr Top Cell Regul. 1978;13:179–232. doi: 10.1016/b978-0-12-152813-3.50010-0. [DOI] [PubMed] [Google Scholar]
- Pate J. S., Layzell D. B., McNeil D. L. Modeling the transport and utilization of carbon and nitrogen in a nodulated legume. Plant Physiol. 1979 Apr;63(4):730–737. doi: 10.1104/pp.63.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. S., Sharkey P. J., Atkins C. A. Nutrition of a developing legume fruit: functional economy in terms of carbon, nitrogen, water. Plant Physiol. 1977 Mar;59(3):506–510. doi: 10.1104/pp.59.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K. R., Engelke J. A., Russell S. A., Evans H. J. Hydrogen reactions of nodulated leguminous plants: I. Effect of rhizobial strain and plant age. Plant Physiol. 1977 Nov;60(5):651–654. doi: 10.1104/pp.60.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K. R., Evans H. J. Hydrogen evolution: A major factor affecting the efficiency of nitrogen fixation in nodulated symbionts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1207–1211. doi: 10.1073/pnas.73.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]



