Abstract
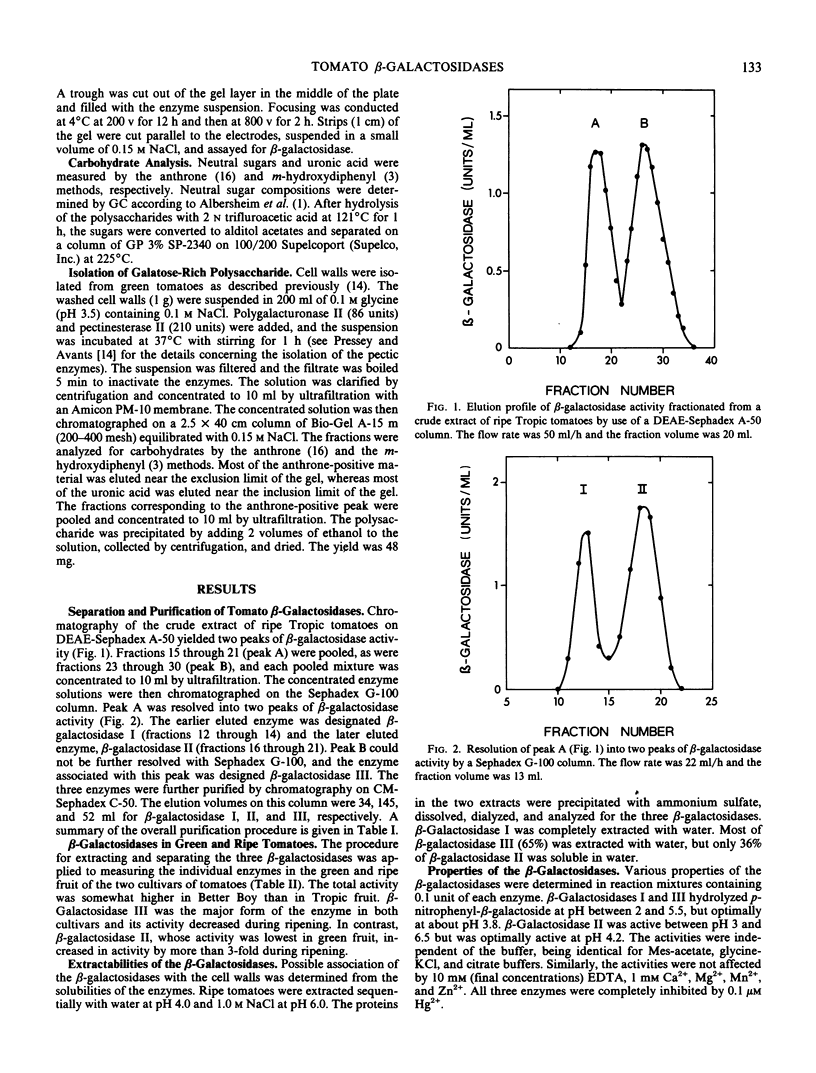
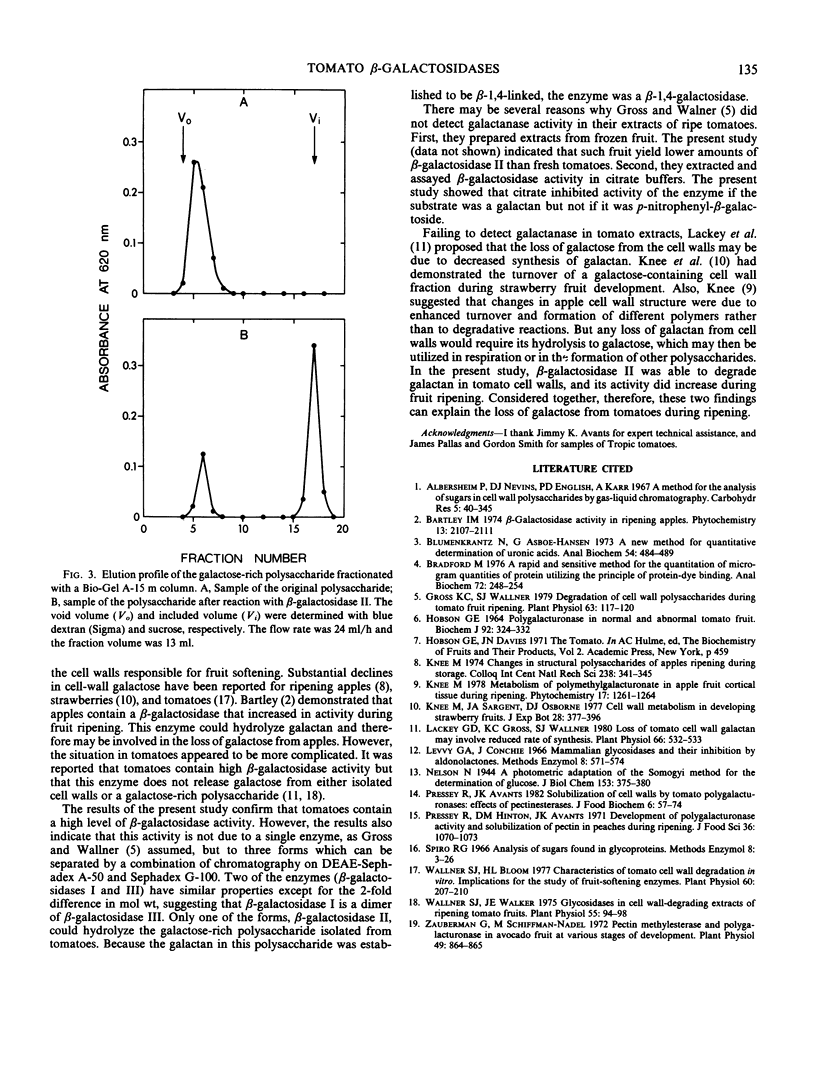
Tomatoes (Lycopersicon esculentum L.) contained a high level of β-galactosidase activity which was due to three forms of the enzyme. During tomato ripening, the sum of their activities remained relatively constant, but the levels of the individual forms of β-galactosidase changed markedly. The three enzymes were separated by a combination of chromatography of DEAE-Sephadex A-50 and Sephadex G-100. During ripening of tomatoes, β-galactosidases I and III levels decreased but the β-galactosidase II level increased more than 3-fold. The three enzymes were optimally active near pH 4, and all were inhibited by galactose and galactonolactone. However, the enzymes differed in molecular weight, Km value with p-nitrophenyl-β-galactoside, and stability with respect to pH and temperature. β-Galactosidase II was the only enzyme capable of hydrolyzing a polysaccharide that was isolated from tomatoes and that consisted primarily of β-1, 4-linked galactose. The ability of β-galactosidase II to degrade the galactan and the increase in its activity during tomato ripening suggest a possible role for this enzyme in tomato softening.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Gross K. C., Wallner S. J. Degradation of Cell Wall Polysaccharides during Tomato Fruit Ripening. Plant Physiol. 1979 Jan;63(1):117–120. doi: 10.1104/pp.63.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson G. E. Polygalacturonase in normal and abnormal tomato fruit. Biochem J. 1964 Aug;92(2):324–332. doi: 10.1042/bj0920324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackey G. D., Gross K. C., Wallner S. J. Loss of tomato cell wall galactan may involve reduced rate of synthesis. Plant Physiol. 1980 Sep;66(3):532–533. doi: 10.1104/pp.66.3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner S. J., Bloom H. L. Characteristics of tomato cell wall degradation in vitro: implications for the study of fruit-softening enzymes. Plant Physiol. 1977 Aug;60(2):207–210. doi: 10.1104/pp.60.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner S. J., Walker J. E. Glycosidases in Cell Wall-degrading Extracts of Ripening Tomato Fruits. Plant Physiol. 1975 Jan;55(1):94–98. doi: 10.1104/pp.55.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauberman G., Schiffmann-Nadel M. Pectin methylesterase and polygalacturonase in avocado fruit at various stages of development. Plant Physiol. 1972 May;49(5):864–865. doi: 10.1104/pp.49.5.864. [DOI] [PMC free article] [PubMed] [Google Scholar]


