TO THE EDITOR:
Purine analogs (PNAs), most of all cladribine (2-chlorodeoxyadenosine, 2CdA) or pentostatin, represent the frontline treatment of choice for hairy cell leukemia (HCL), given their ability to control the disease for significantly long periods.1, 2, 3, 4, 5, 6 Patients treated with 2CdA, however, relapse in nearly half of the cases within the first 10 years, although much longer treatment-free intervals can be seen in patients who attain a deeper response after frontline therapy. When 2CdA fails to control the disease, treatment-free periods become much shorter, and half of patients in partial response (PR) require further treatment within 5 years.5,6 There may be room for repeating PNAs, provided sufficient time (at least 2 years) has elapsed since their previous administration.7 Despite that, many patients do not qualify for retreatment with these agents, especially if relapses are frequent, if the bone marrow appears markedly hypocellular after 1 or more courses of PNAs, or if it is highly infiltrated by leukemic cells. Both reduced marrow cellularity and high disease burden are, in fact, predictors of profound and long-lasting cytopenia under treatment with PNAs.8
For this reason, there has always been a need for agents other than chemotherapy to multiply relapsing HCL. The dense expression of the CD20 antigen on the surface of hairy cells has attracted the attention of clinical investigators9,10; weekly administered single-agent rituximab has demonstrated its efficacy in pretreated patients with HCL in early reports, with documented activity in peripheral blood and bone marrow.11,12 Consequently, it appeared as a feasible treatment for patients relapsing after repeated courses of PNAs and, more importantly, when they were clinically contraindicated.
Here, we report our single-center experience with single-agent rituximab in patients with HCL with symptomatic disease relapse and who have failed at least 1 previous course of PNAs. Rituximab was administered intravenously at the dose of 375 mg/m2 once weekly for 4 weeks. Responses have been categorized according to the Consensus Resolution Criteria13 integrated with bone marrow immunohistochemistry for CD20, as reported previously (supplemental Methods).4 The main study objectives were overall response rate (ORR), time-to-next treatment (TTNT), and overall survival. The study was approved by our institutional board (Ethical Committee AVEC of Bologna, approval ID 1043/2021/Oss/AOUBo).
Thirty-three patients received 39 courses of rituximab between 1999 and 2019. Four patients received it twice during the course of their disease, and 1 patient received it 3 times. It was administered as a median third line of therapy (range, 2-8). First rituximab was given at a median age of 60.9 years (range, 35.4-81.7) and at a median time from diagnosis of 65.1 months (range, 1.9-374.7). Most of the patients were neutropenic or thrombocytopenic at rituximab start (Table 1), with a median bone marrow cellularity and disease infiltration of 30% and 80%, respectively. Thirty-one patients were male, and 2 were female. In most cases (71.8%), rituximab was applied as a second line (12 patients) or third or fourth line (9 and 7 patients, respectively). 2CdA represented the most widely applied agent before rituximab administration: all patients treated with rituximab as second line received 2CdA as frontline approach, as well as 89% and 86% of those treated in third and fourth line, respectively.
Table 1.
Clinical details and outcomes by line of treatment
| Second line | Third line | Fourth line | Fifth line | Sixth line | Seventh line | Eighth line | |
|---|---|---|---|---|---|---|---|
| Patients, n | 12 | 9 | 7 | 5 | 4 | 1 | 1 |
| Male, n | 12/12 | 8/9 | 6/7 | 5/5 | 4/4 | 1/1 | 1/1 |
| Leukocytes (mm−3) | 2 250 | 1 740 | 2 600 | 1 800 | 2 950 | 1 600 | 5 900 |
| Neutrophils (mm−3) | 660 | 1 160 | 1 300 | 740 | 1 350 | 1 072 | 2 000 |
| Hemoglobin (g/dL) | 13.4 | 11.3 | 13.1 | 11.6 | 12.7 | 11.7 | 7.6 |
| Platelets (mm−3) | 72 000 | 89 000 | 56 000 | 100 000 | 97 000 | 77 000 | 21 000 |
| Splenomegaly | 33% | 33% | 0 | 20% | 0 | 0 | 0 |
| Last therapy before rituximab | Cladribine (100%) | Cladribine (89%) Rituximab (11%) |
Cladribine (86%) Interferon (14%) |
Cladribine (60%) Interferon (40%) |
Rituximab (50%) Cladribine (25%) Pentostatin (25%) |
Rituximab (100%) | Cladribine (100%) |
| Early interruption of rituximab, n | 1 (death) | None | 1 (cytopenia) | None | 1 (cytopenia) 1 (infusion reaction) |
None | None |
| Next therapy after rituximab | Cladribine (50%) Rituximab (25%) Vemurafenib (25%) |
Cladribine (60%) Interferon (20%) Rituximab + vemurafenib (20%) |
Vemurafenib (20%) Pentostatin (40%) Chlorambucil (20%) Cladribine (20%) |
Rituximab (50%) Cladribine (25%) Interferon (25%) |
Interferon (50%) Cladribine (25%) Rituximab (25%) |
None | None |
| Overall response | 75.0% | 88.9% | 57.1% | 80.0% | 50.0% | 100% | 0 |
| Complete response | 41.7% | 33.3% | 0 | 20.0% | 25.0% | 100% | 0 |
| Further treatment | 36.4% | 55.6% | 71.4% | 80.0% | 100% | 0 | 0∗ |
| Month to relapse, range | 2.6-24.9 | 2.5-109.0 | 1.2-28.8 | 10.0-37.5 | 1.5-193.7 | N/A | N/A∗ |
N/A, not assessable.
Patient deceased in the post-rituximab follow-up.
Out of 39 courses, a complete response (CR) was obtained in 28.2% of cases, a PR in 23.1% and a minimal response (MR) in 20.5%, yielding an ORR of 71.8%. In 28.2% of patients, we observed no response. Among the 12 patients treated in the second line, the ORR was 75.0%, with a CR in 41.7% of the cases. All these patients received 2CdA as frontline treatment of HCL, obtaining with this agent a CR, PR, and MR rate of 8.3%, 33.3% and 16.7%, respectively. Rituximab was given at a median time from 2CdA of 5.98 (range, 1.23-115.8) months. More specifically, 4 patients (2 unresponsive to 2CdA and 1 in PR) received rituximab within 3 months from the initial 2CdA, and all but 1 obtained a CR. The only patient who obtained a CR with 2CdA received rituximab after 65.2 months, although with no response. Likewise, a patient in PR after 2CdA who received rituximab after 115.8 months from initial treatment did not achieve a response. Importantly, 63.6% of the patients in this group did not require any further treatment for HCL after salvage rituximab.
ORR with rituximab remains high in patients treated in the third or higher line, although the higher the line, the lower the proportion of CR. Similarly, the proportion of patients who require further treatment after rituximab increases along with the number of previous lines of treatment they have received. Notably, significantly long treatment-free periods may be exceptionally obtained in highly pretreated cases: 109.0 months in a patient treated with rituximab in the second line, 114.5 months in 1 treated in the fourth line, and 193.7 in 1 treated in the sixth line.
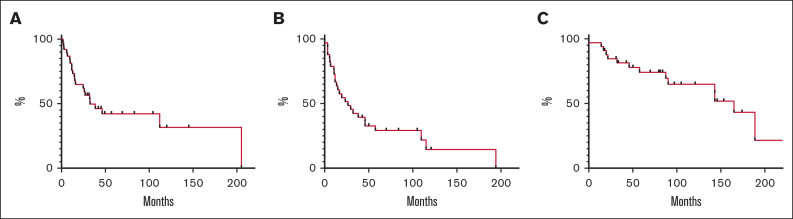
Median TTNT calculated on all 39 courses was 33 months, with a proportion of patients of 65% and 32% being treatment free at 2 and 15 years, respectively. Median progression-free survival was 24 months (15% at 15 years), whereas the median overall survival was 154 months (43% at 15 years) (Figure 1). Fourteen patients had died, mainly because of disease progression (85.7%). One patient who underwent splenectomy earlier during the disease died because of pneumonia-related septic shock, whereas 1 patient died because of secondary acute myeloid leukemia.
Figure 1.
Time to next treatment and survival analysis. (A) TTNT calculated on each treatment received (it includes also patients who received rituximab more than once). (B) Progression-free survival determined on 33 patients from the initiation of the (first) treatment with rituximab to the date of progression, death, or last follow-up. (C) Overall survival determined on 33 patients from the initiation of the (first) treatment with rituximab to the date of death or last follow-up.
Among the 5 patients who received rituximab more than once, all responded after the first course, obtaining a CR in 80.0% and a PR in 20.0% of the cases. Nevertheless, the ORR after the second or later course was only 50.0%, with a CR, PR, and MR rate of 16.7% each, respectively. The median TTNT following the first rituximab was 38.5 months in these patients, ranging from 15.0 to 205.0 months. In all but 1 case, rituximab was given in 2 consecutive lines of treatment, given the favorable response obtained with the first course and the lack of valid alternatives at the time it was administered again. The patient who received rituximab 3 times was treated on his fifth, sixth, and eighth lines, with an initial PR and no response thereafter.
Literature on rituximab in HCL is rather scarce, and the available data mainly refer to a limited number of patients treated as first-line salvage (supplemental Table).12,14, 15, 16, 17, 18 Our monocentric study, to the best of our knowledge, is the largest experience with rituximab in the treatment of relapsed or refractory HCL, exploring its activity both as first salvage and later rescue treatment, and it also concentrates on rituximab retreatment. Patients with less than CR or no response to frontline 2CdA could improve their status with subsequent rituximab in half of the cases. Importantly, the highest impact was seen when administered earlier after the conclusion of the frontline course of 2CdA, as more than 60% of the patients treated as second line did not require further treatment for HCL after rituximab. Retreatment was, in contrast, rather unsuccessful, despite the high quality of responses (CR in 80.0% and PR in 20.0%) obtained upon first administration: CR and PR were each achieved in 1 patient of 6 retreatments, and in half of the cases, no response was obtained. There is no unequivocal explanation why rituximab did not work in previous responders; a possible mechanism could be the downregulation of the CD20 antigen because of previous rituximab exposure.
Published studies mainly report on rituximab administered for 4 weeks,12,14,15,17,18 whereas 1 report by Thomas et al16 deals with rituximab administered for 8 consecutive weeks. Comparisons among studies are difficult because of the heterogeneity of disease characteristics at treatment inception. Most patients treated with rituximab × 8 as their first salvage, for example, had a previous favorable response to 2CdA, and on the contrary, patients treated with rituximab as the second line in our study did not; this may account for the higher CR rate obtained in the former series.16 Having said that, it is therefore hard to draw a conclusion on which rituximab schedule should be regarded as the most effective.
The bulk of our data has been collected in the past 20 years, often before the introduction of new drugs and in a setting of poor treatment alternatives besides interferon or repeated PNAs. More recently, in fact, vemurafenib and moxetumomab have modified the treatment scenario of patients with relapsing and refractory HCL.19, 20, 21 In addition to these, ibrutinib and zanubrutinib have also shown some activity in the context of multiply relapsing disease.22,23 For this reason, rituximab now seems mainly confined to frontline treatment, concomitantly with 2CdA, or as an early rescue in case PNAs fail to maintain a negative level of minimal residual disease.24
Although effective, most of the newer agents have never received formal approval for HCL treatment by regulatory authorities. Only moxetumomab was approved by the Food and Drug Administration (FDA) in 2018 for the treatment of adult patients with relapsed and refractory HCL after at least 2 previous treatment lines, including a PNA. Despite that, the producer has recently decided to remove the drug from the market, not because of its efficacy or safety, but because of its very low clinical uptake since FDA approval because of the availability of treatment alternatives and possibly to the complexity of administration. New agents are therefore not easily available in many geographic areas, including several, if not all, European countries. This means that the treatment of relapsed and refractory HCL in many places in the world still relies on the use of more conventional agents, among which rituximab should play a pivotal role.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Acknowledgments
Acknowledgments: The authors thank Massimo Agostini for data entry and AIL OdV for funding his activity (prot 2CSAIL21 Argnani).
The work reported in this publication was funded by the Italian Ministry of Health, RC-2023-2778897 project.
Contribution: A.B. and P.L.Z. conceived the work; A.B. and L.A. wrote the manuscript; L.A. performed statistical analyses; L.N., V.S., C.P., B.C., G.G., M.C., P.E.C., and G.B. provided patients and study materials; all authors read and approved the final version of the manuscript after revising it critically; all authors have access to the final database; and P.L.Z. reviewed the manuscript and gave approval for publication.
Footnotes
Data are available on request from coauthor, Alessandro Broccoli (alessandro.broccoli6@unibo.it).
The study was approved by our institutional board (Ethical Committee AVEC of Bologna, approval ID 1043/2021/Oss/AOUBo). All participants gave written informed consent (when applicable) in accordance with the Declaration of Helsinki to retrospectively collect their data.
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Else M, Dearden CE, Matutes E, et al. Long-term follow-up of 233 patients with hairy cell leukaemia, treated initially with pentostatin or cladribine, at a median of 16 years from diagnosis. Br J Haematol. 2009;145(6):733–740. doi: 10.1111/j.1365-2141.2009.07668.x. [DOI] [PubMed] [Google Scholar]
- 2.Cornet E, Tomowiak C, Tanguy-Schmidt A, et al. Long-term follow-up and second malignancies in 487 patients with hairy cell leukaemia. Br J Haematol. 2014;166(3):390–400. doi: 10.1111/bjh.12908. [DOI] [PubMed] [Google Scholar]
- 3.Zinzani PL, Stefoni V, Broccoli A, et al. Is it really possible to cure hairy cell leukemia patients only with frontline therapy? Ann Hematol. 2014;93(9):1565–1569. doi: 10.1007/s00277-014-2081-5. [DOI] [PubMed] [Google Scholar]
- 4.Broccoli A, Argnani L, Nanni L, et al. The treatment of hairy cell leukemia with a focus on long lasting responses to cladribine: a 30-year experience. Am J Hematol. 2021;96(10):1204–1210. doi: 10.1002/ajh.26287. [DOI] [PubMed] [Google Scholar]
- 5.Broccoli A, Argnani L, Cross M, et al. A 3-decade multicenter European experience with cladribine as upfront treatment in 384 patients with hairy cell leukemia. Blood Adv. 2022;6(14):4224–4227. doi: 10.1182/bloodadvances.2022007854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pagano L, Criscuolo M, Broccoli A, et al. Long-term follow-up of cladribine treatment in hairy cell leukemia: 30-year experience in a multicentric Italian study. Blood Cancer J. 2022;12(7):109. doi: 10.1038/s41408-022-00702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Troussard X, Maitre E, Cornet E. Hairy cell leukemia 2022: update on diagnosis, risk stratification, and treatment. Am J Hematol. 2022;97(2):226–236. doi: 10.1002/ajh.26390. [DOI] [PubMed] [Google Scholar]
- 8.Grever M, Abdel-Wahab O, Andritsos LA, et al. Consensus guidelines for the diagnosis and management of patients with classic hairy cell leukemia. Blood. 2017;129(5):553–560. doi: 10.1182/blood-2016-01-689422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foucar K, Falini B, Stein H. In: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised. 4th ed. Swerdlow SH, Campo E, Harris NL, et al., editors. IARC press; 2017. Hairy cell leukaemia; pp. 226–228. [Google Scholar]
- 10.Juliusson G, Lenkei R, Liliemark J. Flow cytometry of blood and bone marrow cells from patients with hairy cell leukemia: phenotype of hairy cells and lymphocyte subsets after treatment with 2-chlorodeoxyadenosine. Blood. 1994;83(12):3672–3681. [PubMed] [Google Scholar]
- 11.Zinzani PL, Ascani S, Piccaluga PP, Bendandi M, Pileri S, Tura S. Efficacy of rituximab in hairy cell leukemia treatment. J Clin Oncol. 2000;18(22):3875–3877. doi: 10.1200/JCO.2000.18.22.3875. [DOI] [PubMed] [Google Scholar]
- 12.Lauria F, Lenoci M, Annino L, et al. Efficacy of anti-CD20 monoclonal antibodies (Mabthera) in patients with progressed hairy cell leukemia. Haematologica. 2001;86(10):1046–1050. [PubMed] [Google Scholar]
- 13.Catovsky D, Quesada JR, Golomb HM. Consensus resolution: proposed criteria for evaluation of response to treatment in hairy cell leukemia. Leukemia. 1987;1(4):405–406. [PubMed] [Google Scholar]
- 14.Hagberg H, Lundholm L. Rituximab, a chimaeric anti-CD20 monoclonal antibody, in the treatment of hairy cell leukaemia. Br J Haematol. 2001;115(3):609–611. doi: 10.1046/j.1365-2141.2001.03143.x. [DOI] [PubMed] [Google Scholar]
- 15.Nieva J, Bethel K, Saven A. Phase 2 study of rituximab in the treatment of cladribine-failed patients with hairy cell leukemia. Blood. 2003;102(3):810–813. doi: 10.1182/blood-2003-01-0014. [DOI] [PubMed] [Google Scholar]
- 16.Thomas DA, O’Brien S, Bueso-Ramos C, et al. Rituximab in relapsed or refractory hairy cell leukemia. Blood. 2003;102(12):3906–3911. doi: 10.1182/blood-2003-02-0630. [DOI] [PubMed] [Google Scholar]
- 17.Zenhäusern R, Simcock M, Gratwohl A, et al. Rituximab in patients with hairy cell leukemia relapsing after treatment with 2-chlorodeoxyadenosine (SAKK 31/98) Haematologica. 2008;93(9):1426–1428. doi: 10.3324/haematol.11564. [DOI] [PubMed] [Google Scholar]
- 18.Leclerc M, Suarez F, Noël MP, et al. Rituximab therapy for hairy cell leukemia: a retrospective study of 41 cases. Ann Hematol. 2015;94(1):89–95. doi: 10.1007/s00277-014-2175-0. [DOI] [PubMed] [Google Scholar]
- 19.Tiacci E, Park JH, De Carolis L, et al. Targeting mutant BRAF in relapsed or refractory hairy-cell leukemia. N Engl J Med. 2015;373(18):1733–1747. doi: 10.1056/NEJMoa1506583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiacci E, De Carolis L, Simonetti E, et al. Vemurafenib plus rituximab in refractory or relapsed hairy-cell leukemia. N Engl J Med. 2021;384(19):1810–1823. doi: 10.1056/NEJMoa2031298. [DOI] [PubMed] [Google Scholar]
- 21.Kreitman RJ, Dearden C, Zinzani PL, et al. Moxetumoomab pasudotox in heavily pre-treated patients with relapsed/refractory hairy cell leukemia (HCL): long-term follow-up from the pivotal trial. J Hematol Oncol. 2021;14(1):35. doi: 10.1186/s13045-020-01004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers KA, Andritsos LA, Wei L, et al. Phase 2 study of ibrutinib in classic and variant hairy cell leukemia. Blood. 2021;137(25):3473–3483. doi: 10.1182/blood.2020009688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tam CS, Trotman J, Opat SS, et al. Zanubrutinib in patients with relapsed/refractory hairy cell leukemia. Blood Adv. 2023;7(12):2884–2887. doi: 10.1182/bloodadvances.2022008990. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chihara D, Arons E, Stetler-Stevenson M, et al. Randomized phase II study of first-line cladribine with concurrent or delayed rituximab in patients with hairy cell leukemia. J Clin Oncol. 2020;38(14):1527–1538. doi: 10.1200/JCO.19.02250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.