Abstract
A new autotrophic Thiomicrospira strain, MA-3, was isolated from the surface of a polymetal sulfide deposit collected at a Mid-Atlantic Ridge hydrothermal vent site. The DNA homology among three vent isolates, Thiomicrospira crunogena, Thiomicrospira sp. strain L-12, and Thiomicrospira sp. strain MA-3, was 99.3% or higher, grouping them as the same species, T. crunogena (type strain, ATCC 35932). The fact that T. crunogena and Thiomicrospira sp. strain L-12 were isolated from Pacific vent sites demonstrates a cosmopolitan distribution of this species.
Sulfur-oxidizing chemolithotrophs isolated from hydrothermal environments are described as mesophiles and facultative or obligate autotrophs (6, 7, 10, 14, 19) with diverse oxidative capabilities (12, 13, 15). Twelve of ninety-five sulfur-oxidizing isolates from the Galapagos hydrothermal vent site were physiologically described as Thiomicrospira strains (19), and one Mid-Atlantic Ridge (MAR) isolate was phylogenetically placed in the same genus (17). Combined molecular and cultivation approaches have greatly furthered the concept of Thiomicrospira diversity in marine as well as freshwater habitats (2). Presently fully described sulfur-oxidizing chemolithoautotrophs isolated from deep-sea hydrothermal vents are limited to two species of Thiomicrospira (14, 18) and one species of Thiobacillus (7), none of which is acidophilic. Furthermore, natural microbial populations as well as certain MAR isolates and the two previously described Thiomicrospira isolates from Pacific vent sites (T. crunogena and Thiomicrospira sp. strain L-12) have been shown to oxidize sulfidic minerals at near-neutral pH (8, 23). The most active of these Atlantic vent isolates (MAR strain MA-3) has been physiologically and phylogenetically characterized and compared to the two different Pacific vent site Thiomicrospira isolates.
Strain MA-3 was isolated from enrichments of surface scrapings of a polymetal sulfide rock collected from a depth of 3,620 m at the MAR Trans-Atlantic Geotransverse (TAG) hydrothermal vent site. T. crunogena and Thiomicrospira sp. strain L-12 were regrown from our culture collection. The medium used for enrichment and laboratory experimentation was artificial seawater (ASW) containing vitamins, trace elements, and phenol red indicator (8). The pH of filter-sterilized (0.2-μm pore size) medium was set at 7.4 with HCl. The reduced sulfur sources for autotrophic growth were sodium thiosulfate (10 mM [T-ASW]), elemental sulfur (1% [wt/vol]), sodium sulfide (500 μM) (5), sodium sulfite (1 and 5 mM), and natural and commercial metal sulfides (e.g., pyrite and chalcopyrite) as ground and sterilized slurries (4% [wt/vol]) (8). The organic substrates were glucose, lactate, galactose, peptone, yeast extract, and Casamino Acids, tested individually at 0.1% in ASW medium. Agar (1.5%) medium made with T-ASW was used for the initial isolation, counting of CFU, and stock culture maintenance. Growth was determined by acridine orange epifluorescent cell counts (11), pH indicator change, elemental sulfur deposition, and incorporation of radiolabeled 14CO2. The latter was done in T-ASW or metal sulfide-amended ASW medium with radiolabeled NaH14CO3 (8, 21, 23). In testing for the possible excretion of acid-stable products during CO2 incorporation in growing cultures at 24°C, the procedure of Ruby and Jannasch (18) was modified by acidifying the filtrate to pH 3.0 and then sparging with air to remove residual radiolabeled bicarbonate. Maximum growth rates were determined at 24°C in 100 and 10% air-saturated media by direct cell counts and by 14CO2 incorporation. The growth temperature range was determined with liquid cultures (1°C increments above 38°C). The pH range for growth was tested in T-ASW set at initial pH values in the range of 4.5 to 9.0 at 0.5-pH intervals. Iron oxidation supporting CO2 incorporation was studied with ASW medium with 10 mM ferrous sulfate at pH 7.0 with a gaseous headspace of 20, 2, or 1% oxygen (balanced by N2). T-ASW with 1% oxygen in the gas phase served as the positive control. Iron toxicity was tested in oxygen-free (N2 gassed) ASW medium containing from 0 to 90 mM ferrous sulfate at near-neutral pH and inoculated with pregrown, centrifuged, and washed cells. Viability, as CFU, was assessed by plating of subsamples at various time points on T-ASW agar medium. Nitrate respiration was tested by counting cell number increase in anoxic T-ASW medium supplemented with 2 mM KNO3. The requirement for sodium ions and the possible substitution of potassium were tested in NaCl-free T-ASW medium (18). Effects of ambient versus in situ pressure (1 × 105 Pa and 350 × 105 Pa) on growth were measured by CO2 incorporation at 24°C (14, 18). The 16S rRNA-encoding gene from isolate MA-3 was amplified by using primers and PCR conditions as described by Muyzer et al. (17). The 16S rRNA sequence of strain MA-3 was aligned manually, by using the sequence editor SEQAPP (9), to sequences of other bacteria obtained from the Ribosomal Database Project (RDP [16]), and from GenBank (1). The DNA base composition for G+C, the ubiquinone analysis, and the DNA-DNA hybridizations were all carried out as described in detail by Brinkhoff et al. (3).
Strain MA-3 is an autotrophic, obligately aerobic, gram-negative, motile (by phase microscopy) and slightly vibrioid bacterium measuring 0.5 to 0.7 by 1.3 to 2.0 μm. The colonies on T-ASW agar are white, smooth, and entire and produce elemental sulfur and sulfate (i.e., sulfuric acid). The strain grows aerobically on reduced sulfur compounds (H2S, sulfur, and thiosulfate) and sulfidic minerals, all of which may be available in its natural vent habitat, but not on sulfite. It did not demonstrate nitrate respiration. The growth rate on thiosulfate at 24°C is 0.8 h−1 (doubling time of 51 min) in the presence of either 100 or 10% air-saturated medium. It did not grow heterotrophically on any of the organic compounds tested. Strain MA-3 of the species T. crunogena has been deposited as ATCC 700270 in the American Type Culture Collection.
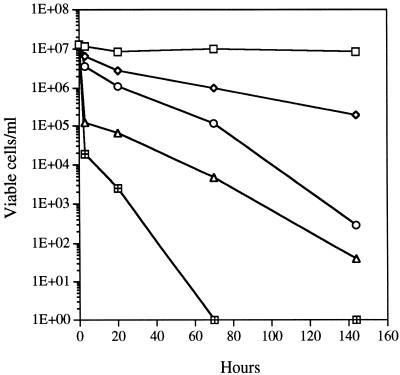
At near-neutral pH, strain MA-3 will utilize commercial pyrite or natural metal sulfides (primarily chalcopyrite) collected from MAR vent sites. Growth on these minerals is optimal at pH 6.5 to 7.0, with doubling times averaging from 10 to 50 h, depending on the mineral, and ceases when the pH drops below 6.0 to 5.5. On thiosulfate, strain MA-3 grows optimally at pH 7.5 and ceases growth at comparably low pHs. Strain MA-3 did not grow autotrophically on ferrous iron as a substrate. In fact, ferrous iron was significantly toxic at increasing concentrations (Fig. 1). In the iron-free control, cells remained almost completely viable (as CFU) over the experimental time period. Growth of this isolate at a hydrostatic pressure of its normal habitat in the deep sea (ca. 350 × 105 Pa) did not show a barophilic adaptation; however, the organism is quite barotolerant, demonstrating a rate of CO2 fixation at 350 × 105 Pa approximately 83% of the rate measured at 1 × 105 Pa. The reduction in growth rate is similar to that reported for T. crunogena and Thermomicrospira sp. strain L-12 at their in situ pressure of 250 × 105 Pa (14, 18). Strain MA-3 grows over a temperature range of 4 to 41°C (optimum of 28 to 32°C). This maximum growth temperature of 41°C is the highest reported for the three species. Cells did not grow at 42°C, but the organism survived for 4 days at this temperature and then grew at a lower temperature (24°C), while cells incubated at 44°C for an equivalent time period did not grow upon transfer. Sodium is required for growth of strain MA-3, because no growth occurred at concentrations of less than 50 mM NaCl over 1 week. Optimum growth occurred at 200 to 400 mM NaCl. Potassium, as KCl, was unable to substitute for sodium. In T-ASW medium, strain MA-3 excreted approximately 4 to 5% of its fixed carbon as acid-stable organic compounds, of unknown composition, during exponential growth and a maximum of 12% in the stationary phase.
FIG. 1.
Survival (CFU) of pregrown cells of strain MA-3 exposed to varied concentrations of Fe2+ at near-neutral pH under anoxic conditions at 23°C. Symbols represent concentrations of iron: □, none; ◊, 10 mM; ○, 30 mM; ▵, 60 mM; ⊞, 90 mM.
Most physiological characteristics of strain MA-3 are similar to those of the two Thiomicrospira isolates described earlier, T. crunogena and Thiomicrospira sp. strain L-12 (e.g., motility and utilization of the same reduced sulfur sources), including the presence of ubiquinone Q-8 with traces of Q-7. Differences among the three isolates are listed in Table 1. In its maximum rate of growth on thiosulfate and its growth temperature range, strain MA-3 resembles T. crunogena more than Thiomicrospira sp. strain L-12. Similarly, slight differences hold for the excretion of fixed carbon, pH optima, minimum salinities, and G+C contents. The 16S rRNA-encoding gene of strain MA-3 was nearly completely sequenced to determine its phylogenetic affiliation. By parsimony analysis of the 16S rRNA sequences, the new isolate was grouped with other Thiomicrospira species isolated from hydrothermal vent communities, i.e., T. crunogena and Thiomicrospira sp. strain L-12. The closest relative is T. crunogena (14), to which it has a sequence similarity value higher than 99%. Only 2 bp differences could be found between the sequences of T. crunogena and strain MA-3: at Escherichia coli positions (4) 978 and 1015, there was a G in the sequence of T. crunogena versus an A in the sequence of strain MA-3. The DNA-DNA homologies among the three strains are presented in Table 2. Strain MA-3 has a 99.3% homology to the other two strains. The two Pacific vent strains, T. crunogena and Thiomicrospira sp. strain L-12, share 100% homology to each other. The type species of the genus Thiomicrospira, T. pelophila, shows only a 27 to 33.5% homology to all three vent strains. This value, being below the homology threshold of 60 to 70%, defines (20) T. pelophila as a separate species.
TABLE 1.
Growth characteristics of three Thiomicrospira isolates from deep-sea hydrothermal vents
| Organism | Source | Maximum growth rate (h−1) | °C temp range (optimum) | pH range (optimum) | % Fixed carbon excreted (24 h) | % Growth in situ bar/1 bar | Minimum NaCl requirement (mM) | G+C content |
|---|---|---|---|---|---|---|---|---|
| T. crunogenaa | Vestimentiferan tube worm casing, 21°N EPR | 0.8 | 4–38.5 (28–32) | 5.0–8.5 (7.75) | 8.5 | 80 | 45 | 44.2 ± 0.2c |
| Thiomicrospira sp. strain L-12b | Mussel periostracum, Galapagos Rift | 0.32 | 10–35 (25) | 5.5–8.5 (8.0) | 9.0 | 75 | 80 | 44.4 ± 0.2c |
| Thiomicrospira sp. strain MA-3 | Polymetal sulfide rock, TAG site, MAR | 0.8 | 4–41 (28–32) | 5.5–8.5 (7.5) | 12 | 83 | 50 | 44.6 ± 0.3 |
TABLE 2.
Percentages of DNA-DNA homology for three Thiomicrospira isolates from deep-sea hydrothermal vents and T. pelophila, the type species of the genus
| Isolate | % Homology to:
|
||
|---|---|---|---|
| T. pelophila | Thiomicrospira sp. strain L-12 | T. crunogena | |
| Thiomicrospira sp. strain L-12 | 27 | ||
| T. crunogena | 33.5 | 100 | |
| Thiomicrospira sp. strain MA-3 | 27 | 99.3 | 99.3 |
This study has resulted in two new findings. (i) Despite physiological differences among the three isolates compared, their almost identical 16S rRNA sequences and DNA-DNA hybridization values define them as one species (20, 22), T. crunogena (type strain, ATCC 35932). (ii) The identification of these isolates from MAR and East Pacific Rise (EPR) deep-sea hydrothermal vent sites indicates a highly cosmopolitan occurrence of this species. In view of the 16S RNA sequence similarity of the three isolates compared in this paper, a further strain isolated from the MAR, MA2-6 (17), has a similarity value of about 97% with T. crunogena, indicating it is a separate species (20). On the other hand, a new intertidal Thiomicrospira isolate, strain JB-B2, shows a 99% similarity to both T. crunogena and Thiomicrospira sp. strain L-12 (2) and, therefore, may be yet another strain of the species T. crunogena.
In view of the greater than 99.3% DNA homology of the three T. crunogena strains compared, the observed differences of a physiological nature under equal growth conditions (Table 1) indicate a certain range of phenotypic adaptations to environmentally different vent sites. The metabolic capability of metal sulfide oxidation affords these chemolithotrophs an additional and stable source of energy in environments where massive polymetal sulfide deposits exist, namely at deep-sea hydrothermal vent sites. This versatility may contribute to the fact that the genus Thiomicrospira appears to dominate, by molecular analysis (17), the sulfur-oxidizing bacterial communities at deep-sea hydrothermal vents. The present work presents a striking case of wide geographic distribution of a single sulfur-oxidizing bacterial species at Atlantic and Pacific deep-sea vent sites. If this can indeed be extended toward intertidal zones, as suggested by the genetic relatedness of strain JB-B2 (2), a truly worldwide distribution of the species T. crunogena would be apparent.
Nucleotide sequence accession number.
The 16S rRNA sequence of strain MA-3 is available from GenBank under accession no. AF069959.
Acknowledgments
The ubiquinones were identified by B. Tindall, and the G+C determination and DNA-DNA hybridizations were done by J. Burghardt, both of the DSMZ identification service (Braunschweig, Germany).
This work was supported by grants OCE 92-000458, OCE 9615830, and OCE 9714195 from the National Science Foundation and by financial support from the Max-Planck-Society, Munich, Germany.
Footnotes
Contribution no. 9695 of the Woods Hole Oceanographic Institution.
REFERENCES
- 1.Benson D A, Boguski M S, Lipman D J, Ostell J. GenBank. Nucleic Acids Res. 1997;25:1–6. doi: 10.1093/nar/25.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinkhoff T, Muyzer G. Increased species diversity and extended habitat range of sulfur-oxidizing Thiomicrospira spp. Appl Environ Microbiol. 1997;63:3789–3796. doi: 10.1128/aem.63.10.3789-3796.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinkhoff, T., G. Muyzer, C. O. Wirsen, and J. Kuever. Characterization of Thiomicrospira kuenenii sp. nov. and Thiomicrospira frisia sp. nov., two mesophilic obligately chemolithoautotrophic sulfur-oxidizing bacteria from an intertidal mud flat. Int. J. Syst. Bacteriol., in press. [DOI] [PubMed]
- 4.Brosius J, Dull T J, Sleeter D D, Noller H F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981;148:107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- 5.Cline J D. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol Oceanogr. 1969;14:454–458. [Google Scholar]
- 6.Durand P, Benyagoub A, Prieur D. Numerical taxonomy of heterotrophic sulfur-oxidizing bacteria isolated from southwestern Pacific hydrothermal vents. Can J Microbiol. 1994;40:690–697. [Google Scholar]
- 7.Durand P, Reysenbach A, Prieur D, Pace N. Isolation and characterization of Thiobacillus hydrothermalis sp. nov., a mesophilic obligately chemolithotrophic bacterium isolated from a deep-sea hydrothermal vent in Fiji Basin. Arch Microbiol. 1993;159:764–766. [Google Scholar]
- 8.Eberhard C, Wirsen C O, Jannasch H W. Oxidation of polymetal sulfides by chemolithoautotrophic bacteria from deep-sea hydrothermal vents. Geomicrobiol J. 1995;13:145–164. [Google Scholar]
- 9.Gilbert D G. SeqApp—a bio-sequence analysis application. Bloomington: Indiana University; 1992. [Google Scholar]
- 10.Gugliandolo C, Maugeri T L. Chemolithotrophic, sulfur-oxidizing bacteria from a marine, shallow hydrothermal vent of Vulcano (Italy) Geomicrobiol J. 1993;11:109–120. [Google Scholar]
- 11.Hobbie J E, Daley R J, Jasper S. Use of Nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977;33:1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber H, Stetter K O. Thiobacillus prosperus sp. nov. represents a new group of halotolerant metal-mobilizing bacteria isolated from a marine geothermal field. Arch Microbiol. 1989;151:479–485. [Google Scholar]
- 13.Huber H, Stetter K O. Thiobacillus cuprinus sp. nov., a novel facultatively organotrophic metal-mobilizing bacterium. Appl Environ Microbiol. 1990;56:315–322. doi: 10.1128/aem.56.2.315-322.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jannasch H W, Wirsen C O, Nelson D C, Robertson L A. Thiomicrospira crunogena sp. nov., a colorless sulfur-oxidizing bacterium from a deep-sea hydrothermal vent. Int J Syst Bacteriol. 1985;35:422–424. [Google Scholar]
- 15.Javor B J, Wilmot D B, Vetter R D. pH-dependent metabolism of thiosulfate and sulfur globules in the chemolithotrophic marine bacterium Thiomicrospira crunogena. Arch Microbiol. 1990;154:231–238. [Google Scholar]
- 16.Maidak B L, Olsen G J, Larsen N, Overbeek R, McCaughey M J, Woese C R. The RDP (Ribosomal Database Project) Nucleic Acids Res. 1997;25:109–110. doi: 10.1093/nar/25.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muyzer G, Teske A, Wirsen C O, Jannasch H W. Phylogenetic relationships of Thiomicrospira species and their identification in deep-sea hydrothermal vent samples by denaturing gradient gel electrophoresis of 16S rDNA fragments. Arch Microbiol. 1995;164:165–172. doi: 10.1007/BF02529967. [DOI] [PubMed] [Google Scholar]
- 18.Ruby E G, Jannasch H W. Physiological characteristics of Thiomicrospira sp. strain L-12 isolated from deep-sea hydrothermal vents. J Bacteriol. 1982;149:161–165. doi: 10.1128/jb.149.1.161-165.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruby E G, Wirsen C O, Jannasch H W. Chemolithotrophic sulfur-oxidizing bacteria from the Galapagos Rift hydrothermal vents. Appl Environ Microbiol. 1981;42:317–342. doi: 10.1128/aem.42.2.317-324.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stackebrandt E, Goebel B M. Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int J Syst Bacteriol. 1994;44:846–849. [Google Scholar]
- 21.Tuttle J H, Jannasch H W. Thiosulfate stimulation of microbial dark assimilation of carbon dioxide in shallow marine environments. Microb Ecol. 1977;4:9–25. doi: 10.1007/BF02010426. [DOI] [PubMed] [Google Scholar]
- 22.Wayne L G, Brenner D J, Colwell R R, Grimont P A D, Kandler O, Krichevsky M J, Moore L H, Moore W E C, Murray R G E, Stackebrandt E, Starr M P, Trüper H G. Report of the Ad Hoc Committee on Reconciliation of Approaches to Bacterial Systematics. Int J Syst Bacteriol. 1987;37:463–464. [Google Scholar]
- 23.Wirsen C O, Jannasch H W, Molyneaux S J. Chemosynthetic microbial activity at Mid-Atlantic Ridge hydrothermal vent sites. J Geophys Res. 1993;98:9693–9703. [Google Scholar]