Abstract
Cisplatin is a chemotherapeutant widely used in treating solid tumors, with the common side effect of acute kidney injury (AKI). Developing effective useful agent for preventing or treating cisplatin-induced AKI is of great importance. In this study, we investigate the protective effect of vaccarin, a chemical entity of flavonoid glycoside, against cisplatin-induced AKI. Cisplatin-treated C57BL/6J mice and human kidney-2 (HK-2) cells were used as the model of cisplatin-induced AKI. The levels of blood urea nitrogen (BUN) and creatine (Cr) levels and periodic acid-Schiff staining (PAS) scores decreased when vaccarin was administrated. Vaccarin had no impact on renal platinum accumulation, which was detected by the ICP-MS 6 h after cisplatin injection. Moreover, vaccarin can significantly alleviate the product of reactive oxygen species and the expression of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 4 (NOX4) in cisplatin-induced AKI, both in vivo and in vitro. In addition, vaccarin decreased the receptor-interacting protein kinase 1 (RIPK1) related programmed necrosis (necroptosis), cell apoptosis (shown by the protein levels of cleaved-caspase3 and flow cytometry) and inflammation (shown by the decreased levels of NLRP3, p-P65 and the mRNA of several inflammatory factors). NOX4 inhibitor GLX351322 (GLX) and NOX4 kowndown by siRNA have equivalent protective effect of vaccarin in vitro. When vaccarin was administered together with GLX or NOX4 siRNA, this protective effect of vaccarin did not further increase, as indicating by the index of oxidative stress, cell viability, necroptosis and apoptosis. In conclusion, vaccarin can alleviate cisplatin-induced AKI via inhibiting NOX4.
Keywords: Vaccarin, Acute kidney injury, Cisplatin, Inflammation, NOX4
1. Introduction
Cisplatin is a chemotherapeutant widely used in treating solid tumors, with the rate of the side effect of acute kidney injury (AKI) reaching 30 % [1]. Cisplatin mainly causes damage to renal proximal tubules, resulting in edema and degeneration of tubular epithelial cells, thickening of the basemembrane and mild fibrosis of renal interstitium [2]. Recently, it was found that apoptosis and necroptosis of renal proximal tubule epithelial cells is the main pathological form of cisplatin-induced AKI [3]. Moreover, oxidative stress, damage of DNA structure, inflammatory response, the activation of necroptosis enzymes and mitochondrial dysfunction are the potential key nodes that trigger apoptosis or necroptosis [4]. Presently, hydration therapy with diuretic is often used to prevent AKI [5]. However, the renal toxicity still happens and the preventive procedure is not applicable for the patients with autoimmune diseases, kidney dysfunction, cardiac insufficiency and pleural effusion [6]. Therefore, it is of great significance to develop effective drugs for the preventing or treating cisplatin-induced AKI.
Vaccarin is a kind of natural pharmaceutical compound which belongs to flavonoid glycoside and is found in the seeds of a Chinese herbal Vaccaria hispanica (Mill.) [7]. Recently, vaccarin was found to hasten wound healing by promoting angiogenesis [8]. Additionally, vaccarin facilitated the integrity of intestinal barrier in type 2 diabetic mice through the activation of extracellular regulated protein kinases and myosin light chain kinase [9]. Moreover, vaccarin protected endothelial cells against oxidized low-density lipoprotein-induced endothelial-mesenchymal transition, inflammation and apoptosis by reducing reactive oxygen species (ROS) [10]. However, the pharmacological function of vaccarin is not fully understood, especially to AKI. In the current study, the in vitro and in vivo effects of vaccarin on cisplatin-induced AKI were evaluated and the potential pharmacological function was investigated.
2. Materials and methods
2.1. Materials
The compound vaccarin, with the chemical structure shown in Fig. 1A, was obtained from Pulis Biology Technology (Chengdu, China). Cisplatin (purity≥99 %) and MTT were obtained from Sigma (U.S.A.). GLX351322 was obtained from MedChem Express (Monmouth Junction, NJ). The GSH assay kit, MDA assay kit, BUN assay kit and Cr assay kit were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Primary antibody of RIPK3 was obtained from HUABIO (Shangzhou, China). Human phospho-mixed lineage kinase domain-like protein (p-MLKL) antibody was obtained from Cell Signaling Technology (U.S.A.) and mouse p-MLKL antibody was obtained from HUABIO. RIPK1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Kidney injury molecule-1(KIM-1) and NOX4 was obtained from Bioss Biotechnology (Beijing, China). NLRP3 was obtained from Wanleibio (Shenyang, China). Cleaved-caspase-3 were from Cell Signaling Technology (U.S.A.). PCR Amplification Kit and reverse transcription kit were obtained from TAKARA (Japan).
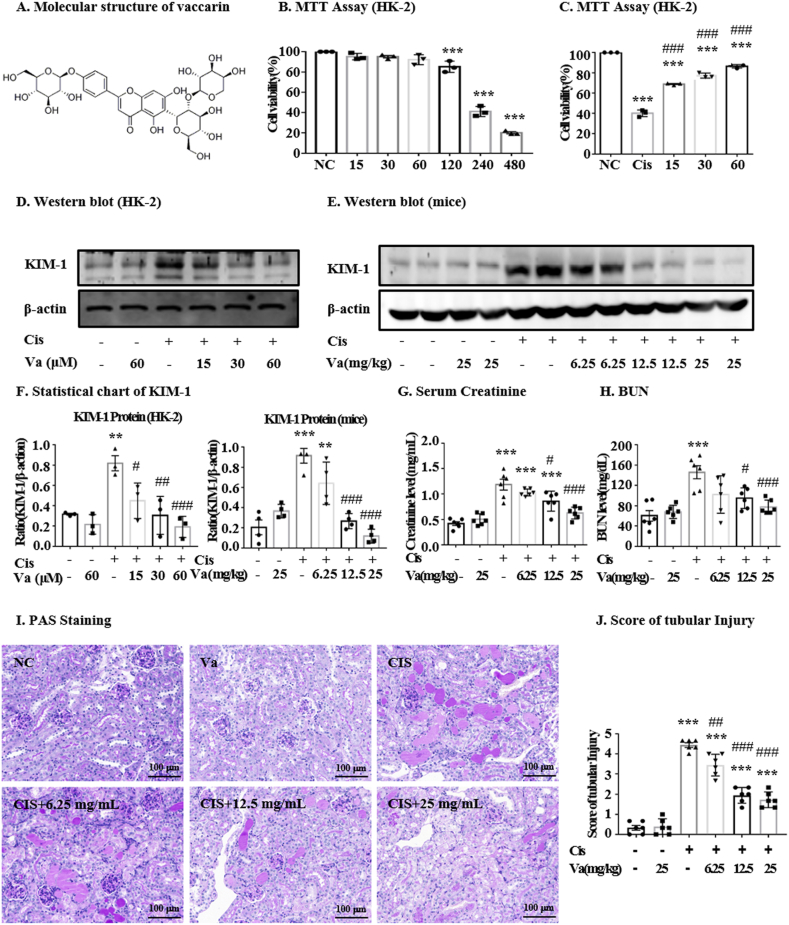
Fig. 1.
Effect of vaccarin on the viability of HK-2 cells and renal damage. For cell experiments, the suspended HK-2 cells were firstly seeded in 96-well plates. After starving for 12 h in DMEM/F12 medium containing 0.5 % FBS, the cells were pretreated with vaccarin for 12 h, followed by 24 h treatment with or without cisplatin. (A) The molecular structure of vaccarin. (B) The effect of different concentrations of vacation on the viability of HK-2 cells (n = 3). (C) Protective effect of different concentrations of vaccarin on HK-2 cells treated with cisplatin (n = 3). (D, E, F) Western blot and quantitative analysis of KIM-1 (n = 4). (G) Creatinine assay, (H) BUN assay (I, J) and the results of PAS staining showed that vaccarin could alleviate cisplatin-induced renal damage (n = 6). Results were presented as mean ± S.E.M. in vivo experiments. ***p < 0.001, **p < 0.01 and *p < 0.05 versus the control group; ###p < 0.001, ##p < 0.01 and #p < 0.05 versus the cisplatin-treated group.
2.2. Cell culture
HK-2 cells were cultured in Dulbecco's modified eagle medium/nutrient mixture F-12 containing 5 % fetal bovine serum (FBS) in a 37 °C incubator under 5 % CO2 with humidity. The cells cultured were suspended and seeded in different plates. The cells were firstly incubated with different drugs for indicated time. Then, cell viability assay, flow cytometry assay, immunofluorescence assay and the detection of oxidative stress were implemented or the cells were harvested for determining the levels of protein and mRNA.
2.3. Cell viability assay
HK-2 cells were seeded in a plate of 96-well (4000 cells/well) with the culture medium containing 5 % FBS. Subsequently, cells were treated with drugs. Finally, MTT was added into each well and incubated for 4 h in the 37 °C incubator under 5 % CO2 with humidity. The absorbance data was obtained using a microplate reader.
2.4. Flow cytometry
Apoptosis of cells was detected by flow cytometry according to the instruction of manufacturer (BD FACSVerse, USA). Briefly, after incubation with drugs, HK-2 cells were harvested and centrifuged at 800 rpm for 5 min. Annexin V-fluorescein isothiocyanate and propidium iodide were added to the cells kept away from light. The samples were immediately assayed by flow cytometry.
2.5. Mouse model
Male C57BL/6J mice were obtained from the Animal Center of Anhui Medical University. All animal experiments were conducted in accordance with the Regulations on the Control of Laboratory Animals promulgated by the State Science and Technology Commission. All animal procedures were approved by the Animal Experiment Ethics Committee of Anhui Medical University (LLSC 20231354). The mice were reared in a room where temperature, humidity and ray of light were controlled. 36 mice (18–22 g) were randomly divided into the following groups: control group, vaccarin group, model group, treatment group (with low, medium and high dose of vaccarin). In model group, mice were intraperitoneally injected with a single dose of cisplatin (20 mg/kg). The NS was given as vehicle control. Vaccarin (6.25, 12.5 and 25 mg/kg) was intraperitoneally injected 0.5 h before cisplatin or NS administration and another two doses of vaccarin were injected in the next two days at the same time point. 72 h after the administration of cisplatin, mice were anesthetized for blood and tissue collection.
2.6. Kidney histology
The middle segment of mice kidney was fixed, embedded in paraffin block and subsequently cut into sections at a thickness of 4 μm. The slices were then stained with periodic acid-Schiff to evaluate the severity of renal tubular injury including cast formation, tubule dilatation and tubular atrophy. Blinded to the group assignment, three expert renal pathologists scored the degree of tubular damage. PAS sections were assigned scores according to the percentage of tubules that were damaged: 6 = more than 96 %; 5 = 75–95 %; 4 = 51–75 %; 3 = 26–50 %; 2 = 10–25 %; 1 = >0 and < 10 %; 0 = normal.
2.7. RNA extraction and polymerase chain reaction (PCR)
A RNeasy Isolation Kit (Qiagen, Valencia, CA, U.S.A.) was used for extracting the total RNA from the kidney homogenate and HK-2 cells as per the instructions. A Nano-Drop 2000 Spectrophotometer (Thermo Scientific, Waltham, MA, U.S.A.) was used for detecting the concentration. A Real Master Mix system (Toyobo, Osaka, Japan) was used for reverse transcribing the total RNA to complementary DNA. The levels of TNF-α, MCP-1, IL-1β and IL-6 mRNA were measured using a CFX96 real-time RT-PCR detection system (Bio-Rad Laboratories, Hercules, CA, U.S.A.) through SYBR-Green Quantitative Real-time PCR. The sequence of the primers used in this project was listed in Table 1. The normalization of the quantity of the mRNA of interest to that of β-actin was listed as the mean of the ratio ± SEM.
Table 1.
Primers sequences for realtime RT-PCR
| Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| hTNF-α | CAGCCGATGGGTTGTACCTT | GTGTGGGTGAGGAGCACGTA |
| hIL-1β | CAACCAACAAGTGATATTCTCCATG | CAACCAACAAGTGATATTCTCCATG |
| hMCP-1 | CCAAAGAAGCTGTGATCTTCAA | TGGAATCCTGAACCCACTTC |
| hIL-6 | CGGGAACGAAAGAGAAGCTCTA | GAGCAGCCCCAGGGAGAA |
| hβ-actin | CGCCGCCAGCTCACCATG | CACGATGGAGGGGAAGACGG |
| mTNF-α | CATCTTCTCAAAATTCGAGTGACAA | TGGGAGTAGACAAGGTACAACCC |
| mIL-1β | CAACCAACAAGTGATATTCTCCATG | GATCCACACTCTCCAGCTGCA |
| mMCP-1 | GTCTGTGCTGACCCCAAGAAG | TGGTTCCGATCCAGGTTTTTA |
| mIL-6 | GAGGATACCACTCCCAACAGACC | AAGTGCATCATCGTTGTTCATACA |
| mβ-actin | CATTGCTGACAGGATGCAGAA | ATGGTGCTAGGAGCCAGAGC |
2.8. Western blot analysis
Radio immunoprecipitation assay lysis buffer (Beyotime, Jiangsu, China) was used for extracting proteins from mice kidneys and HK-2 cells. After adding loading buffer to the lysis solution, the solution system was heated to 99 °C for 10 min. Next, the protein sample was separated by electrophoresis, and was transferred electrochemically to a polyvinylidene difluoride membrane. 5 % bovine serum albumin (BSA) in tris-buffered saline buffer (TBST; 20 mM Tris-HCl, 150 mM NaCl, and 0.1 % Tween 20) was used for blocking non-specific binding. Thereafter, the membranes were incubated overnight at 4 °C in primary antibodies solution such as RIPK1, RIPK3, p-MLKL, cle-caspase-3, KIM-1, NOX4, p-P65, P65 and NLRP3. After washing for three times, the membranes were incubated at 37°Cwith horseradish peroxidase-conjugated secondary antibody in 1 % BSA/TBST for 1.5 h. The membranes were washed again and then visualized using ChemiDoc MP Imaging System (Bio-Rad).
2.9. Detection of oxidative stress
2′, 7′-dichlorofluorescein diacetate (DCFH-DA) and dihydroethidium (DHE) dye (Beyotime, Jiangsu, China) were used to detect the cellular ROS according to the instructions from the manufacturer.
2.10. Knockdown of NOX4 by siRNA transfection
Non-targeting siRNA (GenePharm, Shanghai, China) and NOX4-siRNA (GeneChem Co. Ltd., Shanghai, China) were transfected using Lipofectamine™2000 reagent (Invitrogen, Carlsbad, CA, USA) follow the instructions. Briefly, when the cell density in the six-well plate reached 60 %, discarded the original medium and washing twice with PBS. 5 μL siRNA and 5 μL Lipofectamine™2000 were added to 200 μL serum-free Opti-MEM, respectively. After incubated for 5 min at room temperature, the two mixed solution were mixed and kept at room temperature for 20 min. Then, the above final mixture and 600 μL Opti-MEM were added to a well of 6-well plate. After 12 h incubating in the 37 °C incubator under 5 % CO2 with humidity, the culture medium was replaced with the medium containing 5 % FBS.
2.11. Detection of cisplatin accumulation
For the detection of renal cisplatin accumulation, mice were intraperitoneally injected with 20 mg/kg CP 30 min after 25 mg/kg vaccarin or NS administration. The mice were euthanized 6 h after cisplatin injection and the renal blood was drained by cardiac perfusion with NS. The renal cortex tissues were incised, weighted, and then digested with 3 % nitric acid for platinum (Pt) analysis by ICP-MS, according to our previous study [11].
2.12. Molecular docking
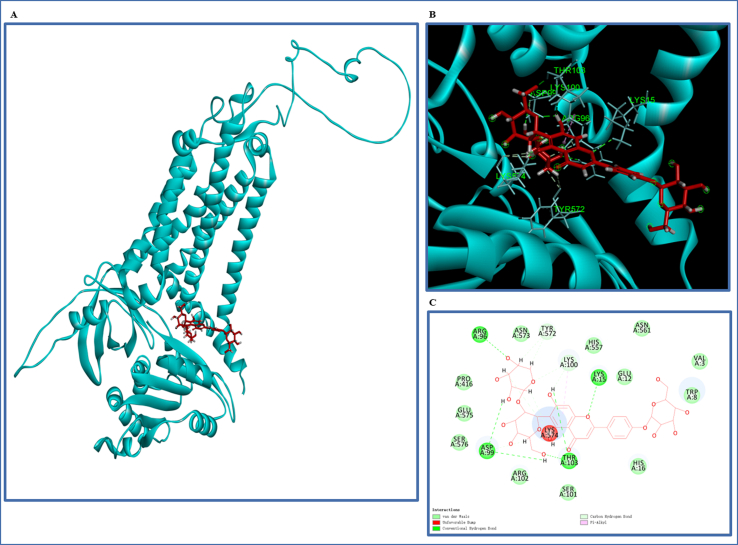
Discovery Studio 2017 R2 was used to carry out molecular docking to further observe the possible binding interaction and relationship between vaccarin and NOX4. We obtained the predicted structure of NOX4 from Alpha Fold Protein Structure Database (https://alphafold.ebi.ac.uk/, Q9NPH5). Molecular vaccarin energy minimization was used the Minimize Ligands protocol and docking program was performed by Lib Dock module. Other parameters were set as default.
2.13. Statistical analysis
Statistical significance was analyzed by one-way analysis of variance (ANOVA), followed by Tukey's multiple comparisons test using GraphPad Prism 7 software (GraphPad, La Jolla, CA, USA). Data are expressed as the mean ± S.E.M.
3. Results
3.1. Vaccarin alleviated the renal toxicity induced by cisplatin
The toxicity of vaccarin, as well as its protective effects on HK-2 cells against cisplatin, was assessed by MTT experiments. The results showed that concentrations of vaccarin lower than 120 μM did not significantly affect cell viability (Fig. 1B). Moreover, the concentrations of 15, 30, and 60 μM rescued the cell viability in cisplatin treatment in a dose-dependent manner (Fig. 1C). The result of Western blot demonstrated the high expression of renal injury biomarker KIM-1 in HK-2 cells and kidneys treated with cisplatin. Vaccarin was found to reduce KIM-1 levels in a dose-dependent pattern (Fig. 1D–F). In mice, vaccarin was able to dose-dependently reverse the cisplatin-induced up-regulation of Cr (Fig. 1G) and BUN (Fig. 1H). The periodic acid-Schiff staining (PAS) results showed that the administration of vaccarin (6.25, 12.5, and 25 mg/kg) could alleviate tubular necroptosis, tubular expansion and glycogen deposition, compared to the cisplatin-treated group (Fig. 1I and J). Overall, vaccarin was shown with a beneficial effect on cisplatin-induced renal damage. The concentration of 80 μM was chosen for in vitro experiments and the dose of 25 mg/kg for the in vivo experiments.
3.2. Vaccarin alleviated cisplatin-induced oxidative stress
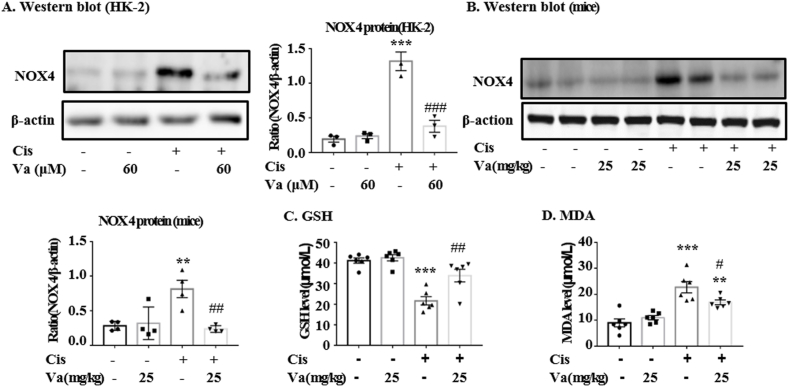
Vaccarin was found to largely reduce the high expression of NOX4 protein in cisplatin-treated HK-2 cells (Fig. 2A). The analysis of DHE or DCFH-DA dying illustrated that vaccarin significantly alleviated the cellular levels of reactive oxidative species (ROS), which were induced by cisplatin (Fig. 2D and E). In the current study, the levels of GSH (an endogenic cellular anti-oxidant), MDA (a natural product of the oxidation of lipids) and the protein NOX4 were detected in mice renal tissues. The levels of the above indexes all increased in cisplatin-treated mice, whereas decreased after the pre-treatment of vaccarin (Fig. 2B–D).
Fig. 2.
Vaccarin alleviated cisplatin-induced oxidative stress. (A, B) Western blot and quantitative analysis of NOX4 in HK-2 cells (n = 3) and mice (n = 4). (C) Glutathione (GSH) assay in vivo (n = 6). (D) Malondialdehyde (MDA) assay in vivo (n = 6). The results were presented as mean ± S.E.M. ***p < 0.001, **p < 0.01 and *p < 0.05 versus the control group; ###p < 0.001, ##p < 0.01 and #p < 0.05 versus the cisplatin-treated group.
3.3. Vaccarin alleviated cisplatin-induced necroptosis and apoptosis
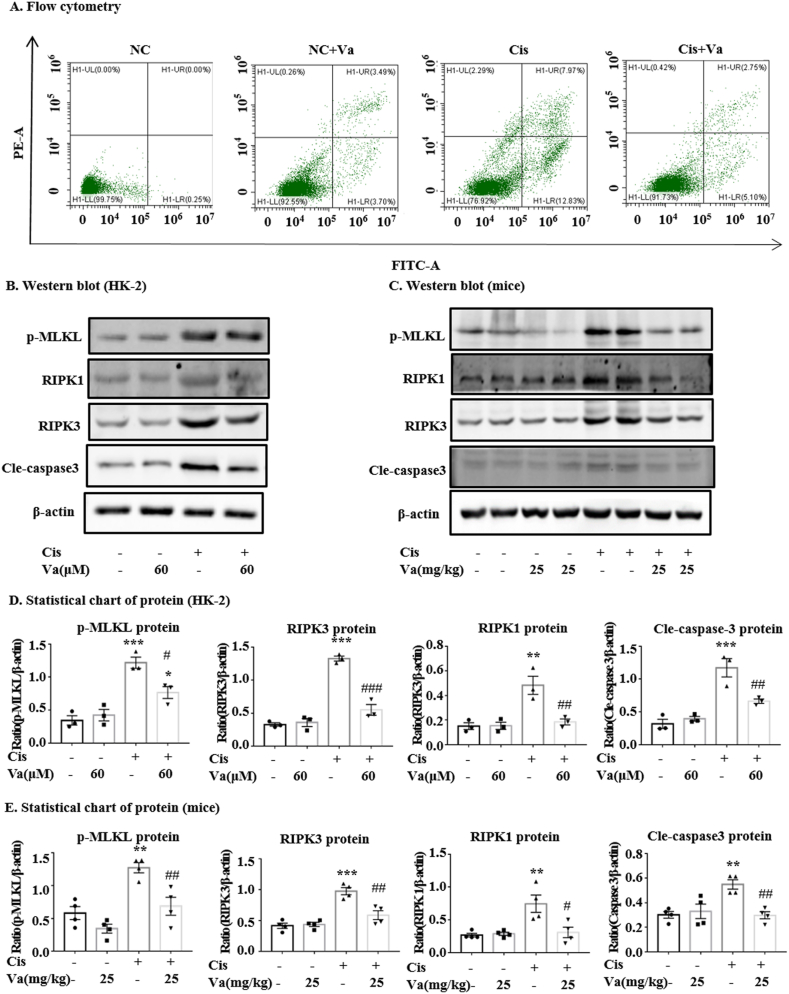
Flow cytometry analysis showed that vaccarin alleviated cell apoptosis induced by cisplatin (Fig. 3A). Vaccarin also significantly reduced the expression of cle-caspase3 both in vitro and in vivo (Fig. 3B and C). The levels of necroptosis-related signals including RIPK1, RIPK3 and p-MLKL increased upon the cisplatin treatment. However, they decreased moderately when vaccarin was pretreated (Fig. 3B and C).
Fig. 3.
Vaccarin alleviated cell necroptosis and apoptosis induced by cisplatin. (A) The flow cytometry of PI/Annexin V stained HK-2 cells. (B, C, D, E) Western blot and quantitative analysis of both necroptosis and apoptosis signals (RIPK1, RIPK3, MLKL, p-MLKL and cleaved-caspase3). The in vitro experiments were repeated for three times and presented as mean ± S.E.M. The in vivo results were presented as mean ± S.E.M. of 4 mice. ***p < 0.001, **p < 0.01 and *p < 0.05 versus the control group. ###p < 0.001, ##p < 0.01 and #p < 0.05 versus the cisplatin-treated group.
3.4. Vaccarin alleviated cisplatin-induced inflammation
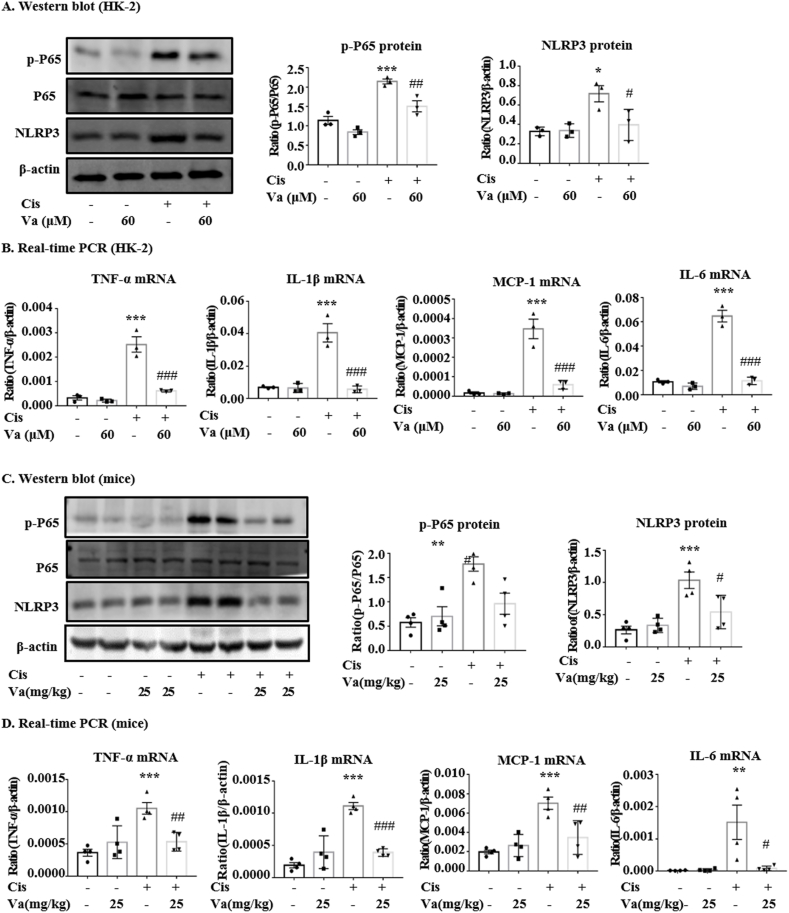
The results of Western blot and RT-qPCR showed that vaccarin could alleviate the inflammation induced by cisplatin (Fig. 4A–D). The protein levels of NLRP3, which is the key intracellular receptors that sense and trigger inflammation, increased in cisplatin-treated HK-2 cells (Fig. 4A) and mice (Fig. 4C), but decreased to nearly normal level when vaccarin was used. Moreover, vaccarin reduced the phosphorylation of P65, which is also highly related to inflammatory response. In addition, we found that vaccarin could significantly reverse the expression of pro-inflammatory genes including TNF-α, IL-1β, IL-6, and MCP-1 in cisplatin-treated HK-2 cells (Fig. 4B) and mice (Fig. 4D).
Fig. 4.
Vaccarin alleviated the inflammation both in vitro and in vivo. (A, C) Western blot and quantitative analysis of p-P65/P65 and NLRP3 (B, D) The results of RT-qPCR of TNF-α, IL-1β, IL-6, and MCP-1 in HK-2 cells and mice. The in vitro experiments were repeated for three times and presented as mean ± S.E.M. The in vivo results were presented as mean ± S.E.M. of 4 mice. ***p < 0.001, **p < 0.01 and *p < 0.05 versus the control group; ###p < 0.001, ##p < 0.01 and #p < 0.05 versus the cisplatin-treated group.
3.5. Vaccarin had on impact on renal accumulation of cisplatin
To determine whether vaccarin interfere with the renal cisplatin transporting, the accumulation of Pt in the cortex tissues 6 h after cisplatin injection was determined by the method of ICP-MS. The accumulation of Pt in the mice administered with cisplatin is 9.74 ± 1.23 ng/g, which showed no statistic difference with the mice co-administered with cisplatin and vaccarin (9.80 ± 1.07 ng/g).
3.6. Vaccarin alleviated necroptosis and cell apoptosis via NOX4
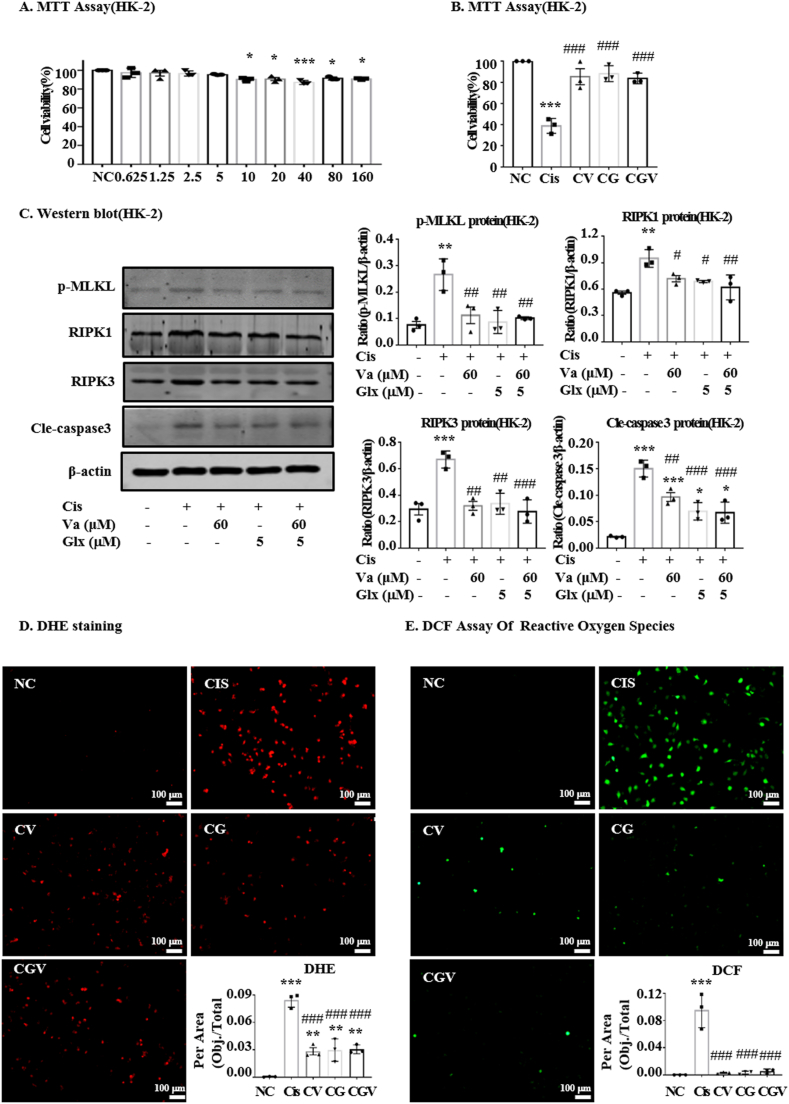
The cytotoxic effects of GLX and its protective effects against the cisplatin-induced toxicity were assessed by MTT in HK-2 cells. The results showed that concentrations of GLX lower than 10 μM had no impact on cell viability (Fig. 5A). Moreover, the panels of vaccarin, GLX and the combination of vaccarin and GLX were shown with comparable effects on cell viability (Fig. 5B). In addition, we detected the expression levels of p-MLKL, RIPK1, RIPK3 and cle-caspase3 (Fig. 5C). Also, GLX, vaccarin or the combination of GLX and vaccarin could reduce the levels of these proteins to nearly the same degree. Finally, the detection of ROS levels using the dyes of DHE and DCFH-DA also showed the same tendency (Fig. 5D).
Fig. 5.
The protective effect of vaccarin with GLX in HK-2 cells. (A) The effect of different concentrations of GLX on the viability of HK-2 cells. (B) Protective effect of the three panels of Vaccarin (CV), GLX (CG) and the combination of vaccarin and GLX (CGV) on HK-2 cells treated with cisplatin. The results showed with comparable effects against cisplatin-induced toxicity. (C) Western blot and quantitative analysis of oxidative stress, necroptosis and apoptosis signaling molecules. (D, E) DHE and DCF staining of HK-2. The independent experiment was repeated three times and presented as mean ± S.E.M. ***p < 0.001, **p < 0.01 and *p < 0.05 versus the control group; ###p < 0.001, ##p < 0.01 and #p < 0.05 versus the cisplatin-treated group.
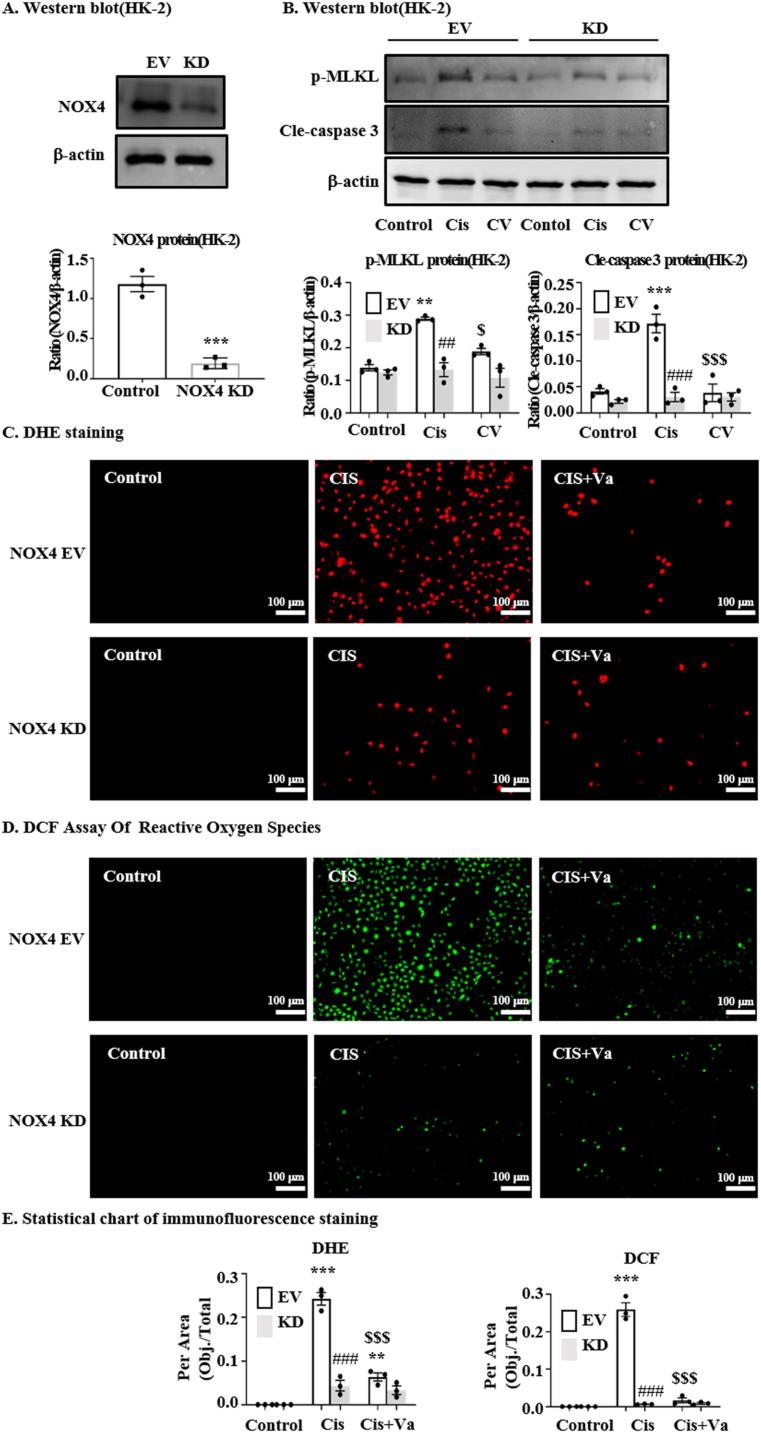
3.7. Knockdown of NOX4 alleviate cisplatin-induced necroptosis and cell apoptosis
To determine whether NOX4 is an important target of vaccarin mediating its protective effect, the cells were transfected with NOX4-siRNA to knock down the expression of NOX4 (the protein level was validated in Fig. 6A). Western blot showed that vaccarin could not further alleviate cisplatin-induced necroptosis (indicated by p-MLKL levels) and cell apoptosis (indicated by cleaved-caspase3 levels) when the NOX4 is knocked down (Fig. 6B). Also, using the dyes of DHE [Fig. 6 (C, E)] and DCFH-DA [Fig. 6 (D, E)], it demonstrated that vaccarin could not further alleviate the ROS induced by cisplatin when the NOX4 is deficient. Moreover, in the Supplementary Fig. 1, it was predicted that vaccarin could directly target to the bioactive region of NOX4 by Molecular docking.
Fig. 6.
The protective effect of vaccarin was influenced by NOX4 knockdown. (A) The protein level of NOX4 was significantly downregulated when HK-2 cells were transfected with NOX4-siRNA. (B) Western blot and quantitative analysis of necroptosis and apoptosis signals (p-MLKL and cleaved-caspase3) in NOX4 knockdown cells. DHE (C) and DCF (D) staining and their quantitative analysis (E) in HK-2. The independent experiment was repeated three times and presented as mean ± S.E.M. ***p < 0.001, **p < 0.01 and *p < 0.05 versus the control group; ###p < 0.001, ##p < 0.01 and #p < 0.05 versus the NOX4-EV group; $$$p < 0.001, $$p < 0.01 and $p < 0.05 versus the cisplatin-treated group.
4. Discussion
Vaccarin is a kind of flavonoid glycoside compounds which are widely found in all kinds of plants. Hesperidin, naringin, baicalin, didymin and Calycosin-7-O-β-D-glucoside also belong to flavonoid glycoside, with the chemical structure similar to vaccarin. Most of these flavonoid glycoside compounds have anti-oxidative effect. Among these chemical compounds, hesperidin [12], naringin [13] and baicalin [14] were reported to attenuate cisplatin-induced nephrotoxicity through their anti-oxidative effect. More importantly, Anees Ahmed Syed et al. [15] found that hesperidin treatment protected rats from oxidative and inflammatory damage through upregulating SIRT1 and inhibiting NOX4. Junwei Zhang [16] reported that naringin ameliorated diabetic nephropathy by inhibiting NOX4. Recently, Chaoxing Ren et al. [17] confirmed that complanatoside A targeted to NOX4 and blocked renal fibrosis in diabetic mice by suppressing NLRP3 inflammasome activation and autophagy. However, the current study on the pharmacological function of vaccarin is limited.
Recently, it was found that ROS had a pivotal role in the pathology of cisplatin-induced nephrotoxicity [12,18]. ROS in the intracellular environment is essential for maintaining normal physiological and biochemical activities of cells, but excessive ROS is harmful to the cells [19]. Uncontrolled ROS was founded in cisplatin, contrast and sepsis-induced AKI [[20], [21], [22]]. The nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) family is the major catalyzing enzyme for ROS, such as H2O2 and O2 [23]. Among these NOX enzymes, NOX4 is mainly expressed in renal tubular cells [24]. Furthermore, our colleagues also found that disrupting Smad3 attenuated cisplatin-induced renal damage, programmed cell death, and inflammation through NOX4-dependent oxidative stress [25]. NOX-derived ROS could function as secondary messengers to activate the NF-κB signaling transduction pathway [26,27]. In the present study, we detected the high expression levels of NOX4 in both HK-2 cells and mice renal tissue. After the pre-treatment with vaccarin, the levels of NOX4, as well as the ROS, decreased significantly, probably suggesting that vaccarin can decrease the NOX4-derived ROS. Concordantly, a previous study reported that vaccarin protected human vascular endothelium endothelial from apoptosis induced by oxidized low-density lipoprotein through inhibiting the production of ROS [28]. Moreover, Zhu et al. [29] also demonstrated the protective effects of vaccarin on high glucose-induced cell apoptosis in human microvascular endothelial cells via inhibiting ROS accumulation and HDAC1 expression.
The NF-κB pathway, which can be induced directly by ROS, is a central contributor of inflammation response in the kidneys. Recently, Yu C et al. [30] found that Danshensu attenuates cisplatin-induced nephrotoxicity through the activation of Nrf2 and the inhibition of NF-κB. Yan Zhang et al. [31] found that inhibiting hepatocyte nuclear factor 1β contributed to cisplatin nephrotoxicity via regulating NF-κB pathway. In addition, NF-κB-dependent NLRP3 signals were also activated during cisplatin nephrotoxicity. The inhibition of NLRP3 has been found with a protective role against cisplatin-induced toxicity in the kidney and liver [[32], [33], [34], [35]]. In addition, some evidence has shown that NOX4-dependent oxidative stress is directly related to the activation of NLRP3 inflammasome [36,37]. In the present study, it was further verified that NLRP3 was significantly induced by cisplatin both in vivo and in vitro and the using of vaccarin effectively down-regulated NLRP3 expression, as well as the mRNA expression of pro-inflammatory factors downstream NF-κB including TNF-α, IL-1β, IL-6 and MCP-1.
Programmed cell necrosis, also named necroptosis, was observed in cisplatin-induced nephrotoxicity. RIPK1, activated by tumor necrosis factor receptor (TNFR), pattern recognition receptor (PRR), or other signals, is widely regarded as the main mediator of necroptosis [38]. After activation, the proteins of RIPK1 further bind to RIPK3 and form necrosomes, which are essential for the phosphorylation or activation of MLKL. Then, p-MLKL formed oligomers and translocated to the plasma membrane. This structure finally caused cell swelling, rupture and the releasing of damage-associated molecular patterns (DAMPs), which further cause inflammation. In the early phase of cisplatin injection, TNF-α and other cytokines were produced through the NF-κB/NLRP3 pathway in renal resident tissues. Although TNF-α is an inducer of necroptosis through TNFR, Yan Fang et al. [39] revealed that cisplatin-induced necroptosis of the renal tubular cells was still observed after the TNF-knockout or the using of TNF-neutralizing antibodies. In addition to TNF-α/TNFR signals, ROS has also been considered as a driving force for necroptosis. Zhang et al. [40] revealed that ROS promoted RIPK1 autophosphorylation on S161 and its necroptosis activity. Moreover, it was found that the three cysteines (cysteine 257, 268 and 586) in RIPK1 are required for, under the function of ROS, the formation of disulfide bond-linked high molecular weight aggregate of RIPK1 to enhance its autophosphorylation, which then facilitated necrosome formation. Thus, it was found in the present study that vaccarin can largely reduce the necroptosis induced by cisplatin, possibly through inhibiting the formation of ROS. In response to oxidative stress, apoptosis and necroptosis, NOX4 inhibitor GLX or the NOX4 knockdown by siRNA has equivalent protective effect to vaccarin. Moreover, when vaccarin was used together with GLX or NOX4-siRNA transfection, the protective effect of vaccarin did not further increase. Thus, it is probably suggested that vaccarin attenuate the cisplatin-induced nephrotoxicity through NOX4-mediated pathway.
Moreover, studies have shown that cisplatin accumulates mainly in proximal renal tubules over time [41]. Organic Cation Transporters (OCTs) [42] and Copper Transporter1 (CTR1) [28] regulates the uptake of cisplatin of proximal renal tubular epithelial cells. P-glycoprotein (P-gp) and MATE1 mainly mediate the efflux of cisplatin [43]. Cisplatin accumulation is the result of a dynamic balance between cellular uptake and efflux. The accumulation of cisplatin in proximal renal tubules could promote the production of ROS, stimulate NF-κB/NLRP3 signals, and cause cell apoptosis and necroptosis [41]. Some drugs that affect the transporting of cisplatin could alleviate the nephrotoxicity of cisplatin. To exclude the possibility that vaccarin alleviated the nephrotoxicity by reducing accumulation of cisplatin, we detected the levels of cisplatin in the cortex tissues of mice kidney at the time of 6 h after cisplatin injection. The results indicated that vaccarin could not affect cisplatin accumulation at this time. Cisplatin largely eliminated from the body within 4 h after injection, thus 6 h is enough to observe cisplatin transporting or accumulation in renal cortex. Since vaccarin has no impact on the renal accumulation of cisplatin, we did not further detect the drug transporters of cisplatin.
There are also several limitations in the present study. Firstly, we did not illustrate the renal protective effect of vaccarin upon NOX4 overexpression or knockdown in vivo. Secondly, the directly targeting of vaccarin to NOX4 needs further confirmation. Moreover, the signals upstream or downstream NOX4 should be investigated through signal blocking to further substantiate the specific molecular pathways of vaccarin. Finally, this study did not explore the protective effect of vaccarin on other animal models of AKI.
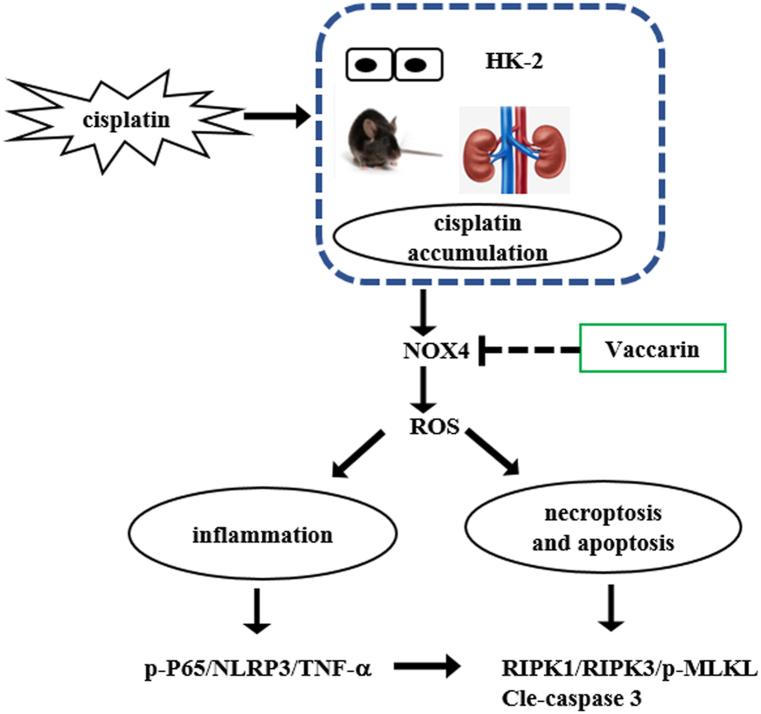
In summary, vaccarin can alleviate cisplatin-induced AKI by inhibiting inflammation, apoptosis and necroptosis possibly through decreasing NOX4-derived ROS and the relative pathway (with the hypothesis of signaling pathway shown in Fig. 7). Vaccarin is a promising adjuvant for the prevention of cisplatin-induced nephrotoxicity. However, its underlying mechanism still requires further investigation.
Fig. 7.
Schematic diagram on possible signaling pathway by which vaccarin alleviates cisplatin-induced AKI.
Ethics statement
All animal procedures were approved by the Animal Experiment Ethics Committee of Anhui Medical University (LLSC 20231354). All animal experiments were conducted in accordance with the Regulations on the Control of Laboratory Animals promulgated by the State Science and Technology Commission.
Consent for publication
All authors Consent for publication.
Funding statement
This work was supported by Grants for National Natural Science Foundation of China (No. U19A2001), National Natural Science Foundation of China (No. 32270424), Pharmaceutical innovative fund of Anhui Medical University (No. YXCX202203), Scientific Research Project of Education Department of Anhui Province (No. YJS20210295) and Key Scientific Research Foundation of the Education Department of Province Anhui (No. 2022AH050767).
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Tingni Wu: Data curation, Investigation, Methodology, Writing – original draft, Formal analysis. Wenxian Ma: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. Weili Lu: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. Zhuofan Huangshen: Conceptualization, Data curation, Methodology. Shiqing Chen: Conceptualization, Data curation, Methodology. Qin Yang: Conceptualization, Data curation, Methodology. Chao Li: Conceptualization, Data curation, Methodology. Zeng Li: Conceptualization, Data curation, Methodology. Ning Li: Formal analysis, Methodology, Resources, Software, Validation. Xiaowen Feng: Formal analysis, Methodology, Resources, Software, Validation. Li Li: Formal analysis, Methodology, Resources, Software, Validation. Yu Miao: Formal analysis, Methodology, Resources, Software, Validation. Jianan Wang: Formal analysis, Methodology, Resources, Software, Validation. Xueqi Liu: Methodology, Validation, Writing – original draft. Yuting Cai: Methodology, Validation, Writing – original draft. Yuan He: Methodology, Validation, Writing – original draft. Xiaoyan He: Methodology, Validation, Writing – original draft. Jun Li: Conceptualization, Project administration, Resources, Writing – review & editing, Data curation. Ren Zhao: Conceptualization, Data curation, Supervision, Writing – review & editing. Jiagen Wen: Conceptualization, Data curation, Funding acquisition, Project administration, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank Xuefu Wang, Cheng Huang, Rui Feng, Rong Li, Lei Di, Xiaofeng Li for their assistance in the acquisition of experimental data. The authors also thank Fenghe Li for the language editing of this article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e21231.
Contributor Information
Ren Zhao, Email: zhaoren@ahmu.edu.cn.
Jiagen Wen, Email: jiagen168@163.com.
Appendix A. Supplementary data
The following are the supplementary data to this article:
figs1.
References
- 1.Sung M.J., Kim D.H., Jung Y.J., Kang K.P., Lee A.S., Lee S., Kim W., Davaatseren M., Hwang J.-T., Kim H.-J., Kim M.S., Kwon D.Y., Park S.K. Genistein protects the kidney from cisplatin-induced injury, Kidney. International. 2008;74:1538–1547. doi: 10.1038/ki.2008.409. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J., Ye Z.W., Tew K.D., Townsend D.M. Cisplatin chemotherapy and renal function. Adv. Cancer Res. 2021;152:305–327. doi: 10.1016/bs.acr.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loren P., Saavedra N., Saavedra K., Zambrano T., Moriel P., Salazar L.A. Epigenetic mechanisms involved in cisplatin-induced nephrotoxicity: an update. Pharmaceuticals. 2021;14:491. doi: 10.3390/ph14060491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volarevic V., Djokovic B., Jankovic M.G., Harrell C.R., Fellabaum C., Djonov V., Arsenijevic N. Molecular mechanisms of cisplatin-induced nephrotoxicity: a balance on the knife edge between renoprotection and tumor toxicity. J. Biomed. Sci. 2019;26:25. doi: 10.1186/s12929-019-0518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li S., He X., Ruan L., Ye T., Wen Y., Song Z., Hu S., Chen Y., Peng B., Li S. Protective effect of mannitol on cisplatin-induced nephrotoxicity: a systematic review and meta-analysis. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.804685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sainamthip P., Saichaemchan S., Satirapoj B., Prasongsook N. The effect of intravenous mannitol combined with normal saline in preventing cisplatin-induced nephrotoxicity: a randomized, double-blind, placebo-controlled trial. JCO. Glob. Oncol. 2022;8 doi: 10.1200/GO.21.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang H., Wang K., Wu J., Chen Y., He P. A new flavonoid glycoside from Vaccaria hispanica. Nat. Prod. Commun. 2011;6:1599–1602. [PubMed] [Google Scholar]
- 8.Hou B., Cai W., Chen T., Zhang Z., Gong H., Yang W., Qiu L. Vaccarin hastens wound healing by promoting angiogenesis via activation of MAPK/ERK and PI3K/AKT signaling pathways in vivo. Acta Cir. Bras. 2020;34 doi: 10.1590/s0102-865020190120000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun J.N., Yu X.Y., Hou B., Ai M., Qi M.T., Ma X.Y., Cai M.J., Gao M., Cai W.W., Ni L.L., Xu F., Zhou Y.T., Qiu L.Y. Vaccarin enhances intestinal barrier function in type 2 diabetic mice. Eur. J. Pharmacol. 2021;908 doi: 10.1016/j.ejphar.2021.174375. [DOI] [PubMed] [Google Scholar]
- 10.Gong L., Lei Y., Liu Y., Tan F., Li S., Wang X., Xu M., Cai W., Du B., Xu F., Zhou Y., Han H., Sun H., Qiu L. Vaccarin prevents ox-LDL-induced HUVEC EndMT, inflammation and apoptosis by suppressing ROS/p38 MAPK signaling. Am. J. Transl. Res. 2019;11:2140–2154. [PMC free article] [PubMed] [Google Scholar]
- 11.Chen S.Q., Hu B.F., Yang Y.R., He Y., Yue L., Guo D., Wu T.N., Feng X.W., Li Q., Zhang W., Wen J.G. The protective effect of rabeprazole on cisplatin-induced apoptosis and necroptosis of renal proximal tubular cells. Biochem. Biophys. Res. Commun. 2022;612:91–98. doi: 10.1016/j.bbrc.2022.04.107. [DOI] [PubMed] [Google Scholar]
- 12.Chen X., Wei W., Li Y., Huang J., Ci X. Hesperetin relieves cisplatin-induced acute kidney injury by mitigating oxidative stress, inflammation and apoptosis. Chem. Biol. Interact. 2019;308:269–278. doi: 10.1016/j.cbi.2019.05.040. [DOI] [PubMed] [Google Scholar]
- 13.Chtourou Y., Aouey B., Aroui S., Kebieche M., Fetoui H. Anti-apoptotic and anti-inflammatory effects of naringin on cisplatin-induced renal injury in the rat. Chem. Biol. Interact. 2016;243:1–9. doi: 10.1016/j.cbi.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Sawicka E., Długosz A., Rembacz K.P., Guzik A. The effects of coenzyme Q10 and baicalin in cisplatin-induced lipid peroxidation and nitrosative stress. Acta. Pol. Pharm. 2013;70:977–985. [PubMed] [Google Scholar]
- 15.Syed A.A., Reza M.I., Yadav H., Gayen J.R. Hesperidin inhibits NOX4 mediated oxidative stress and inflammation by upregulating SIRT1 in experimental diabetic neuropathy. Exp. Gerontol. 2023;172 doi: 10.1016/j.exger.2022.112064. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J., Yang S., Li H., Chen F., Shi J. Naringin ameliorates diabetic nephropathy by inhibiting NADPH oxidase 4. Eur. J. Pharmacol. 2017;804:1–6. doi: 10.1016/j.ejphar.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Ren C., Bao X., Lu X., Du W., Wang X., Wei J., Li L., Li X., Lin X., Zhang Q., Ma B. Complanatoside A targeting NOX4 blocks renal fibrosis in diabetic mice by suppressing NLRP3 inflammasome activation and autophagy. Phytomedicine. 2022;104 doi: 10.1016/j.phymed.2022.154310. [DOI] [PubMed] [Google Scholar]
- 18.Xie X., Wu F., Tian J., Liu Z., He H., Bao D., Li G., Li H., Chen J., Lai Y., Chen Z., Fan J., Chen G., Lai C. Pyrocatechol alleviates cisplatin-induced acute kidney injury by inhibiting ROS production. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/2158644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang S., Lian G. ROS and diseases: role in metabolism and energy supply. Mol. Cell. Biochem. 2020;467:1–12. doi: 10.1007/s11010-019-03667-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y.T., Zhang H., Duan S.B., Wang J., Chen H., Zhan M., Zhang W., Li A.M., Liu Y., Yang Y., Yang S. Mitofusin2 ameliorated ER stress and mitochondrial ROS through maintaining mitochondria-associated ER membrane integrity in cisplatin-induced acute kidney injury. Antioxid. Redox. Signal. 2023 doi: 10.1089/ars.2022.0178. https://pubmed.ncbi.nlm.nih.gov/37053105/ [DOI] [PubMed] [Google Scholar]
- 21.Liu K., Hu C., Yin W., Zhou L., Gu X., Zuo X. An in vivo and in vitro model on the protective effect of cilnidipine on contrast-induced nephropathy via regulation of apoptosis and CaMKⅡ/mPTP pathway. J. Biochem. Mol. Toxicol. 2023;37 doi: 10.1002/jbt.23238. [DOI] [PubMed] [Google Scholar]
- 22.Wang T.T., Du Y.W., Wang W., Li X.N., Liu H.B. Inhibition of xanthine oxidase protects against sepsis-induced acute kidney injury by ameliorating renal hypoxia. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/4326695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xirouchaki C.E., Jia Y., McGrath M.J., Greatorex S., Tran M., Merry T.L., Hong D., Eramo M.J., Broome S.C., Woodhead J.S.T., D'Souza R F., Gallagher J., Salimova E., Huang C., Schittenhelm R.B., Sadoshima J., Watt M.J., Mitchell C.A., Tiganis T. Skeletal muscle NOX4 is required for adaptive responses that prevent insulin resistance. Sci. Adv. 2021;7 doi: 10.1126/sciadv.abl4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajaram R.D., Dissard R., Faivre A., Ino F., Delitsikou V., Jaquet V., Cagarelli T., Lindenmeyer M., Jansen-Duerr P., Cohen C., Moll S., Seigneux S. Tubular NOX4 expression decreases in chronic kidney disease but does not modify fibrosis evolution. Redox Biol. 2019;26:101234. doi: 10.1016/j.redox.2019.101234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang J.N., Yang Q., Yang C., Cai Y.T., Xing T., Gao L., Wang F., Chen X., Liu X.Q., He X.Y., Wei B., Jiang L., Li C., Jin J., Wen J.G., Ma T.T., Chen H.Y., Li J., Meng X.M. Smad3 promotes AKI sensitivity in diabetic mice via interaction with p53 and induction of NOX4-dependent ROS production. Redox Biol. 2020;32 doi: 10.1016/j.redox.2020.101479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Q., Spencer N.Y., Oakley F.D., Buettner G.R., Engelhardt J.F. Endosomal Nox2 facilitates redox-dependent induction of NF-kappaB by TNF-alpha. Antioxid. Redox. Signal. 2009;11:1249–1263. doi: 10.1089/ars.2008.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J., Zhang Z., Wang L., Jiang L., Qin Z., Zhao Y., Su B. Maresin 1 attenuates lipopolysaccharide-induced acute kidney injury via inhibiting NOX4/ROS/NF-κB pathway. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.782660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pabla N., Murphy R.F., Liu K., Dong Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am. J. Physiol. Renal. Physiol. 2009;296:F505–F511. doi: 10.1152/ajprenal.90545.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu X., Lei Y., Tan F., Gong L., Gong H., Yang W., Chen T., Zhang Z., Cai W., Hou B., Wang X., Sun H., Zhou Y., Qiu L. Vaccarin protects human microvascular endothelial cells from apoptosis via attenuation of HDAC1 and oxidative stress. Eur. J. Pharmacol. 2018;818:371–380. doi: 10.1016/j.ejphar.2017.09.052. [DOI] [PubMed] [Google Scholar]
- 30.Yu C., Dong H., Wang Q., Bai J., Li Y.N., Zhao J.J., Li J.Z. Danshensu attenuates cisplatin-induced nephrotoxicity through activation of Nrf2 pathway and inhibition of NF-κB. Biomed. Pharmacother. 2021;142 doi: 10.1016/j.biopha.2021.111995. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y., Hao J., Du Z., Li P., Hu J., Ruan M., Li S., Ma Y., Lou Q. Inhibition of hepatocyte nuclear factor 1β contributes to cisplatin nephrotoxicity via regulation of nf-κb pathway. J. Cell Mol. Med. 2021;25:2861–2871. doi: 10.1111/jcmm.16316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komada T., Muruve D.A. The role of inflammasomes in kidney disease. Nat. Rev. Nephrol. 2019;15:501–520. doi: 10.1038/s41581-019-0158-z. [DOI] [PubMed] [Google Scholar]
- 33.Li S., Lin Q., Shao X., Mou S., Gu L., Wang L., Zhang Z., Shen J., Zhou Y., Qi C., Jin H., Pang H., Ni Z. NLRP3 inflammasome inhibition attenuates cisplatin-induced renal fibrosis by decreasing oxidative stress and inflammation. Exp. Cell Res. 2019;383 doi: 10.1016/j.yexcr.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Jiang S., Zhang H., Li X., Yi B., Huang L., Hu Z., Li A., Du J., Li Y., Zhang W. Vitamin D/VDR attenuate cisplatin-induced AKI by down-regulating NLRP3/Caspase-1/GSDMD pyroptosis pathway. J. Steroid Biochem. Mol. Biol. 2021;206 doi: 10.1016/j.jsbmb.2020.105789. [DOI] [PubMed] [Google Scholar]
- 35.Qu X., Gao H., Tao L., Zhang Y., Zhai J., Song Y., Zhang S. Autophagy inhibition-enhanced assembly of the NLRP3 inflammasome is associated with cisplatin-induced acute injury to the liver and kidneys in rats. J. Biochem. Mol. Toxicol. 2018 doi: 10.1002/jbt.22228. [DOI] [PubMed] [Google Scholar]
- 36.Zhai L., Pei H., Yang Y., Zhu Y., Ruan S. NOX4 promotes Kupffer cell inflammatory response via ROS-NLRP3 to aggravate liver inflammatory injury in acute liver injury. Aging. (Albany NY) 2022;14:6905–6916. doi: 10.18632/aging.204173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moon J.S., Nakahira K., Chung K.P., DeNicola G.M., Koo M.J., Pabón M.A., Rooney K.T., Yoon J.H., Ryter S.W., Stout-Delgado H., Choi A.M. NOX4-dependent fatty acid oxidation promotes NLRP3 inflammasome activation in macrophages. Nat. Med. 2016;22:1002–1012. doi: 10.1038/nm.4153. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Linkermann A. Nonapoptotic cell death in acute kidney injury and transplantation, Kidney. Int. 2016;89:46–57. doi: 10.1016/j.kint.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Xu Y., Ma H., Shao J., Wu J., Zhou L., Zhang Z., Wang Y., Huang Z., Ren J., Liu S., Chen X., Han J. A role for tubular necroptosis in cisplatin-induced AKI. J. Am. Soc. Nephrol. 2015;26:2647–2658. doi: 10.1681/ASN.2014080741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y., Su S.S., Zhao S., Yang Z., Zhong C.Q., Chen X., Cai Q., Yang Z.H., Huang D., Wu R., Han J. RIP1 autophosphorylation is promoted by mitochondrial ROS and is essential for RIP3 recruitment into necrosome. Nat. Commun. 2017;8 doi: 10.1038/ncomms14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peres L.A., da Cunha A.D., Jr. Acute nephrotoxicity of cisplatin: molecular mechanisms. J. Bras. Nefrol. 2013;35:332–340. doi: 10.5935/0101-2800.20130052. [DOI] [PubMed] [Google Scholar]
- 42.Ciarimboli G., Ludwig T., Lang D., Pavenstädt H., Koepsell H., Piechota H.J., Haier J., Jaehde U., Zisowsky J., Schlatter E. Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am. J. Pathol. 2005;167:1477–1484. doi: 10.1016/S0002-9440(10)61234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeda F., Oda M., Terasaki M., Ichimura Y., Kojima H., Saitoh H. Downregulated expression of intestinal P-glycoprotein in rats with cisplatin-induced acute kidney injury causes amplification of its transport capacity to maintain "gatekeeper" function. Toxicol. Appl. Pharmacol. 2021;423 doi: 10.1016/j.taap.2021.115570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.