Abstract
A pyogenic liver abscess (PLA) is a space-occupying lesion in the liver that is associated with significant morbidity and mortality. We herein present the case of a Japanese 76-year-old man who visited our hospital with fever and back pain lasting 3 weeks after endoscopic treatment for common bile duct stones. He was accompanied by poorly controlled diabetes mellitus (DM) with an HbA1c of 9.7 %. Laboratory tests disclosed elevated C-reactive protein level (22.1 mg/dL) and white cell count (11,910/μL). Abdominal computed tomography (CT) revealed hypodense lesions in the right liver lobe, with abdominal ultrasonography showing an echogenicity-mixed hypoechoic lesion. Percutaneous needle aspiration of a liver lesion was performed under suspicion of a PLA. Subsequent enhanced CT and magnetic resonance imaging confirmed the hepatic lesions in the right lobe as well as a septic pulmonary embolism, right hepatic vein thrombosis, spondylodiscitis, and a retroperitoneal abscess. Gram staining of the abscess drainage revealed gram-negative bacteria. The above findings indicated invasive liver abscess syndrome (ILAS) caused by Klebsiella pneumoniae. However, further examination of blood, urine, and abscess drainage cultures revealed positivity for Klebsiella oxytoca. This case illustrates that K. oxytoca may cause ILAS-like symptoms. Screening for systemic metastatic infection should be considered in patients with PLA due to K. oxytoca in whom therapeutic intervention has been delayed, especially in patients with poorly controlled DM.
Keywords: Pyogenic liver abscess, Klebsiella oxytoca, Invasive liver abscess syndrome
1. Introduction
Invasive liver abscess syndrome (ILAS) is a clinical condition characterized by the presence of a pyogenic liver abscess (PLA) caused by Klebsiella pneumoniae along with such extrahepatic complications as endophthalmitis, meningitis, necrotizing fasciitis, and spondylodiscitis [1,2]. Klebsiella is a gram-negative, anaerobic, rod-shaped enterobacterium known to attack immune-compromised patients [3]. This opportunistic pathogen is widely distributed and often has a commensal presence in the human nasopharynx and intestinal tract, animal bowel, water, and soil [4]. Among the Klebsiella species (spp), Klebsiella oxytoca is second only to K. pneumoniae in frequency of isolation. K. oxytoca can cause a wide range of diseases, including colitis and infective endocarditis, as well as common urinary tract and respiratory tract infections [5]. However, there are few reports of K. oxytoca being detected in liver abscesses, with no cases as the metastatic focus of infection.
This report describes the case of a PLA caused by K. oxytoca resulting in a septic pulmonary embolism, right hepatic vein thrombosis, spondylodiscitis, and a retroperitoneal abscess, which presented symptoms similar to those in ILAS.
1.1. Case report
A 76-year-old Japanese man was admitted to our hospital for endoscopic treatment of common bile duct stones. Endoscopic retrograde cholangiopancreatography confirmed a filling defect within the lower common bile duct. The stones were extracted from the common bile duct during endoscopic sphincterotomy using balloon sweep. One week after discharge, he suffered fever and back pain that persisted for 3 weeks. The fever rose to 39.1 °C, but the patient self-medicated with an over-the-counter antipyretic. The patient was seen in the outpatient clinic on the originally scheduled date. He had a history of diabetes mellitus (DM) and hypertension and was taking several oral medications, including cilnidipine and metformin hydrochloride. He had no known allergies, but reported smoking 20 cigarettes per day for 47 years and consuming 70 g of ethanol daily in that period. His height, weight, and body mass index were 168 cm, 54 kg, and 19.2 kg/m2, respectively. Upon admission, physical examination showed a temperature of 37.8 °C with normal vital signs. The liver was not tender and was not palpable. There was no evidence of endocarditis, including heart murmur, Osler's nodes, or Janeway lesions. No tenderness was noted upon palpation of the spine or bilateral costovertebral angles. Furthermore, there were no indications of muscle weakness or sensory impairments, and the Straight Leg Raise test yielded negative results bilaterally.
Laboratory findings revealed elevated C-reactive protein (CRP) level (22.1 mg/dL) and white cell count (11,910/μL) along with low platelet count (10.3 × 104/μL). His serum aspartate aminotransferase level was normal at 15 U/L, alanine aminotransferase was normal at 14 IU/L, alkaline phosphatase was elevated at 275 U/L, and gamma-glutamyltranspeptidase was normal at 20 U/L. His serum albumin level was low at 1.7 g/dL. Renal function and coagulation capacity were within normal limits. Fasting blood glucose of 280 mg/dL and HbA1c of 9.3 % indicated poorly controlled DM. T-SPOT Mycobacterium tuberculosis-specific IFN-γ, HBs antigen, anti-HCV antibody, and anti-HIV antigen/antibody were all negative. The string test was negative. The tumor markers CEA, CA19-9, AFP, and PIVKA-2 were all within normal range (Table 1).
Table 1.
Laboratory data on admission.
| Hematology | Biochemistry | |||||||
|---|---|---|---|---|---|---|---|---|
| WBC | 11910 | /μL | TP | 4.8 | g/dL | HbA1c | 9.3 | % |
| Neu | 93.2 | % | Alb | 1.7 | g/dL | CRP | 22.1 | mg/dL |
| Lym | 3.5 | % | AST | 15 | U/L | |||
| Eosino | 0.1 | % | ALT | 14 | U/L | Infection marker | ||
| RBC | 348 × 104 | /μL | ALP | 275 | U/L | Β-D glucan | - | |
| Hemoglobin | 10.7 | g/dL | γ-GTP | 20 | U/L | T-SPOT | - | |
| HCT | 30.6 | % | T-Bil | 1.18 | mg/dL | HIV-Ab/Ab | - | |
| MCV | 87.9 | fL | BUN | 18.7 | mg/dL | HBs-Ag | - | |
| Platelet | 10.3 × 104 | /μL | Cre | 0.87 | mg/dL | HCV-Ab | - | |
| Na | 135 | mEq/L | ||||||
| Coagulation | K | 2.7 | mEq/L | |||||
| PT-INR | 1.17 | Cl | 98 | mEq/L | ||||
| APTT | 34.7 | sec | Glucose | 280 | mg/dL | |||
Alb, albumin; ALT, alanine aminotransferase; ALP, alkaline phosphatase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Cre, creatinine; CRP, C-reactive protein; Eosino, Eosinophils; GGTP, gamma-glutamyl transpeptidase; HbA1c, hemoglobin A1c; HCT, hematocrit; Lym, lymphocytes; MCV, mean corpuscular volume; Neu, neutrophils; PT-INR, prothrombin time-international normalized ratio; RBC, red blood cells; T-Bil, total bilirubin; TP, total protein; WBC, white blood cells.
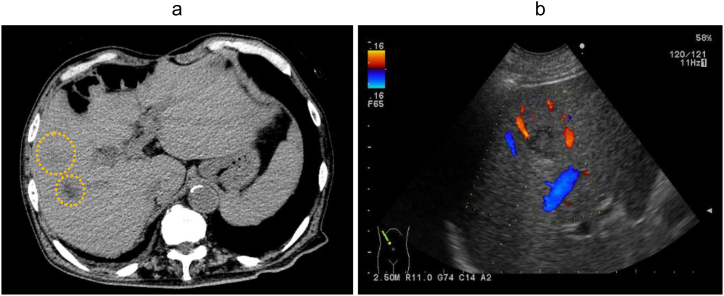
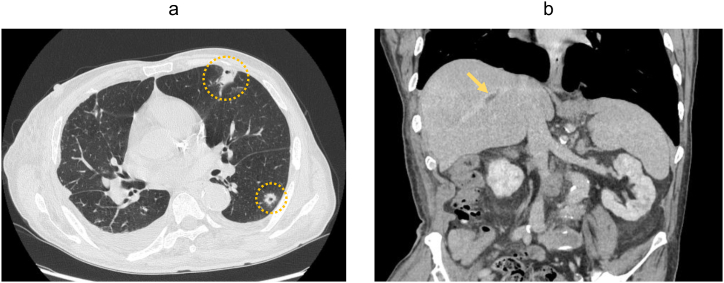
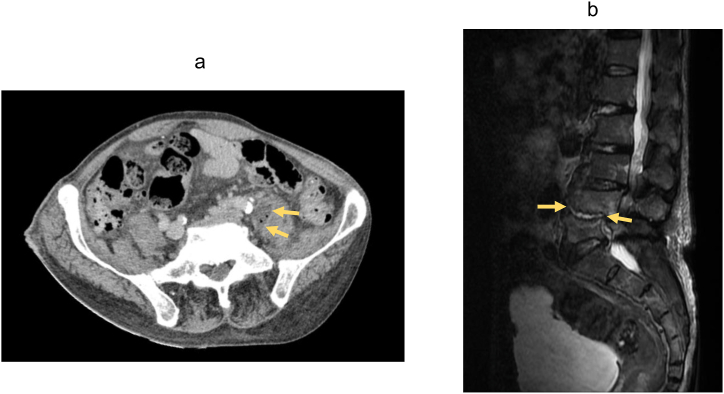
Abdominal computed tomography (CT) revealed 2 hypodense lesions in the right liver lobe (Fig. 1a), while abdominal ultrasonography detected an echogenicity-mixed hypoechoic lesion (Fig. 1b). These imaging findings did not suggest any signs of biliary tract illness, including biliary obstruction, gallstones, or gallbladder wall thickening. Under suspicion of a PLA, percutaneous needle aspiration of the liver lesion was performed for microbiological examination. The patient was started on cefoperazone/sulbactam (2.0 g/day) following re-admission. Subsequent enhanced CT and magnetic resonance imaging uncovered a septic pulmonary embolism (Fig. 2a), right hepatic vein thrombosis (Fig. 2b), a retroperitoneal abscess (Fig. 3a) and spondylodiscitis (L4/5) (Fig. 3b) in addition to the hepatic lesions in the right lobe.
Fig. 1.
(a) Abdominal computed tomography revealed 2 hypodense lesions in the right liver lobe (yellow circles). (b) Doppler abdominal ultrasonography showed an echogenicity-mixed hypoechoic lesion without blood flow.
Fig. 2.
(a) Chest computed tomography detected multiple cavitated nodules (yellow circles). (b) Enhanced abdominal computed tomography showed thrombosis of the hepatic vein (yellow arrow).
Fig. 3.
(a) Abdominal computed tomography showed a low-density area of left-sided iliopsoas muscle swelling (yellow arrow). (b) Short tau inversion recovery magnetic resonance imaging depicted enlarged high-signal areas in the L4/5 intervertebral disc (yellow arrow) and in the upper and lower vertebral bodies.
Gram staining revealed gram-negative bacilli, leading to the assumption that the patient's findings were due to ILAS caused by a PLA from K. pneumoniae. The antibiotic was changed to cefmetazole (4 g/day) and anticoagulant therapy with edoxaban tosilate hydrate (30 mg/day) was initiated. Intraocular and echocardiographic examination to rule out Klebsiella endophthalmitis and tricuspid valve warts, respectively, were negative. Unexpectedly, aspirated abscess fluid, blood, and urine cultures taken on admission were all positive for K. oxytoca. MALDI-ToF mass spectrometry (Bruker Daltonics, Bremen, Germany) employing the BDAL 9.0 database was used for identification of bacterial species. Results of antimicrobial susceptibility of K. oxytoca are shown in Table 2.
Table 2.
Antimicrobial susceptibility of Klebsiella oxytoca.
| Antimicrobial agent | MIC (mg/mL) | SIR result |
|---|---|---|
| Ampicillin | >16 | R |
| Ampicillin/sulbactam | 8 | S |
| Amoxicillin/clavulanic acid | ≦ 8 | S |
| Piperacillin | 16 | S |
| Piperacillin-tazobactam | ≦ 8 | S |
| Cefazolin | >16 | R |
| Cefmetazole | ≦ 16 | S |
| Ceftriaxone | ≦ 1 | S |
| Cefepime | ≦ 2 | S |
| Cefoperazone/sulbactam | ≦ 16 | S |
| Meropenem | ≦ 1 | S |
| Amikacin | ≦ 4 | S |
| Sulfamethoxazole-trimethoprim | ≦ 2 | S |
| Levofloxacin | ≦ 0.5 | S |
Susceptibility testing of K. oxytoca was performed according to Clinical and Laboratory Standards Institute (CLSI) document (ver. 26).
I, intermediate; MIC, minimum inhibitory concentration; R, resistant; S, susceptible.
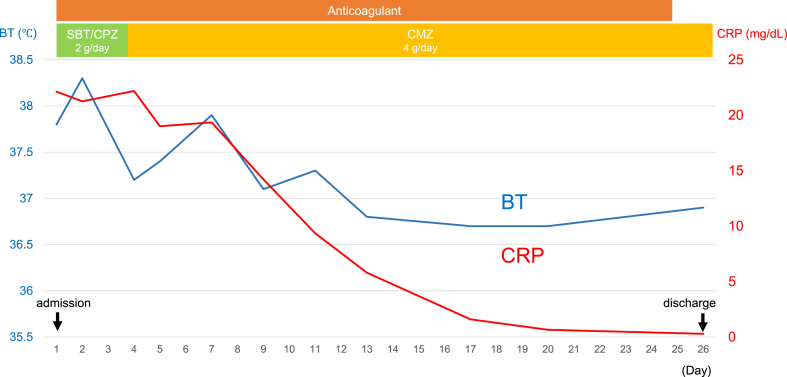
After receiving antibiotics and anticoagulants, the patient's back pain improved, and his elevated CRP and fever decreased (Fig. 4). On the 25th day of hospitalization, CT and abdominal ultrasonography showed reduction of the lung lesion and retroperitoneal abscess and disappearance of the right hepatic vein thrombus. Anticoagulation treatment was discontinued, and the patient was transferred to his referring physician. After the hospital transfer, he received antibiotic therapy with levofloxacin (500 mg/day) for a total of 8 weeks based on the antimicrobial susceptibility (Table 2), with no symptom flare-ups.
Fig. 4.
Clinical course. BT, body temperature; CRP, C-reactive protein.
We searched the English literature using the terms “liver abscess” and “Klebsiella oxytoca” in PubMed/MEDLINE and retrieved 10 relevant references. While 4 reports included the finding of liver abscess caused by K. oxytoca [[6], [7], [8], [9]], none of them described symptoms such as ILAS that were associated with metastatic infection (Table 3).
Table 3.
Reported cases of liver abscess caused by Klebsiella. oxytoca.
| No | Case | Diabetes mellitus | Background | References |
|---|---|---|---|---|
| 1 | 64, Male | + | Cholecystitis | Paasch C et al |
| 2 | 80, Male | + | Cholangitis/Endoscopic procedure | Lee JY et al |
| 3 | 84, Male | - | Liver cyst | Surani A et al |
| 4 | 74, Male | - | Cholelithiasis/Cholecystectom | Osório C et al |
| This case | 76, Male | + | Choledocholithiasis/Endoscopic procedure |
2. Discussion
We successfully managed a patient with a PLA caused by K. oxytoca that led to a septic pulmonary embolism, right hepatic vein thrombosis, spondylodiscitis, and a retroperitoneal abscess. To the best of our knowledge, this is the first report of a PLA caused by K. oxytoca presenting with metastatic infection similar to that of ILAS.
In a study of 540 cases of intra-abdominal abscesses including intra- and retroperitoneal lesions, liver abscesses made up 13 % of all intra-abdominal abscesses and 48 % of visceral abscesses [10]. The incidence of intra-abdominal abscesses varies significantly by region, ranging from 3.6 to 13.52 per 100,000 individuals in Taiwan, Germany, and United States [[11], [12], [13]]. The most common underlying diseases are reportedly DM (35.3 %), cholelithiasis (13.1 %), and hepatocellular carcinoma (9.8 %) [14].
In K. pneumoniae, K1/K2 serotypes, hypermucoviscosity, and expression of the magA gene are strongly related to high virulence [15,16]; K1/K2 serotypes and expression of the magA gene lack the capsular sugar sequences recognized by macrophages [17], while hypermucoviscosity causes increased resistance to complement-mediated serum killing [18]. Therefore, a certain number of individuals with a PLA caused by K. pneumoniae display ILAS symptoms. ILAS was first reported in Taiwan in the 1980s [19]. Subsequently, a considerable number of cases have been confirmed in East Asian countries. Several cases have also been reported in the Americas, with the same characteristics as those reported in Asia, confirming the worldwide spread of the disease [20]. Endophthalmitis, meningitis, and brain abscess are the most typical symptoms of ILAS [14,21]. Other signs include psoas abscess, lung abscess, spondylodiscitis, and septic pulmonary emboli [2,11,22]. Patients afflicted with liver abscesses resulting from Klebsiella spp. may experience thrombophlebitis within the hepatic venous system, which is considered a plausible cause of septic pulmonary embolism [23]. ILAS is prone to misdiagnosis due to non-specific symptoms, atypical presentations, overlapping clinical conditions, and lack of awareness. To reduce misdiagnosis in ILAS, health care providers should increase awareness of ILAS, utilize advanced imaging techniques, and ensure invasive access to the abscess area when necessary.
The major surface antigens of Klebsiella spp. are capsular polysaccharide (CPS; K antigen) and lipopolysaccharide (LPS; containing O antigen), which are common virulence factors of K. pneumoniae. Interestingly, recent reports of genome analysis indicate that the O and K antigen types of K. oxytoca overlap with those of K. pneumoniae [24]. However, a definitive association between K. oxytoca and ILAS by K. pneumoniae has not been established in the existing literature. It is also important to note that in the present case, the infection was prolonged without effective treatment after endoscopy, which may have enhanced the infectivity of K. oxytoca regardless of its intrinsic virulence. Further studies are needed to determine the exact mechanism of invasive infection by K. oxytoca.
K. oxytoca is a commensal bacterium and was not recognized as a common pathogen of bacteremia. However, numerous studies and cases of bacteremia caused by K. oxytoca in patients of all ages have recently been reported [5,25]. Most K. oxytoca bacteremia cases are associated with certain underlying diseases, such as DM, malignancy, chemotherapy, chronic obstructive pulmonary disease, and chronic renal failure [5,25]. Furthermore, the majority of K. oxytoca bacteremia was secondary to infection of other sites, with hepatobiliary infection (58 % of patients) being the most common original site of infection [26]. Considering these characteristics of K. oxytoca bacteremia, it is highly likely that the present case had bacteremia secondary to hepatobiliary infection with K. oxytoca in a diabetic background.
A possible mechanism of thrombus formation in vascular vessels includes increased coagulability caused by endotoxin from K. oxytoca and blood flow stagnation due to venous drainage of the liver abscess. In this case, the liver abscess was in contact with the right hepatic vein and the thrombus was continuously formed at the same site, suggesting that direct spillover of inflammation into the vein was the main cause of the thrombus.
In conclusion, we need to recognize that K. oxytoca can cause ILAS-like symptoms and that a systematic search for metastatic infection should be considered in patients with PLA due to K. oxytoca, especially in poorly controlled DM patients with delayed treatment initiation.
Funding
This research was funded by the Japan Agency for Medical Research and Development, Program for Basic and Clinical Research on Hepatitis (project number: 23fk0210125h).
Ethical approval
Not applicable.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Takanobu Iwadare: Conceptualization, Data curation, Investigation, Writing – original draft. Takefumi Kimura: Conceptualization, Data curation, Investigation, Writing – review & editing. Ayumi Sugiura: Writing – review & editing. Risa Takei: Investigation. Masato Kamakura: Investigation. Shun-ichi Wakabayashi: Investigation. Taiki Okumura: Investigation. Daichi Hara: Investigation. Akira Nakamura: Supervision. Takeji Umemura: Funding acquisition, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Trevor Ralph for his English editorial assistance.
References
- 1.Siu L.K., Yeh K.M., Lin J.C., Fung C.P., Chang F.Y. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect. Dis. 2012;12:881–887. doi: 10.1016/S1473-3099(12)70205-0. [DOI] [PubMed] [Google Scholar]
- 2.Wakabayashi S.I., Kimura T., Tanaka N., Pham J., Tanaka T., Higuchi S., et al. Invasive liver abscess syndrome accompanied by spondylodiscitis: a case report and review of the literature. Clin J Gastroenterol. 2020;13:927–934. doi: 10.1007/s12328-020-01161-0. [DOI] [PubMed] [Google Scholar]
- 3.Podschun R., Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 1998;11:589–603. doi: 10.1128/cmr.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh L., Cariappa M.P., Kaur M. Klebsiella oxytoca: an emerging pathogen? Med. J. Armed Forces India. 2016;72:S59–s61. doi: 10.1016/j.mjafi.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim B.N., Ryu J., Kim Y.S., Woo J.H. Retrospective analysis of clinical and microbiological aspects of Klebsiella oxytoca bacteremia over a 10-year period. Eur. J. Clin. Microbiol. Infect. Dis. 2002;21:419–426. doi: 10.1007/s10096-002-0738-9. [DOI] [PubMed] [Google Scholar]
- 6.Paasch C., Wilczek S., Strik M.W. Liver abscess and sepsis caused by Clostridium perfringens and Klebsiella oxytoca. Int J Surg Case Rep. 2017;41:180–183. doi: 10.1016/j.ijscr.2017.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee J.Y., Lee H.N., Lee W.H., Shin H.C., Kim S.S., Hwang J.A. Liver abscess caused by Klebsiella oxytoca with hepatic artery pseudoaneurysm: a case report. Taehan Yongsang Uihakhoe Chi. 2020;81:1448–1452. doi: 10.3348/jksr.2019.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Surani A., Slama E.M., Thomas S., Ross R.W., Cunningham S.C. Raoultella ornithinolytica and Klebsiella oxytoca pyogenic liver abscess presenting as chronic cough. IDCases. 2020;20 doi: 10.1016/j.idcr.2020.e00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osório C., Silva D., Teles L., Ferreira T., Nora M. An unusual fatal outcome of laparoscopic cholecystectomy: a case report. Cureus. 2023;15 doi: 10.7759/cureus.34365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altemeier W.A., Culbertson W.R., Fullen W.D., Shook C.D. Intra-abdominal abscesses. Am. J. Surg. 1973;125:70–79. doi: 10.1016/0002-9610(73)90010-x. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y.C., Lin C.H., Chang S.N., Shi Z.Y. Epidemiology and clinical outcome of pyogenic liver abscess: an analysis from the national health insurance research database of taiwan, 2000-2011. J. Microbiol. Immunol. Infect. 2016;49:646–653. doi: 10.1016/j.jmii.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 12.Meddings L., Myers R.P., Hubbard J., Shaheen A.A., Laupland K.B., Dixon E., et al. A population-based study of pyogenic liver abscesses in the United States: incidence, mortality, and temporal trends. Am. J. Gastroenterol. 2010;105:117–124. doi: 10.1038/ajg.2009.614. [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann L., Wendt S., Lübbert C., Karlas T. Epidemiology of pyogenic liver abscesses in Germany: analysis of incidence, risk factors and mortality rate based on routine data from statutory health insurance. UEG Journal. 2021;9:1039–1047. doi: 10.1002/ueg2.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin D., Ji C., Zhang S., Wang J., Lu Z., Song X., et al. Clinical characteristics and management of 1572 patients with pyogenic liver abscess: a 12-year retrospective study. Liver Int. 2021;41:810–818. doi: 10.1111/liv.14760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsay R.W., Siu L.K., Fung C.P., Chang F.Y. Characteristics of bacteremia between community-acquired and nosocomial Klebsiella pneumoniae infection: risk factor for mortality and the impact of capsular serotypes as a herald for community-acquired infection. Arch. Intern. Med. 2002;162:1021–1027. doi: 10.1001/archinte.162.9.1021. [DOI] [PubMed] [Google Scholar]
- 16.Yu W.L., Ko W.C., Cheng K.C., Lee H.C., Ke D.S., Lee C.C., et al. Association between rmpA and magA genes and clinical syndromes caused by Klebsiella pneumoniae in Taiwan. Clin. Infect. Dis. 2006;42:1351–1358. doi: 10.1086/503420. [DOI] [PubMed] [Google Scholar]
- 17.Lin J.C., Chang F.Y., Fung C.P., Xu J.Z., Cheng H.P., Wang J.J., et al. High prevalence of phagocytic-resistant capsular serotypes of Klebsiella pneumoniae in liver abscess. Microbes Infect. 2004;6:1191–1198. doi: 10.1016/j.micinf.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Alvarez D., Merino S., Tomás J.M., Benedí V.J., Albertí S. Capsular polysaccharide is a major complement resistance factor in lipopolysaccharide O side chain-deficient Klebsiella pneumoniae clinical isolates. Infect. Immun. 2000;68:953–955. doi: 10.1128/iai.68.2.953-955.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y.C., Cheng D.L., Lin C.L. Klebsiella pneumoniae liver abscess associated with septic endophthalmitis. Arch. Intern. Med. 1986;146:1913–1916. [PubMed] [Google Scholar]
- 20.Cardenas-Alvarez J., Balayla G., Triana A., Diaz Lankenau R., Franco-Paredes C., Henao-Martínez A.F., et al. Clinical spectrum and outcomes of cryptogenic Klebsiella pneumoniae liver abscess in the Americas: a scoping review. Pathogens. 2023;12:661. doi: 10.3390/pathogens12050661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Setsu T., Tsuchiya A., Yamagiwa S., Terai S. Invasive liver abscess syndrome caused by Klebsiella pneumoniae. Intern. Med. 2017;56:3121–3122. doi: 10.2169/internalmedicine.9014-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y., Wang H., Liu Z., Chang Z. The incidence of septic pulmonary embolism in patients with Klebsiella pneumoniae liver abscess: a systematic review and meta-analysis. Gastroenterol Res Pract. 2022;2022 doi: 10.1155/2022/3777122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maffiolo C., Novellas S., Chevallier P., Brunner P., Mourou M.Y., Bruneton J.N. Thrombophlebitis of the hepatic veins: complication of a Klebsiella liver abscess. Clin. Imag. 2006;30:63–65. doi: 10.1016/j.clinimag.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 24.Long H., Hu Y., Feng Y., Zong Z. Genome analysis of Klebsiella oxytoca complex for antimicrobial resistance and virulence genes. Antimicrob. Agents Chemother. 2022;66 doi: 10.1128/aac.02183-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mineau S., Kozak R., Kissoon M., Paterson A., Oppedisano A., Douri F., et al. Emerging antimicrobial resistance among Escherichia coli strains in bloodstream infections in Toronto, 2006-2016: a retrospective cohort study. CMAJ Open. 2018;6:E580–E586. doi: 10.9778/cmajo.20180039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin R.D., Hsueh P.R., Chang S.C., Chen Y.C., Hsieh W.C., Luh K.T. Bacteremia due to Klebsiella oxytoca: clinical features of patients and antimicrobial susceptibilities of the isolates. Clin. Infect. Dis. 1997;24:1217–1222. doi: 10.1086/513637. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.