Abstract
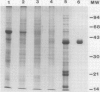
A family of immunologically identical glycoproteins with apparent molecular weights of approximately 40,000 are among the major tuber proteins of potato (Solanum tuberosum L.). These proteins, as purified by ion-exchange and affinity chromatography, have been given the trivial name `patatin.' To determine if patatin can be used as a biochemical marker to study the process of tuberization, its amount was measured in a variety of tissues by rocket immunoelectrophoresis and by enzyme-linked immunosorbent assay (ELISA).
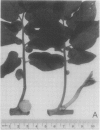
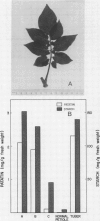
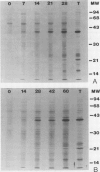
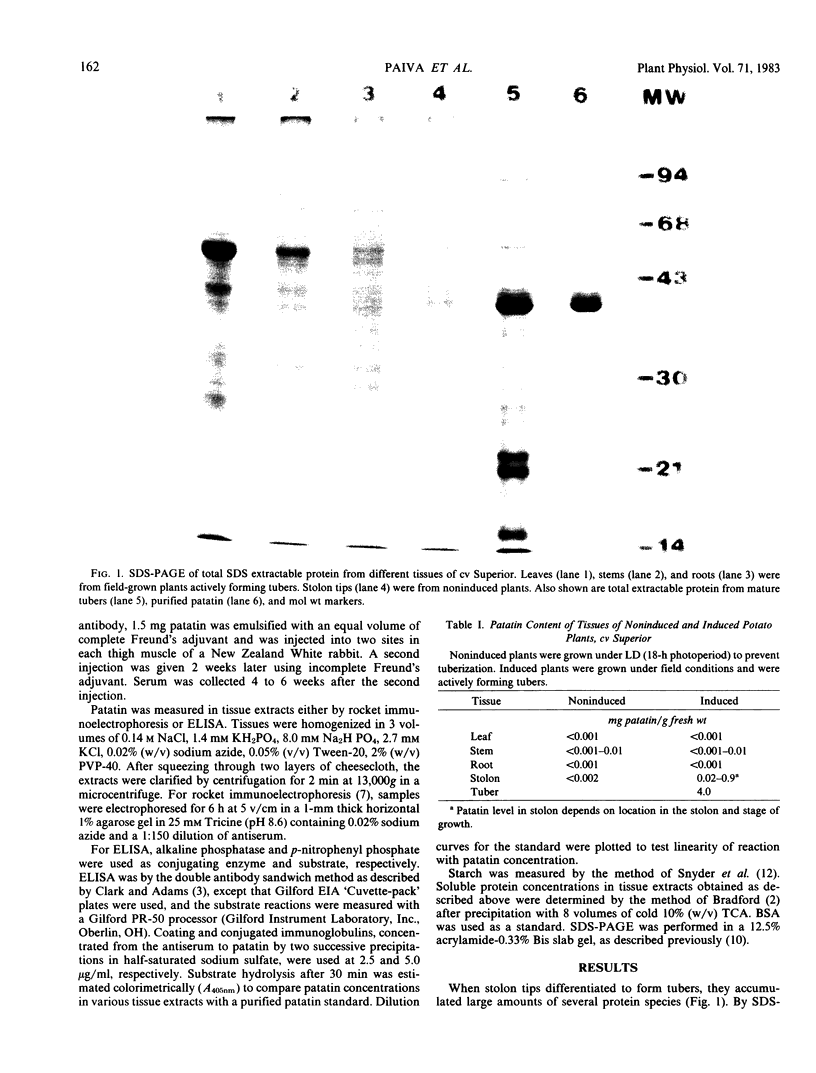
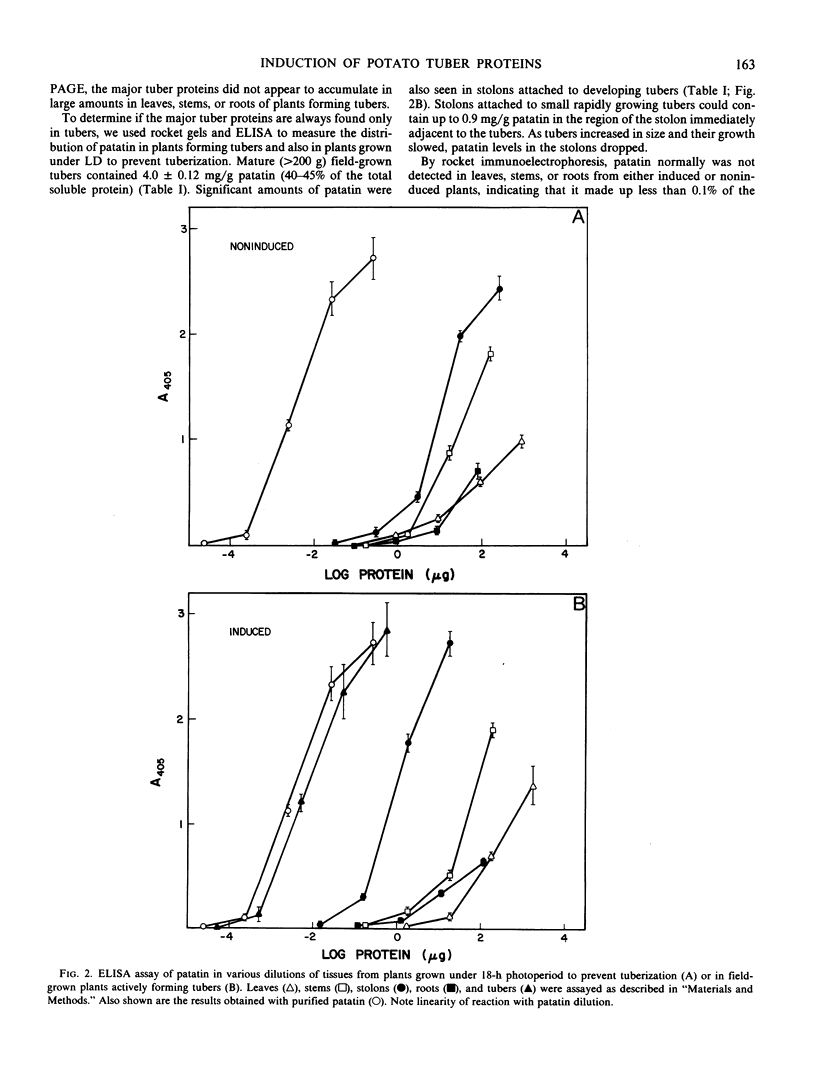
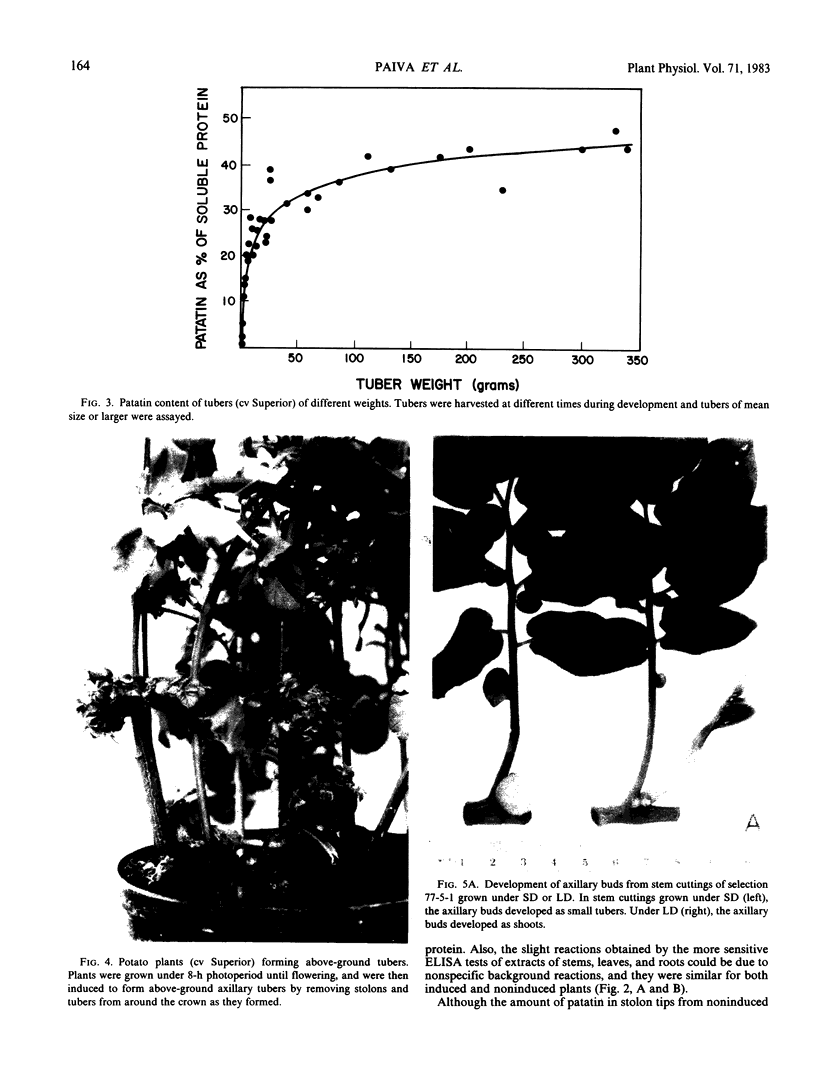
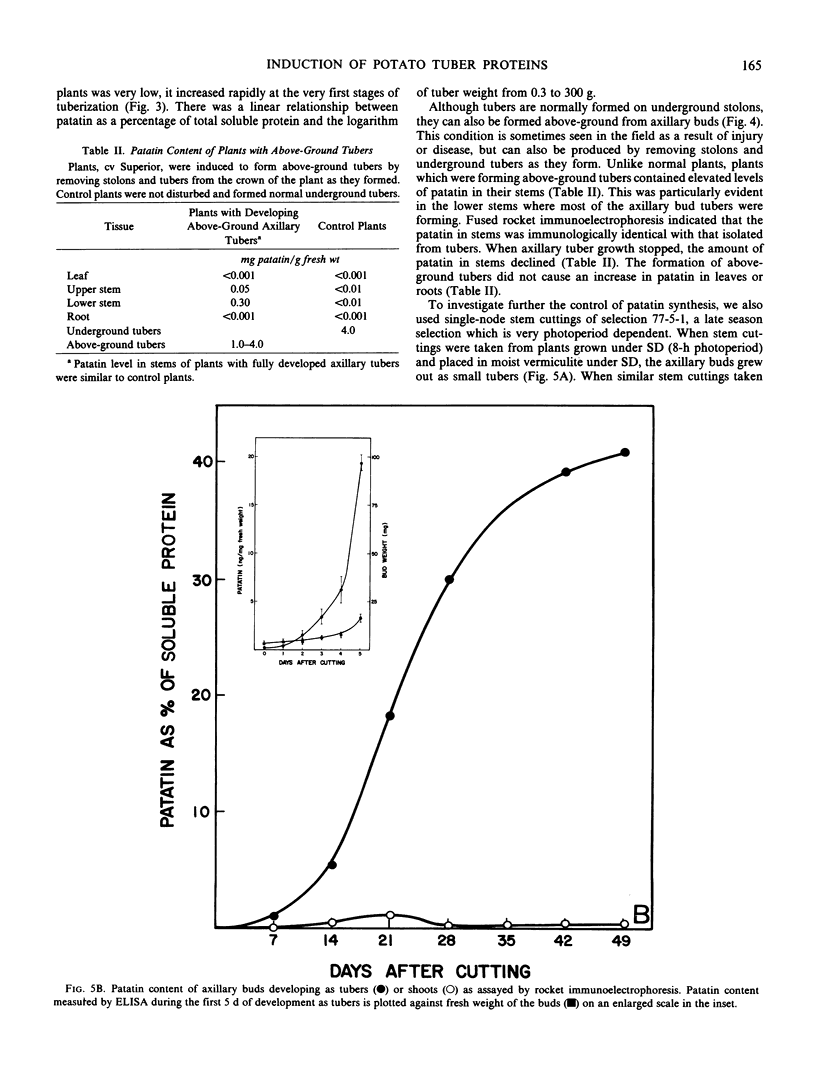
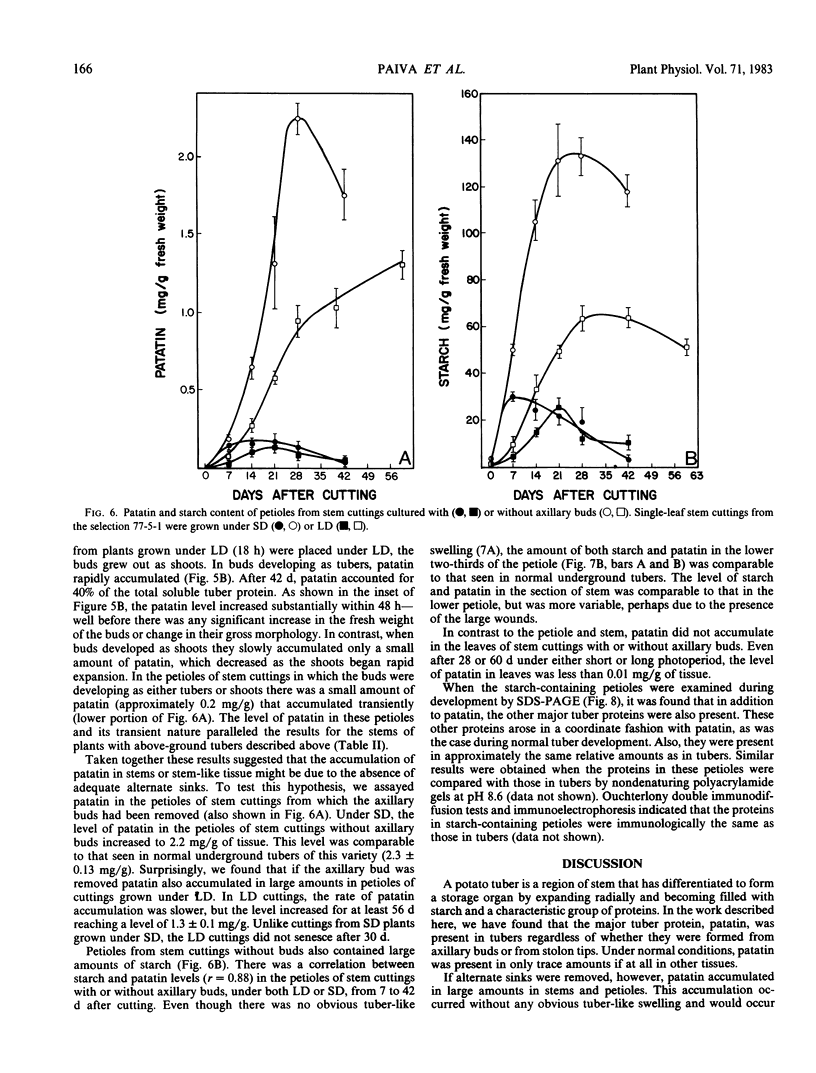
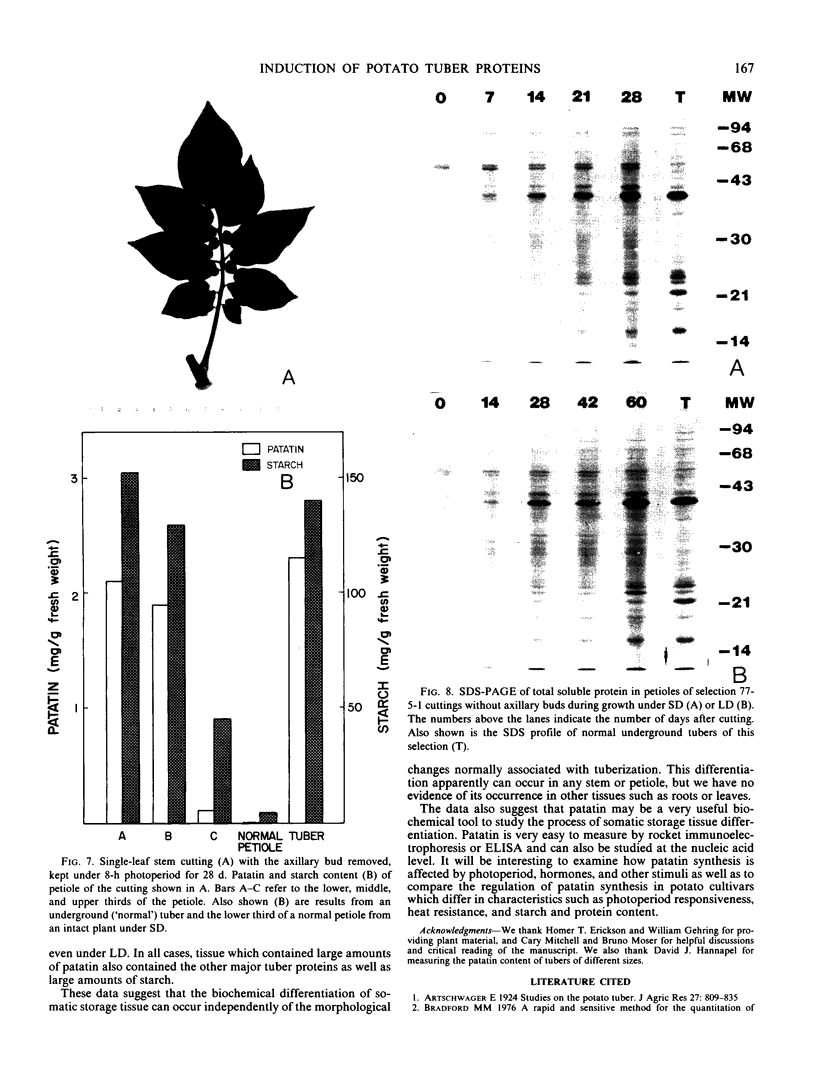
Patatin comprises 40 to 45% of the soluble protein in tubers regardless of whether they are formed on underground stolons or from axillary buds of stem cuttings. Under normal conditions, patatin is present in only trace amounts, if at all, in leaves, stems, or roots of plants which are either actively forming tubers or which have been grown under long days to prevent tuberization. However, if tubers and axillary buds are removed, patatin can accumulate in stems and petioles. This accumulation occurred without any obvious tuber-like swelling and would occur even under long days. In all tissues containing large amounts of patatin, the other tuber proteins were also found as well as large amounts of starch.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clark M. F., Adams A. N. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J Gen Virol. 1977 Mar;34(3):475–483. doi: 10.1099/0022-1317-34-3-475. [DOI] [PubMed] [Google Scholar]
- Lilley R. M. Isolation of Functionally Intact Rhodoplasts from Griffithsia monilis (Ceramiaceae, Rhodophyta). Plant Physiol. 1981 Jan;67(1):5–8. doi: 10.1104/pp.67.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park W. D., Lewis E. D., Rubenstein I. Heterogeneity of zein mRNA and protein in maize. Plant Physiol. 1980 Jan;65(1):98–106. doi: 10.1104/pp.65.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]