Abstract
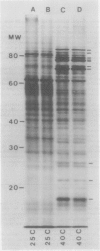
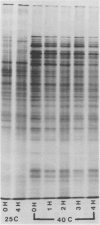
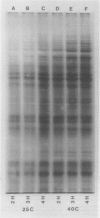
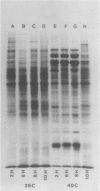
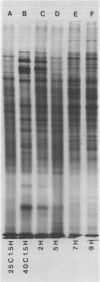
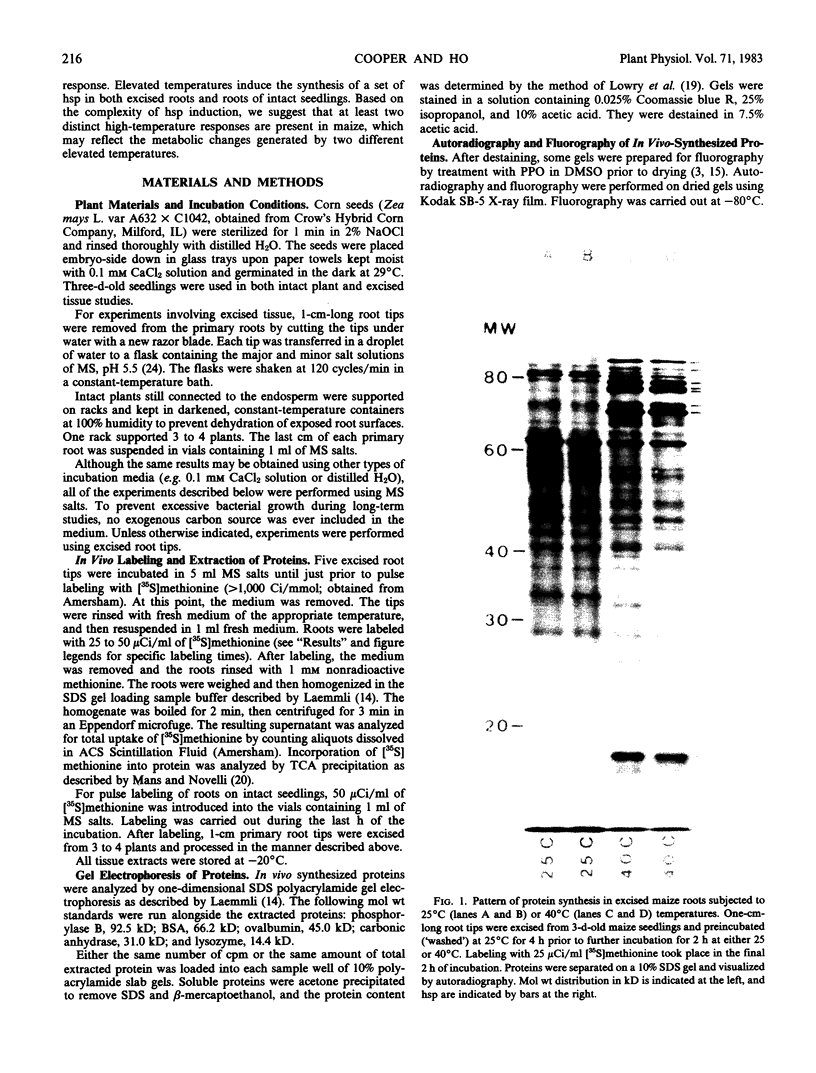
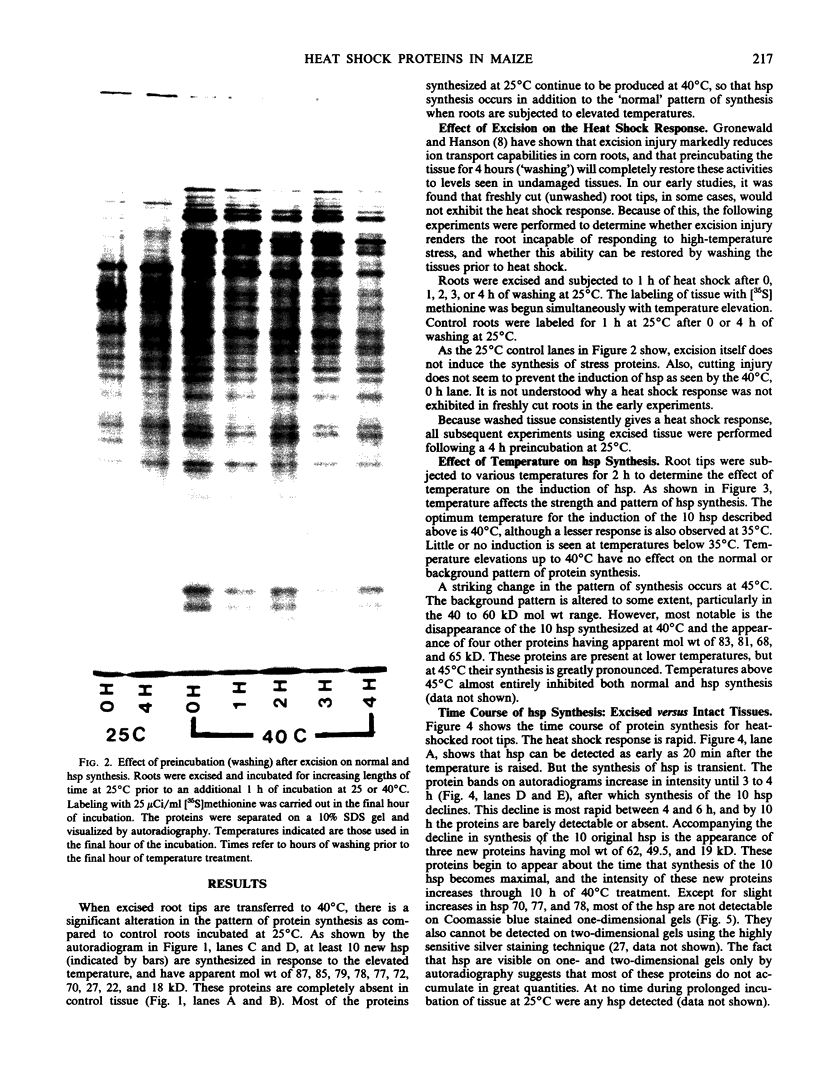
The pattern of protein synthesis in roots of 3-day-old maize seedlings (Zea mays L.) is rapidly and dramatically altered when the incubation temperature is raised from 25 to 40°C. One-dimensional sodium dodecyl sulfate gels reveal that although synthesis of the proteins observed at 25°C continues at 40°C, a new set of `heat shock proteins' (hsp) is induced within 20 minutes of the temperature transition. The hsp have molecular weights of 87, 85, 79, 78, 77, 72, 70, 27, 22, and 18 kilodaltons. The 10 hsp are visible on autoradiograms but not on stained gels, suggesting that the proteins do not accumulate to any great extent.
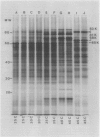
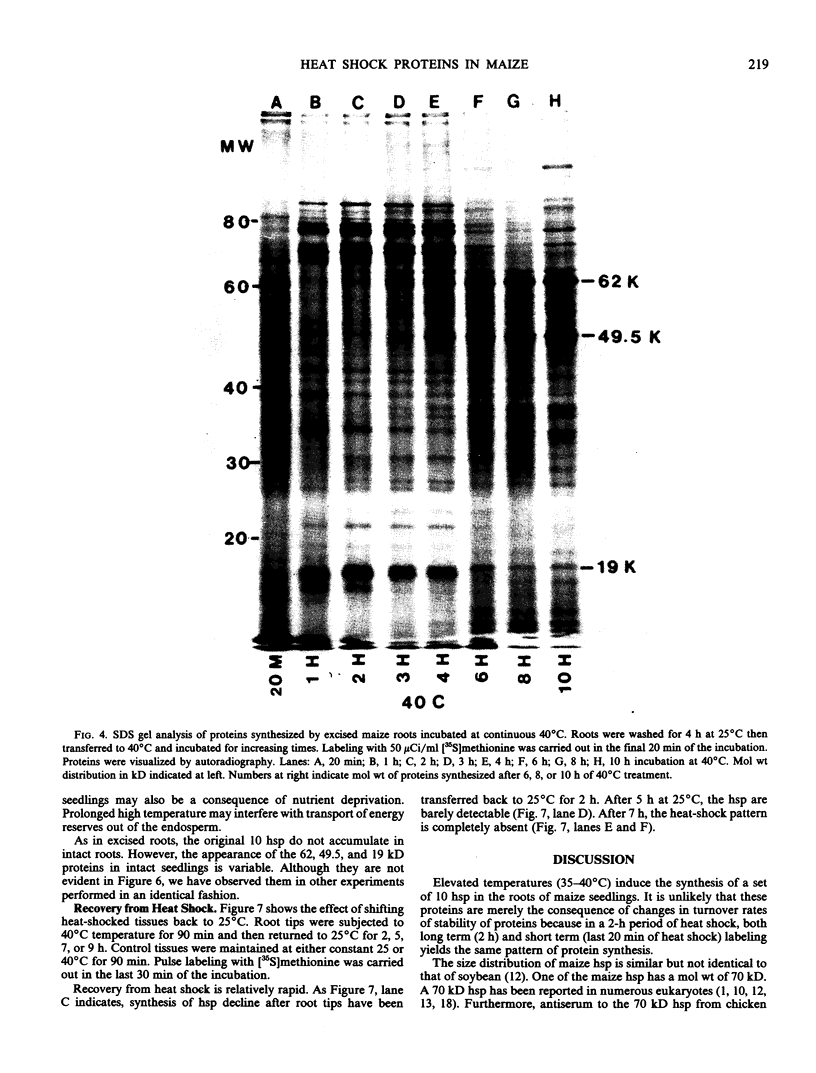
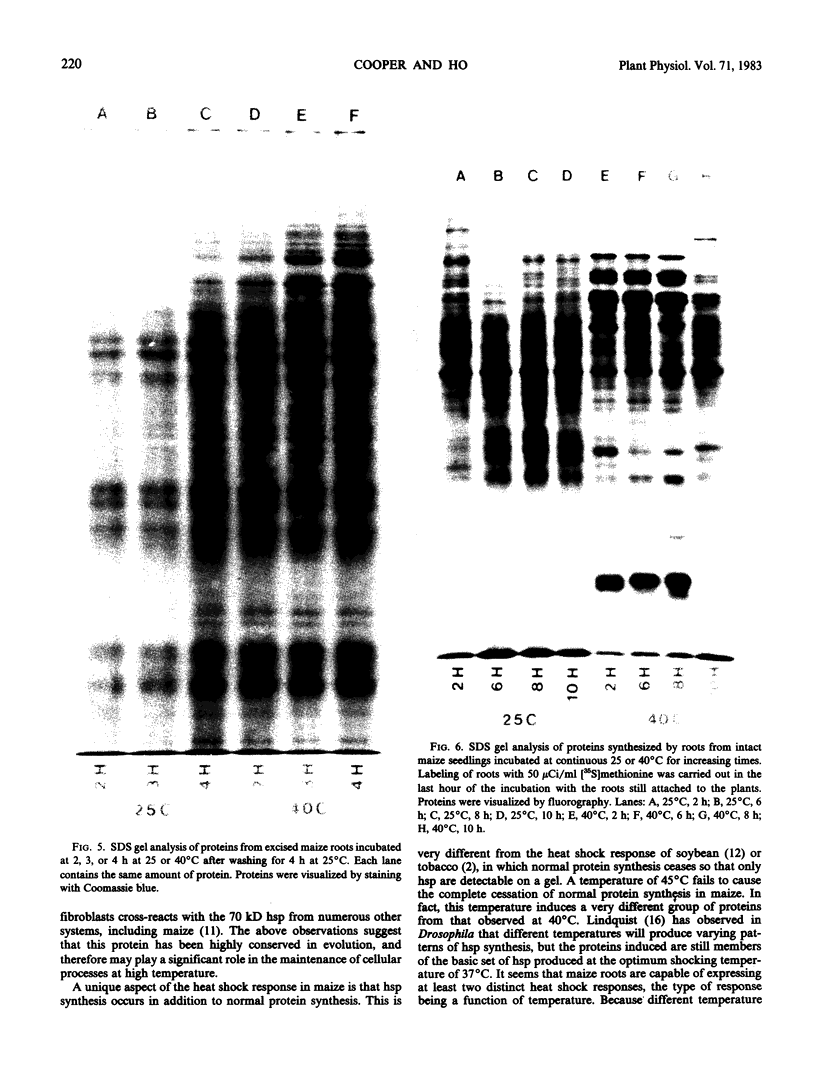
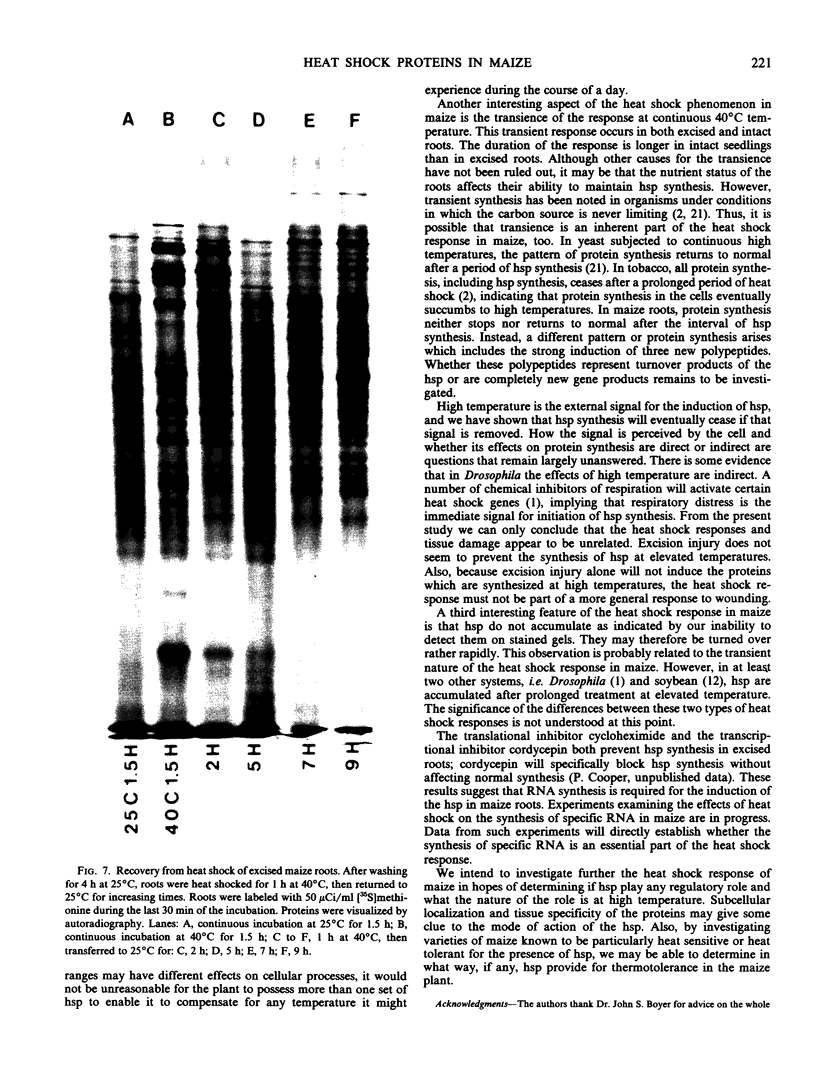
The induction of the hsp is transitory. With prolonged high temperature treatment, the synthesis of hsp continues for 4 hours in excised roots and for 8 hours in the roots of intact seedlings before declining sharply. Coincident to the decline in synthesis of the 10 hsp is the gradual increase in intensity of three new polypeptides having molecular weights of 62, 49.5, and 19 kilodaltons. These proteins begin to appear about the time that synthesis of the other 10 hsp becomes maximal.
Shifting the temperature back to 25°C also causes a decline in synthesis of hsp, but this decline occurs more rapidly than that seen during prolonged heat shock. A decrease in hsp synthesis becomes apparent 2 hours after the roots are returned to 25°C.
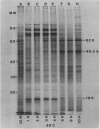
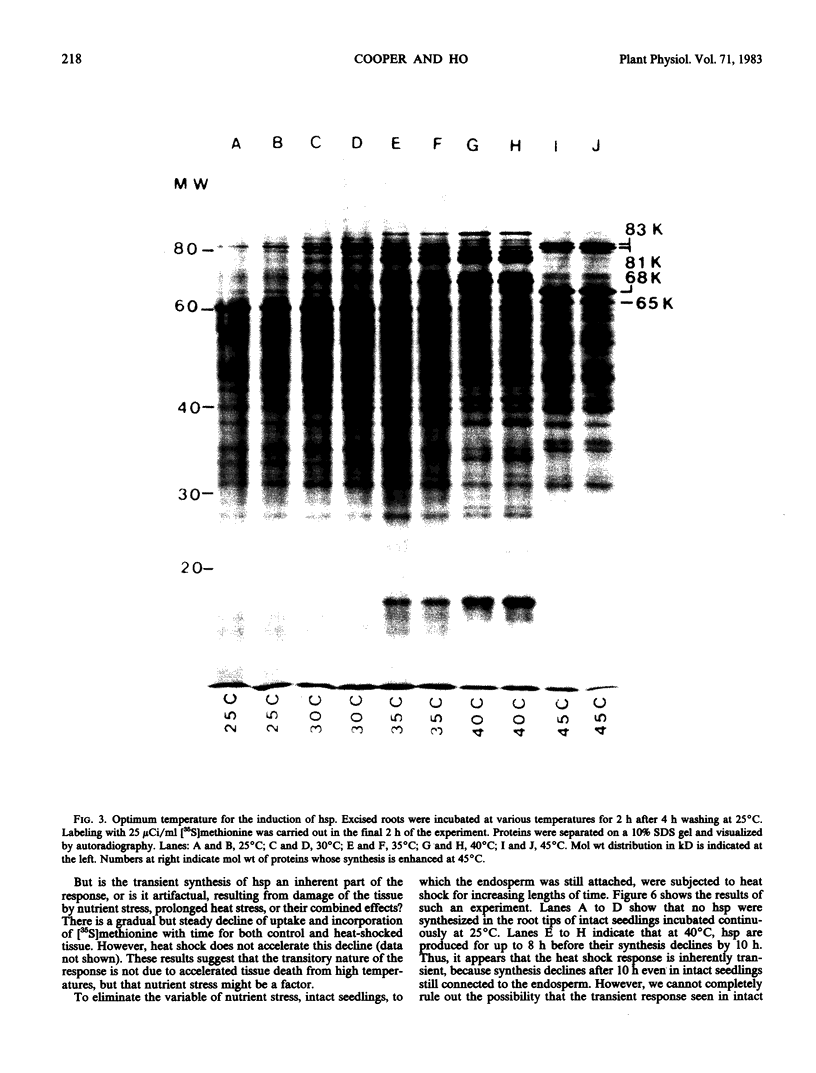
Shifting the temperature from 25 to 45°C results in a pattern of protein synthesis different from that observed after a shift to 40°C. Normal protein synthesis continues, except four proteins, which are produced in small amounts at lower temperatures, show greatly enhanced synthesis at 45°C. These proteins have apparent molecular weights of 83, 81, 68, and 65 kilodaltons. Also, the 10 hsp listed above are not synthesized. It is suggested that at least two distinct high-temperature responses are present in maize, which may reflect the metabolic changes generated at different elevated temperatures.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Dhindsa R. S., Bewley J. D. Plant desiccation: polysome loss not due to ribonuclease. Science. 1976 Jan 16;191(4223):181–182. doi: 10.1126/science.1246604. [DOI] [PubMed] [Google Scholar]
- Dhindsa R. S., Cleland R. E. Water Stress and Protein Synthesis: II. Interaction between Water Stress, Hydrostatic Pressure, and Abscisic Acid on the Pattern of Protein Synthesis in Avena Coleoptiles. Plant Physiol. 1975 Apr;55(4):782–785. doi: 10.1104/pp.55.4.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhindsa R. S., Cleland R. E. Water stress and protein synthesis: I. Differential inhibition of protein synthesis. Plant Physiol. 1975 Apr;55(4):778–781. doi: 10.1104/pp.55.4.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. Antibodies to two major chicken heat shock proteins cross-react with similar proteins in widely divergent species. Mol Cell Biol. 1982 Mar;2(3):267–274. doi: 10.1128/mcb.2.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell. 1978 Dec;15(4):1277–1286. doi: 10.1016/0092-8674(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Key J. L., Lin C. Y., Chen Y. M. Heat shock proteins of higher plants. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3526–3530. doi: 10.1073/pnas.78.6.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S. Regulation of protein synthesis during heat shock. Nature. 1981 Sep 24;293(5830):311–314. doi: 10.1038/293311a0. [DOI] [PubMed] [Google Scholar]
- Lindquist S. Varying patterns of protein synthesis in Drosophila during heat shock: implications for regulation. Dev Biol. 1980 Jun 15;77(2):463–479. doi: 10.1016/0012-1606(80)90488-1. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Wheeler S. Heat shock response of Dictyostelium. Dev Biol. 1980 Oct;79(2):399–408. doi: 10.1016/0012-1606(80)90125-6. [DOI] [PubMed] [Google Scholar]
- MANS R. J., NOVELLI G. D. A convenient, rapid and sensitive method for measuring the incorporation of radioactive amino acids into protein. Biochem Biophys Res Commun. 1960 Nov;3:540–543. doi: 10.1016/0006-291x(60)90171-6. [DOI] [PubMed] [Google Scholar]
- Morilla C. A., Boyer J. S., Hageman R. H. Nitrate Reductase Activity and Polyribosomal Content of Corn (Zea mays L.) Having Low Leaf Water Potentials. Plant Physiol. 1973 May;51(5):817–824. doi: 10.1104/pp.51.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Slater A., Cato A. C., Sillar G. M., Kioussis J., Burdon R. H. The pattern of protein synthesis induced by heat shock of HeLa cells. Eur J Biochem. 1981 Jul;117(2):341–346. doi: 10.1111/j.1432-1033.1981.tb06343.x. [DOI] [PubMed] [Google Scholar]
- Velazquez J. M., DiDomenico B. J., Lindquist S. Intracellular localization of heat shock proteins in Drosophila. Cell. 1980 Jul;20(3):679–689. doi: 10.1016/0092-8674(80)90314-1. [DOI] [PubMed] [Google Scholar]
- Yamamori T., Ito K., Nakamura Y., Yura T. Transient regulation of protein synthesis in Escherichia coli upon shift-up of growth temperature. J Bacteriol. 1978 Jun;134(3):1133–1140. doi: 10.1128/jb.134.3.1133-1140.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]