Abstract
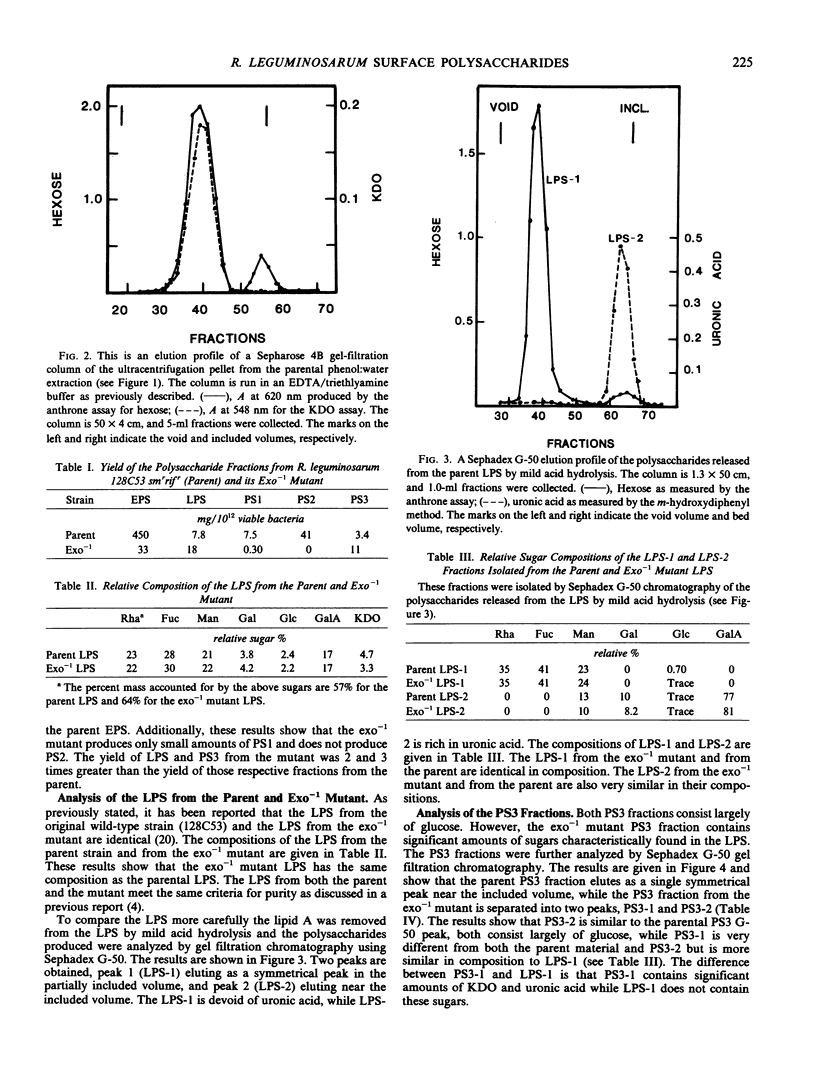
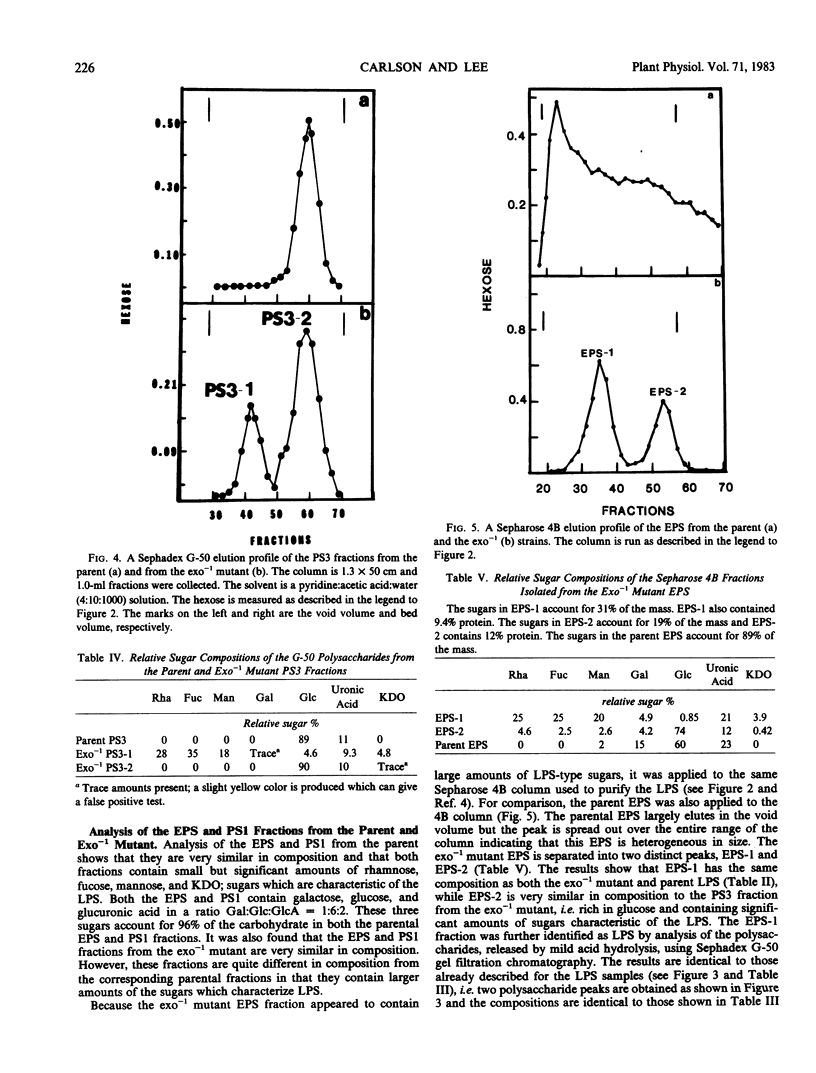
The surface polysaccharides of Rhizobium leguminosarum 128C53 smrrifr (parent) and its exo−1 mutant were isolated and characterized. The parent carries out normal symbiosis with its host, pea, while the exo−1 mutant does not nodulate the pea. The following observations were made. (a) The parent produces lipopolysaccharide (LPS), typical acidic extracellular polysaccharide (EPS), and three additional polysaccharides, PS1, PS2, and PS3. The PS1 and PS2 fractions are likely to be the capsular polysaccharide (CPS) and are identical in composition to the EPS. The PS3 fraction is a small-molecular-weight glucan. (b) The exo−1 mutant produces LPS, EPS, and a PS3 fraction, but does not produce significant amounts of either PS1 or PS2. The LPS from the exo−1 mutant appears to be identical to the parental LPS. Analysis of the EPS from exo−1 shows that it consists of two polysaccharides. One polysaccharide is identical to the LPS and comprises 70% of the exo−1 EPS. The second polysaccharide is identical to the exo−1 PS3 and comprises 30% of the exo−1 EPS. This result shows that the exo−1 mutant does not produce any of the typical acidic parental EPS and that the major polysaccharide released into the media by the exo−1 mutant is intact LPS. The exo−1 mutant PS3 fraction was found to contain two polysaccharides, PS3-1 and PS3-2. The PS3-2 polysaccharide is identical to the parental PS3 described above. The PS3-1 polysaccharide has a composition similar to the polysaccharide portion of the LPS. This result suggests that the exo−1 mutant produces LPS polysaccharide fragments. These LPS polysaccharide fragments are not produced by the parent strain.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Carlson R. W., Sanders R. E., Napoli C., Albersheim P. Host-Symbiont Interactions: III. Purification and Partial Characterization of Rhizobium Lipopolysaccharides. Plant Physiol. 1978 Dec;62(6):912–917. doi: 10.1104/pp.62.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Irvin R. T., Cheng K. J. The bacterial glycocalyx in nature and disease. Annu Rev Microbiol. 1981;35:299–324. doi: 10.1146/annurev.mi.35.100181.001503. [DOI] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Hrabak E. M., Urbano M. R., Dazzo F. B. Growth-phase-dependent immunodeterminants of Rhizobium trifolii lipopolysaccharide which bind trifoliin A, a white clover lectin. J Bacteriol. 1981 Nov;148(2):697–711. doi: 10.1128/jb.148.2.697-711.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann B., Reske K., Jann K. Heterogeneity of lipopolysaccharides. Analysis of polysaccharide chain lengths by sodium dodecylsulfate-polyacrylamide gel electrophoresis. Eur J Biochem. 1975 Dec 1;60(1):239–246. doi: 10.1111/j.1432-1033.1975.tb20996.x. [DOI] [PubMed] [Google Scholar]
- Mort A. J., Bauer W. D. Composition of the Capsular and Extracellular Polysaccharides of Rhizobium japonicum: CHANGES WITH CULTURE AGE AND CORRELATIONS WITH BINDING OF SOYBEAN SEED LECTIN TO THE BACTERIA . Plant Physiol. 1980 Jul;66(1):158–163. doi: 10.1104/pp.66.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Rick P. D. Size heterogeneity of Salmonella typhimurium lipopolysaccharides in outer membranes and culture supernatant membrane fragments. J Bacteriol. 1980 Nov;144(2):630–640. doi: 10.1128/jb.144.2.630-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C., Albersheim P. Rhizobium leguminosarum mutants incapable of normal extracellular polysaccharide production. J Bacteriol. 1980 Mar;141(3):1454–1456. doi: 10.1128/jb.141.3.1454-1456.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Robertsen B. K., Aman P., Darvill A. G., McNeil M., Albersheim P. Host-Symbiont Interactions : V. THE STRUCTURE OF ACIDIC EXTRACELLULAR POLYSACCHARIDES SECRETED BY RHIZOBIUM LEGUMINOSARUM AND RHIZOBIUM TRIFOLII. Plant Physiol. 1981 Mar;67(3):389–400. doi: 10.1104/pp.67.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J. M., Conrad H. E. Structural heterogeneity in the lipopolysaccharide of Salmonella newington. Arch Biochem Biophys. 1974 Jun;162(2):530–535. doi: 10.1016/0003-9861(74)90213-6. [DOI] [PubMed] [Google Scholar]
- Taylor R. L., Conrad H. E. Stoichiometric depolymerization of polyuronides and glycosaminoglycuronans to monosaccharides following reduction of their carbodiimide-activated carboxyl groups. Biochemistry. 1972 Apr 11;11(8):1383–1388. doi: 10.1021/bi00758a009. [DOI] [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]
- York W. S., McNeil M., Darvill A. G., Albersheim P. Beta-2-linked glucans secreted by fast-growing species of Rhizobium. J Bacteriol. 1980 Apr;142(1):243–248. doi: 10.1128/jb.142.1.243-248.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zevenhuizen L. P., Scholten-Koerselman H. J. Surface carbohydrates of Rhizobium. I. Beta-1, 2-glucans. Antonie Van Leeuwenhoek. 1979;45(2):165–175. doi: 10.1007/BF00418581. [DOI] [PubMed] [Google Scholar]


