Abstract
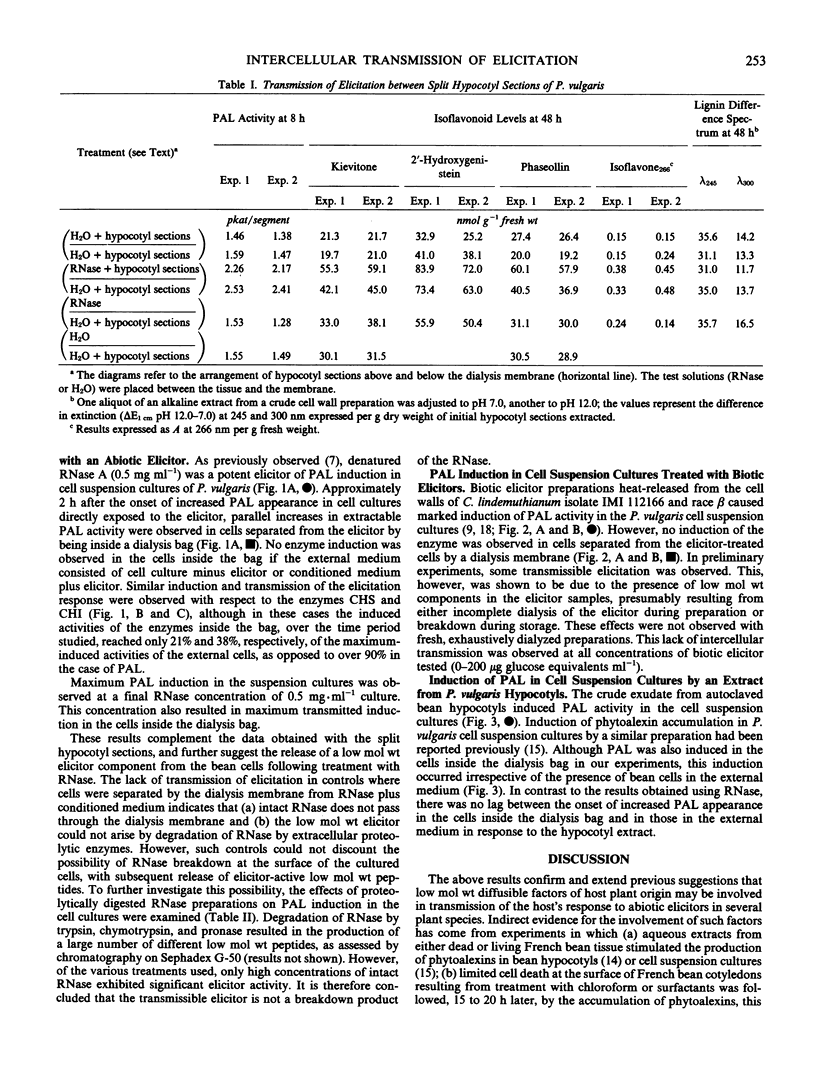
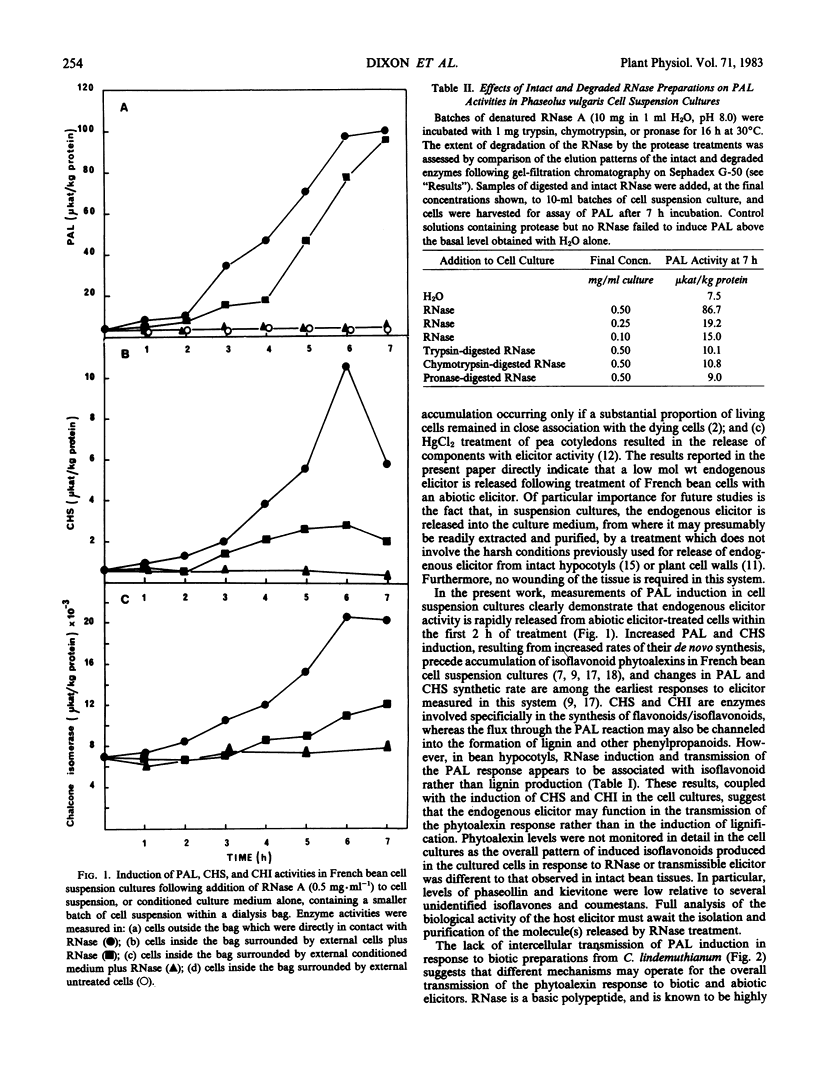
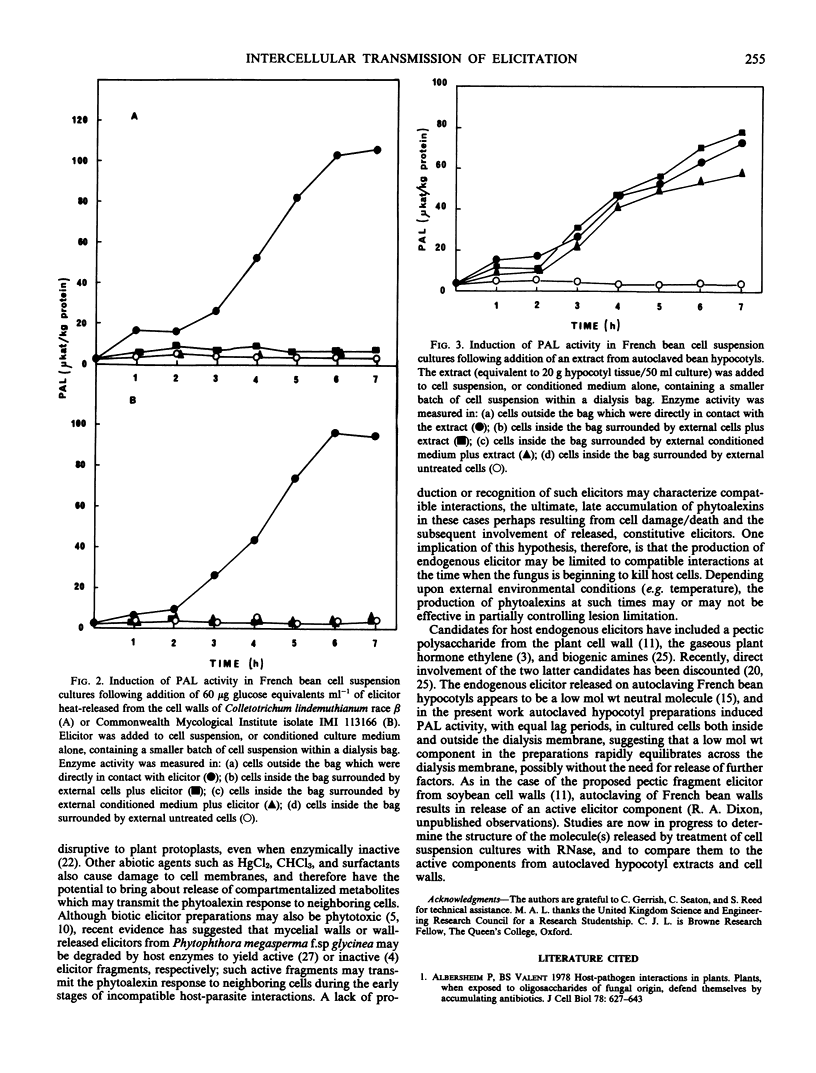
Treatment of hypocotyl sections or cell suspension cultures of dwarf French bean (Phaseolus vulgaris L.) with an abiotic elicitor (denatured ribonuclease A) resulted in increased extractable activity of the enzyme l-phenylalanine ammonia-lyase. This induction could be transmitted from treated cells through a dialysis membrane to cells which were not in direct contact with the elicitor. In hypocotyl sections, induction of isoflavonoid phytoalexin accumulation was also transmitted across a dialysis membrane, although levels of insoluble, lignin-like phenolic material remained unchanged in elicitor-treated and control sections. In bean cell suspension cultures, the induction of phenylalanine ammonia-lyase in cells separated from ribonuclease-treated cells by a dialysis membrane was also accompanied by increases in the activities of chalcone synthase and chalcone isomerase, two enzymes previously implicated in the phytoalexin defense response. Such intercellular transmission of elicitation did not occur in experiments with cells treated with a biotic elicitor preparation heat-released from the cell walls of the bean pathogen Colletotrichum lindemuthianum. The results confirm and extend previous suggestions that a low molecular weight, diffusible factor of host plant origin is involved (in French bean) in the intercellular transmission of the elicitation response to abiotic elicitors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albersheim P., Valent B. S. Host-pathogen interactions in plants. Plants, when exposed to oligosaccharides of fungal origin, defend themselves by accumulating antibiotics. J Cell Biol. 1978 Sep;78(3):627–643. doi: 10.1083/jcb.78.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalutz E., Devay J. E., Maxie E. C. Ethylene-induced Isocoumarin Formation in Carrot Root Tissue. Plant Physiol. 1969 Feb;44(2):235–241. doi: 10.1104/pp.44.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K., Albersheim P. Host-Pathogen Interactions : XVII. HYDROLYSIS OF BIOLOGICALLY ACTIVE FUNGAL GLUCANS BY ENZYMES ISOLATED FROM SOYBEAN CELLS. Plant Physiol. 1981 Jul;68(1):221–228. doi: 10.1104/pp.68.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A., Lamb C. J. Stimulation of de novo synthesis of L-phenylalanine ammonia-lyase in relation to phytoalexin accumulation in Colletotrichum lindemuthianum elicitor-treated cell suspension cultures of french bean (Phaseolus vulgaris). Biochim Biophys Acta. 1979 Sep 3;586(3):453–463. doi: 10.1016/0304-4165(79)90035-7. [DOI] [PubMed] [Google Scholar]
- Hahn M. G., Darvill A. G., Albersheim P. Host-Pathogen Interactions : XIX. THE ENDOGENOUS ELICITOR, A FRAGMENT OF A PLANT CELL WALL POLYSACCHARIDE THAT ELICITS PHYTOALEXIN ACCUMULATION IN SOYBEANS. Plant Physiol. 1981 Nov;68(5):1161–1169. doi: 10.1104/pp.68.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton M. A., Dixon R. A., Lamb C. J. Elicitor modulation of the turnover of L-phenylalanine ammonia-lyase in French bean cell suspension cultures. Biochim Biophys Acta. 1980 Dec 1;633(2):162–175. doi: 10.1016/0304-4165(80)90402-x. [DOI] [PubMed] [Google Scholar]
- Paradies I., Konze J. R., Elstner E. F. Ethylene: indicator but not inducer of phytoalexin synthesis in soybean. Plant Physiol. 1980 Dec;66(6):1106–1109. doi: 10.1104/pp.66.6.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruesink A. W. The plasma membrane of Avena coleoptile protoplasts. Plant Physiol. 1971 Feb;47(2):192–195. doi: 10.1104/pp.47.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C. A., Bishop P., Pearce G. A sycamore cell wall polysaccharide and a chemically related tomato leaf polysaccharide possess similar proteinase inhibitor-inducing activities. Plant Physiol. 1981 Sep;68(3):616–618. doi: 10.1104/pp.68.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford H. A. Differences Between Lignin-like Polymers Formed by Peroxidation of Eugenol and Ferulic Acid in Leaf Sections of Phleum. Plant Physiol. 1960 Jan;35(1):108–114. doi: 10.1104/pp.35.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M., Matama M., Masago H. Release of a Soluble Phytoalexin Elicitor from Mycelial Walls of Phytophthora megasperma var. sojae by Soybean Tissues. Plant Physiol. 1981 May;67(5):1032–1035. doi: 10.1104/pp.67.5.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]


