Abstract
Risk and predisposing factors for viral zoonoses abound in the sub-Saharan Africa (SSA) region with significant public health implications. For several decades, there have been several reports on the emergence and re-emergence of arbovirus infections. The lifetime burden of arboviral diseases in developing countries is still poorly understood. Studies indicate significant healthcare disruptions and economic losses attributed to the viruses in resource-poor communities marked by impairment in the performance of daily activities. Arboviruses have reportedly evolved survival strategies to aid their proliferation in favorable niches, further magnifying their public health relevance. However, there is poor knowledge about the viruses in the region. Thus, this review presents a survey of zoonotic arboviruses in SSA, the burden associated with their diseases, management of diseases as well as their prevention and control, mobility and determinants of infections, their vectors, and co-infection with various microorganisms. Lessons learned from the ongoing coronavirus disease 2019 (COVID-19) pandemic coupled with routine surveillance of zoonotic hosts for these viruses will improve our understanding of their evolution, their potential to cause a pandemic, control and prevention measures, and vaccine development.
Keywords: Arboviruses, vectors, transmission, sub-Saharan Africa, pandemic, epicentre, COVID-19
Introduction
Arboviruses are a complex group of RNA viruses capable of being transmitted to humans and other vertebrates via bites from arthropod vectors such as ticks, mosquitoes, lice, sand flies, and biting midges among others.1 Studies report that more than 100 species of arboviruses are present in most zoonotic diseases recorded in resource-poor settings like sub-Saharan Africa (SSA) and this region continues to bear the brunt of these diseases.2 Most zoonotic arboviruses belong to two major families: Flaviviridae and Togaviridae, as well as the order Bunyavirales, with their infections presenting with various symptoms such as hemorrhagic fever, polyarthralgia, encephalitis, and death in humans and animals. However, the majority of the arboviral infections are asymptomatic.3,4 Also, more families have demonstrated pathogenicity in humans, including the Reoviridae, Rhabdoviridae, and Orthomyxoviridae families.3
Worthy of note is the fact that most arbovirus-infected humans are often categorized as either incidental or dead-end hosts because they do not produce a high level of viremia capable of eliciting a host-vector-host transmission cycle. However, a few infections with dengue and chikungunya viruses are known to cause significant viremia capable of being transmitted to uninfected invertebrate hosts, which initiate the human-vector-human transmission cycle.1 It is now known that almost all mosquito-borne viruses isolated from Africa and recognized as zoonotic have gained intercontinental spread, making them a significant public health challenge. Furthermore, some of these viruses maintain sylvatic cycles with the capacity to infect humans and serve as “time bombs” awaiting future impact.5
The ecology of arboviruses is somewhat complex, including several reservoirs, bridging vectors, and amplifying hosts with the capacity to influence their transmission and potential spill-over into vertebrate hosts. Prior to their international emergence, these viruses were responsible for undetected diseases in Africa, spreading between vectors and vertebrate hosts and extending to sensitive species during climatic events causing severe diseases.4 Geographical dispersions of arboviral diseases are associated with anthropogenic activities and ecological factors. In other words, the abundance of vectors such as Aedes, Culex, Anopheles mosquitoes, rodents, Ixodes ticks and sand flies; forest dispersions, warm eco-climates, moorlands, and steep ecosystems considerably influence their transmission.6 Furthermore, animal reservoirs such as migratory birds, rodents, and nomadic livestock are present in large numbers, especially in areas where arboviral diseases have emerged.3,4 Thus, the availability and epidemiology of vectors, animal reservoirs, and favorable climates are considered significant determinants of arboviral disease outbreaks locally, regionally, and internationally.4
With coronavirus disease 2019 (COVID-19) SSA’s index case reported in Egypt in 2020, available data two years into the pandemic indicates a total of 11,386, 025 cases, 251,845 deaths, and 10,755,951 recoveries as of April 2022, making it the region with the least number of cases and deaths from COVID-19. However, these low figures are in line with the significantly low number of tests which stood at 104,427,090 tests for the same period, less than 20 percent of the total population in the region. The same region is also endemic to human immunodeficiency virus (HIV) and malaria, two infections for which effective cures are still being sought and can even lead to co-infection probably due to geographic overlap among other factors.7 Viral pandemic always seems to be a step ahead of science, thus preventing such epidemics or pandemics appears to be more effective than controlling their occurrence. The concerns and potential of a co-infection with COVID-19 is already reported in Pakistan8 and an increased incidence of arboviruses was documented by the World Health Organization (WHO) and placed at over 1.6 million cases in the first few weeks of 2020 alone in the WHO regions of America. This increase in the incidence of cases was attributable to dengue (97%), chikungunya (>2%), and zika (<1%) viruses.9 While the WHO report was not for SSA, the region is well-known for arboviral diseases that have emerged and re-emerged for several decades.9 There is increasing concern over the potential of co-infection between arboviruses and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which could further worsen control and vaccination efforts, stretch their already fragile and poorly-funded healthcare systems in SSA, and increase co-morbidities. This is an overview of zoonotic arboviruses in SSA, the disease burden, management, prevention and control, mobility, determinants, and vectors of infections, and the potential to co-infect with SARS-CoV-2 and other pandemic viruses.
Diversity of arboviruses in sub-Saharan Africa
First reported by the Rockefeller foundation between the 1930s and 1970, there are well over 100 species of arboviruses belonging to the Flaviviridae, Togaviridae, and Reoviridae families as well as members of Bunyavirales order.2,4 Commonly implicated families include those in the Flaviviridae and Togaviridae families and the order Bunyavirales. The following section highlights the most important arboviruses and their basic biological properties.
Family Togaviridae
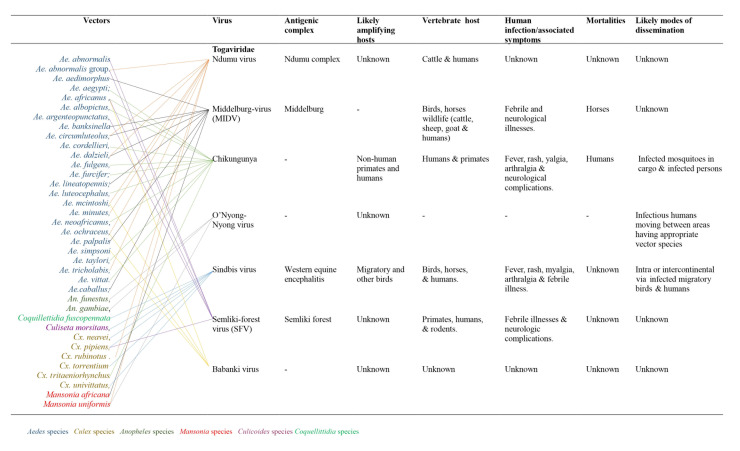
The Togaviridae is a family of small, enveloped viruses with single-stranded, positive-sense RNA genomes of 10-12 kb. This family comprises two genera: Alphavirus and Rubivirus.10 Within the family, the genus Alphavirus includes many diverse species, while the genus Rubivirus contains a single species: the rubella virus.10 The majority of the zoonotic arthropod-borne viruses belonging to the genus Alphavirus have about 30 documented species that exist in well-defined geographical areas, especially where malaria-carrying mosquitoes abound.4 Most alphaviruses which are mosquito-borne are pathogenic to their vertebrate hosts. As a family of enveloped, single-stranded positive RNA viruses, arboviruses are less clustered than flaviviruses, with Culex, Aedes, and Anopheles mosquito species being the most significant vectors.5 Many are significant human and veterinary pathogens, such as the chikungunya virus and eastern equine encephalitis virus. Rubella virus is transmitted through the respiratory tract among humans. Currently, seven viruses from this family are known. The likely amplifying hosts and modes of dissemination are unknown for Ndumu, Middleburg, Semliki virus, and Babanki viruses. Furthermore, the sub-genomic promoters and dual polyproteins in this group of arboviruses are associated with influencing rapid mutations and frequent changes in vectors and hosts, thus enhancing rapid genetic recombination and spread of the virus11 (Figure 1). Figure 1 shows the various viruses, their vectors, antigenic complexes, likely amplifying hosts, vertebrate hosts, human infections and associated mortalities, and likely modes of dissemination of Togaviridae.
Figure 1. Viral hosts, mobility and transmission of Togaviruses.
Family Flaviviridae
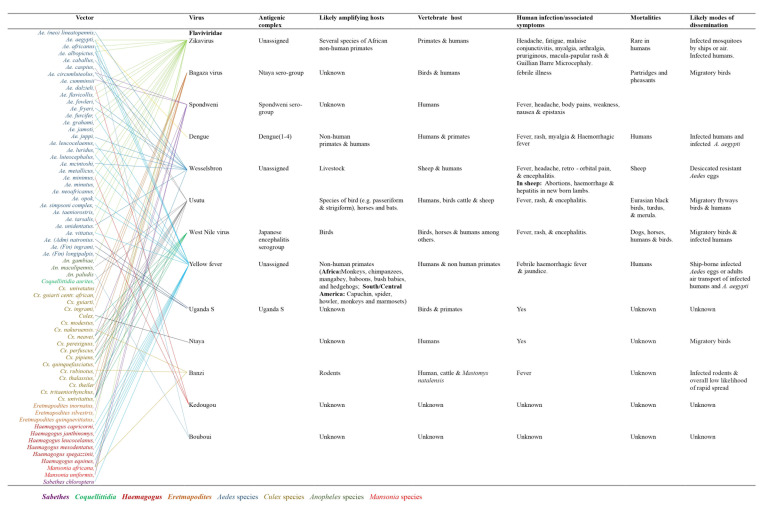
Figure 2 illustrates the biology of Flaviviridae. This family of zoonotic arboviruses is grouped into distinct clusters ranging from non-vectored, unknown vector, tick-borne, and mosquito-borne viruses.4, 5 It is a large family with at least 13 described viruses. This group of viruses is pathogenic to humans and animals. Evidence shows Uganda S, Ntaya, Kedougou, Banzi, Nairobi sheep, and Bouboui viruses have unknown mortalities. Flaviviruses are enveloped single-stranded positive-sense RNA viruses with a genome size of 11 kb.3 Members of this family possess a genome that encodes three significant structural proteins: capsid, pre-membrane, and envelope proteins and seven non-structural proteins. These viruses are known to be pathogenic to animals and humans.12 Figure 2 shows the various viruses, their vectors, antigenic complexes, likely amplifying hosts, vertebrate hosts, human infections and associated mortalities, and likely modes of dissemination.
Figure 2. Viral hosts, mobility and transmission of Flaviviruses.
Order Bunyavirales
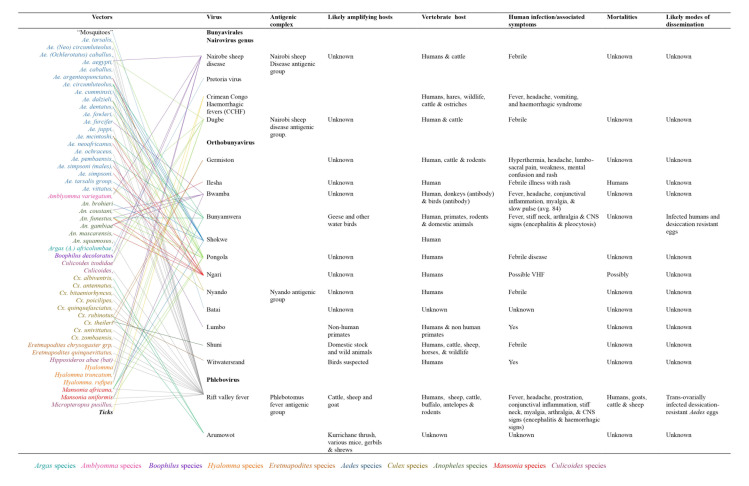
Bunyavirales is a large order comprising a cluster of twelve families with more than 300 clinically significant viruses.4-5 Viruses which induce hemorrhagic fevers belong to the families: Hantaviridae, Phenuiviridae, Arenaviridae, Nairoviridae and Peribunyaviridae with the latter four identified as arthropod-borne and majorly transmitted by vectors including mosquitoes, ticks, and sand flies while the fourth family (Hantaviridae) is non-arthropod-borne.4-5,13 Studies suggest that viruses in the Bunyavirales order originate from arthropods due to their deep node association.10 Their mortality and modes of dissemination are still unknown or elusive. Except for hantavirus, a genus of viruses transmitted by rodents, members of the order Bunyavirales are transmitted by arthropods, particularly mosquitoes, ticks, phlebotomines and biting midges. Although most of these viruses infect vertebrate hosts, including laboratory animals (hamsters and rats), more commonly used for virus isolation, only a few are responsible for zoonotic infection. The most common of these is the Rift Valley fever (RVF) virus
transmitted by mosquitoes to animals in SSA.14 Most of the other viruses have a common tropism for fetal tissues and cause embryonic and fetal death, stillbirths and multiple congenital malformations. These viruses belong to different families, genera and serogroups and are extensive. Among bunyaviruses, Akabane from the Simbu serogroup situate in Australia, Asia, and the Middle East, and Cache Valley from the Bunyamwera virus serogroup is present in North America.14 Among members of the family Nairoviridae, Nairobi sheep virus circulates in East Africa and India. In addition to these viruses, Crimean-Congo hemorrhagic fever virus infects domestic animals from which humans become infected.15-17 This virus is widespread throughout Africa, the Middle East, southern Europe, and Asia, from livestock to birds such as ostriches where they cause asymptomatic infection.16, 18 Figure 3 shows the various viruses, their vectors, antigenic complexes, likely amplifying hosts, vertebrate hosts, human infections and associated mortalities, and likely modes of dissemination.
Figure 3. Viral hosts, mobility and transmission of Bunyaviruses.
Arthropod vectors associated with arboviruses mobility and distribution
Arboviruses are known to have evolved a long-term survival strategy. One such strategy is their ability to utilize a wide range of arthropod vectors globally, with common ones being ticks and mosquitoes.2 The diversity and widespread distribution of arthropod species greatly influence the rapid global spread of arboviruses.3,12,19 According to research, an estimated 300 species of mosquitoes harbor arboviruses.2,3,12 Studies report that ticks are the most prevalent arboviral vectors, with about 116 species currently known to transmit arboviruses.2 There is evidence linking arbovirus-associated diseases with specific vectors, as is the case during epidemics.2 However, in cases where the availability of specific hosts is limited, the vectors may utilize available hosts to continue their transmission cycle. These vectors are shown in Figures 1 to 3 for the various families of the arboviruses.
Although a few of the arboviruses in temperate regions spread among wildlife species, the majority of arboviruses gravely implicated in animal and human diseases in the tropics and sub-tropics have circulated basically where arthropod vectors are in abundance.2, 20 This implies that the nature, type, species, and the number of specific arthropods in a region determines the type and nature (sporadic, endemic, or epidemic) of the prevalence of arboviruses.
The distribution of arboviruses in sub-Saharan Africa
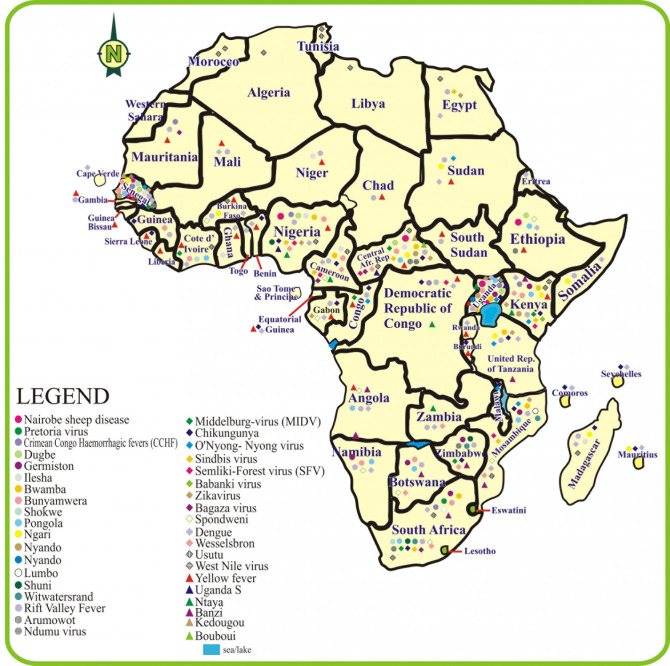
Figure 4 illustrates the distribution of arboviruses showing a wide distribution in SSA. A closer look at the distribution of the viruses indicates that the West African and South African regions have the highest spread or distribution of the virus.
Figure 4. Distribution of arboviruses in sub-Saharan Africa.
Survival potentials of arboviruses
Arboviruses have evolved a series of potentials to ensure their successful long-term survival and dispersal. This significantly reflects their environmental preferences.2 First, the arboviruses survive by maintaining a sylvatic cycle once an epidemic runs its course, sometimes for years, and remain dormant until favorable conditions ensue. A second strategy involves non-viremic transmission in which infected and non-infected arthropods such as ticks feed on small animals in the wild, further enhancing the long-term survival of the viruses. This is possible because it influences the direct transmission of the viruses between these insects.21 Several studies noted the horizontal transfer of viral genome into susceptible hosts (arthropods).2,22-25 Other studies highlight the desiccation of resistant eggs by some arthropod vectors as a crucial survival factor.26 Viral mutation via genome segment recombination or reassortment is reported to not only enhance their survival and efficient transmission by previously inefficient vectors but also leads to the emergence of new viruses with unique virulence and pathological implications.27 In addition to the viral mutation, vector mutation and adaptation have reportedly enhanced the survival and improved their involvement in the spread of arboviruses.5 The survival potentials collectively and individually exhibited by these viruses ensure their survival and possible re-emergence, causing severe epidemics in animals and humans.2
Determinants of arbovirus emergence and transmission
The ability to acquire, maintain and transmit a virus (vector competence) by a vector is a complex phenomenon between the pathogen and the vector. Intrinsic and extrinsic factors influence this phenomenon.28 Studies report that in addition to the virus and the vector, there is a need for appropriate virus replicating hosts such as birds and primates to ensure the perpetuation of the virus cycle.5 Similarly, vector density, survivability, and host density influence the ability of vectors.29 In addition to rapidly adapting to hosts (arthropods, humans, and non-human vertebrates), arboviruses are known for their transmission efficiency, antigenicity, environmental/ecological conditions adaptation, and alteration of receptor specificity. Climate change significantly influences the occurrence of mutation, and human activities are significant determinants of the emergence of arboviruses.2 The reservoir for arboviruses in wild species impedes the control of its emergence. Arthropods transmit arboviruses to humans and vertebrate animals, causing significant mortality. Studies reveal that trans-generational vertical transmission has also been reported among vector species, aiding the transfer of arboviruses from adult vectors to their offsprings.30-33
Most infected arthropod vectors (in varying developmental stages) are dispersed via human travels.34 In addition, more engagements in tourism, humanitarian services, pilgrimages, host density, displaced refugees from arthropod-endemic regions (including Africa, Asia, and the Pacific), import demands, and improved world trade have significantly broadened the global distribution of arboviruses.5 Studies further reveal that most arthropods exhibit significant competitive potential upon arrival, aiding them in successfully establishing a stronghold and dispersing.5,34 In similar studies,35-36 dangers associated with regional movements of livestock are linked to the distribution of most arthropods incriminated in arboviral diseases.
Burden associated with arbovirus-related diseases in sub-Saharan Africa
The burden associated with arboviruses correlates with the occurrence and distribution of arthropod vectors, especially in SSA.5 This is because SSA is home to all types and categories of vectors especially the arthropod vectors, due to the abundance of tropical and sub-tropical climates.2,4,5 Similarly, as a result of lack of adequate facilities and diagnostic tools to aid the evaluation of arbovirus-related diseases in the region, there is an increased risk of misdiagnosis, further limiting the accurate estimation of disease burden in SSA. More complicating is the lack of knowledge of the existence of arbovirus-related diseases in SSA further militating against their isolation. As revealed in a study,37 arboviral infections contribute to a significant proportion of debilitating fever syndromes. Symptoms ranging from asymptomatic infections to severe undifferentiated fever are associated with these viruses in their acute phase.2,4-5
However, a few have been associated with complications comprising meningitis, hemorrhage, encephalitis, which result in long term physical and cognitive impairment and even death.2,4-36,37 Studies show that approximately 100 arboviruses observed to cause diseases in humans and transmitted via varying routes are characterized by their ability to cause encephalitis and/or hemorrhagic fever.37 Currently, more serious and severe cases occur as the viruses spread to new areas. These cases lead to post-infectious, long-term complications such as neurologic, ophthalmologic, and mental impairment, among others.38 The incidence of arbovirus co-infections with diseases including malaria in SSA has further questioned the authenticity of cerebral sequelae due to malaria when almost all the reported arboviruses are capable of inducing neurologic and mental complications in susceptible hosts.
As evident in tropical diseases, a study notes that most minor and disabling complications associated with arboviruses affect resource-poor communities.37 These complications impair individuals’ daily activities and could be chronic depending on the nature of the virus and the available susceptible host. Studies further reveal that the lifetime burden of these diseases, especially in developing countries, is poorly understood due to a lack of long-term longitudinal childhood impact studies of arboviral diseases.37 Clinical data reveal significant healthcare challenges and economic losses associated with arboviral diseases.5,37-38 It is worth noting that only the disease burdens of dengue and Japanese encephalitis are currently in the WHO global burden of disease estimates. The implication is that any significant long-term related morbidity associated with arboviruses other than those captured by WHO remains a substantial health deficit.37 In addition, as the world becomes more globalized, vectors and viruses have continually evolved, making their diagnosis, treatment, and prevention even more challenging.33
Management, prevention, and control of arboviral diseases
There is evidence that arboviral-related infections often occur in epidemics and have pandemic potential.2,5 Adequate planning and routine surveillance of acute-chronic, case-fatality, incidence as well as conversion rates from clinical cases may help reduce the severity of diseases associated with these viruses and help employ preventive approaches.2,5,34 Due to the outbreak potential associated with these viruses, vaccine development for arboviruses, either singly or as a group, remains a necessity in ameliorating incidences and disease burden. Developing therapeutic drugs and vaccines to treat arboviral diseases and simplifying the procedures for establishing the safety and efficacy of antivirals may reduce the risk of arboviral diseases in animals and humans.
Due to a lack of specific treatment for arboviral infections, several studies suggest continuously evolving vector control strategies and public health surveillance for arboviral-related infections as significant steps toward preventing these diseases.2,37 Experimental vector eradication and vaccine development can effectively mitigate the arboviral-related infections in SSA.37 Similarly, utilizing unified vector-controlled strategies that will ensure the survival of wildlife species is the right step in the right direction. Practical procedures to mitigate the risks associated with arthropods may be most effective in reducing the transmission and spread of arboviral-related infections. One way to achieve this is by implementing localized arthropod control measures, especially during epidemics.2,5
The development of training and research programs that will identify the occurrence, pathogenicity, evolution, epidemiology, and disease burden associated with arboviral diseases is also important.2 In addition, promoting and strengthening levels of cooperation between governments, academic institutions, and drug/vaccine development companies will significantly contribute to the eradication of arboviruses. Programmes to educate citizens on local preventive measures, monitoring and evaluating at the community level, border, harbor, airports, and hamlets, among others, may reduce the influx of arboviral vectors to new regions.2,38
Co-infection with SARS-CoV-2
There is evidence that HIV and Ebola virus are not just zoonotic but originated from Africa with significant public health implications.39,40 Furthermore, SARS-CoV-2 has also joined the group of emerging pathogens.40 Although the COVID-19 response has been effective with the arrival of vaccines in less than a year since the pandemic began,7,40 other infections are yet to get a vaccine years after they emerged. Due to the geographical overlap of this infection endemicity, there is a greater risk of co-infections, as evident in HIV and malaria.7 The COVID-19 pandemic exposed the weakness of existing healthcare infrastructures in SSA. With the potential of COVID-19 co-infecting with arboviruses, already reported in Pakistan, there is a pressing need for health policy makers across SSA to take a cue from the COVID-19 response. Throughout SSA, the health systems are inadequate due to a lack of trained staff, infrastructure deficits, facilities, and funding among others. There is a need to consciously build up capacity to avoid co-infection in the region.
Conclusions
Arthropod vectors and arboviruses are endemic in sub-Saharan Africa (SSA). Although most of these viruses do not infect humans, a few have jumped their sylvatic cycles due to several factors that have influenced their effective dissemination to many regions with significant public health implications. Arboviruses have evolved survival strategies creating new favorable niches in the process. These viruses present with disabling syndromes with significant personal and health burdens and losses, yet the lifetime burden of arboviral diseases in developing countries and regions is still poorly understood. Given the potential to lead the next pandemic, there is a need for more studies aimed at the prediction, prevention, and control of arbovirus-related diseases.
Footnotes
Author contributions: ENM and UOE conceived the idea, performed literature searches and drafted the manuscript. HUO, FON, AEE, AO, MC read, revised and approved the final draft. All authors read and approved the final version of the manuscript.
Conflicts of interest: All authors – none to declare.
Funding: None to declare.
Acknowledgement: All the help received is hereby duly acknowledged.
References
- 1.García-Sastre A, Endy TP. Arboviruses. In: Schaechter M, editor. Encyclopedia of microbiology. Oxford: Academic Press; 2009. pp. 313–21. [DOI] [Google Scholar]
- 2.Liang P, Xu Y, Zhang X, et al. CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell. 2015;5:363–72. doi: 10.1007/s13238-015-0153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go YY, Balasuriya UB, Lee CK. Zoonotic encephalitides caused by arboviruses: transmission and epidemiology of alphaviruses and flaviviruses. Clin Exp Vaccine Res. 2014;3:58–77. doi: 10.7774/cevr.2014.3.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Venter M. Assessing the zoonotic potential of arboviruses of African origin. Curr Opin Virol. 2018;28:74–84. doi: 10.1016/j.coviro.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Braack L, Gouveia de Almeida AP, Cornel AJ, Swanepoel R, de Jager C. Mosquito-borne arboviruses of African origin: review of key viruses and vectors. Parasit Vectors. 2018;11:29. doi: 10.1186/s13071-017-2559-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farajollahi A, Fonseca DM, Kramer LD, Kilpatrick AM. “Bird biting” mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect Genet Evol. 2011;11:1577–85. doi: 10.1016/j.meegid.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edet UO, Ebana RUB, Etok CA, Nwamuo LC. Prevalence of human immunodeficiency virus and Plasmodium falciparum dual infection amongst residents of Kaduna South in North Western Nigeria. Int J Trop Dis Health. 2016;17:1–7. doi: 10.9734/IJTDH/2016/25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butt AS. Understanding the implications of pandemic outbreaks on supply chains: an exploratory study of the effects caused by the COVID-19 across four South Asian countries and steps taken by firms to address the disruption. Int J Phys Distrib Logist Manage. 2022;52:370–92. doi: 10.1108/IJPDLM-08-2020-0281. [DOI] [Google Scholar]
- 9.Adams LE, Martin SW, Lindsey NP, et al. Epidemiology of dengue, chikungunya, and Zika virus disease in US States and Territories, 2017. Am J Trop Med Hyg. 2019;101:884–90. doi: 10.4269/ajtmh.19-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Contigiani MS, Diaz LA. Togaviridae. In: Marcondes C, editor. Arthropod borne diseases. Cham: Springer; 2017. pp. 115–35. [DOI] [Google Scholar]
- 11.Marklewitz M, Junglen S. Evolutionary and ecological insights into the emergence of arthropod-borne viruses. Acta Trop. 2019;190:52–8. doi: 10.1016/j.actatropica.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Simmonds P, Becher P, Bukh J, et al. ICTV virus taxonomy profile: Flaviviridae. J Gen Virol. 2017;98:2–3. doi: 10.1099/jgv.0.000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horne KM, Vanlandingham DL. Bunyavirus-vector interactions. Viruses. 2014;6:4373–97. doi: 10.3390/v6114373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Res. 2010;85:328–45. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adelman ZN, Miller DM, Myles KM. Bed bugs and infectious disease: a case for the arboviruses. PLoS Path. 2013;9:e1003462. doi: 10.1371/journal.ppat.1003462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Briese T, Calisher CH, Higgs S. Viruses of the family Bunyaviridae: are all available isolates reassortants? Virol. 2013;446:207–16. doi: 10.1016/j.virol.2013.07.030. [DOI] [PubMed] [Google Scholar]
- 17.Shayan S, Bokaean M, Shahrivar MR, Chinikar S. Crimean-Congo hemorrhagic fever. Lab Med. 2015;46:180–9. doi: 10.1309/LMN1P2FRZ7BKZSCO. [DOI] [PubMed] [Google Scholar]
- 18.Zeller H, Bouloy M. Bunyaviridae and Filoviridae. Rev Sci Tech. 2000;19:79–91. doi: 10.20506/rst.19.1.1208. [DOI] [PubMed] [Google Scholar]
- 19.Ostfeld RS, Keesing F. Effects of host diversity on infectious disease. Ann Rev Ecol Evol Syst. 2012;43:157–82. doi: 10.1146/annurev-ecolsys-102710-145022. [DOI] [Google Scholar]
- 20.Campbell TA, VerCauteren KC. Diseases and parasites [of white-tailed deer] In: Hewitt DG, editor. Biology and management of white-tailed deer. Boca Raton: CRC Press; 2011. pp. 232–63. [DOI] [Google Scholar]
- 21.Slovák M, Kazimírová M, Siebenstichová M, et al. Survival dynamics of tick-borne encephalitis virus in Ixodes ricinus ticks. Ticks Tick Borne Dis. 2014;5:962–9. doi: 10.1016/j.ttbdis.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 22.Villinger J, Mbaya MK, Ouso D, Kipanga PN, Lutomiah J, Masiga DK. Arbovirus and insect‐specific virus discovery in Kenya by novel six genera multiplex high‐resolution melting analysis. Mol Ecol Resour. 2017;17:466–80. doi: 10.1111/1755-0998.12584. [DOI] [PubMed] [Google Scholar]
- 23.Farfan-Ale JA, Loroño-Pino MA, Garcia-Rejon JE, et al. Detection of RNA from a novel West Nile-like virus and high prevalence of an insect-specific flavivirus in mosquitoes in the Yucatan Peninsula of Mexico. Am J Trop Med Hyg. 2009;80:85–95. doi: 10.4269/ajtmh.2009.80.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aranda C, Sánchez-Seco MP, Cáceres F, et al. Detection and monitoring of mosquito flaviviruses in Spain between 2001 and 2005. Vector Borne Zoonotic Dis. 2009;9:171–8. doi: 10.1089/vbz.2008.0073. [DOI] [PubMed] [Google Scholar]
- 25.Sánchez-Seco MP, Vázquez A, Collao X, et al. Surveillance of arboviruses in Spanish wetlands: detection of new flavi-and phleboviruses. Vector Borne Zoonotic Dis. 2010;10:203–6. doi: 10.1089/vbz.2008.0188. [DOI] [PubMed] [Google Scholar]
- 26.Russell RC. Ross River virus: ecology and distribution. Annu Rev Entomol. 2002;47:1–31. doi: 10.1146/annurev.ento.47.091201.145100. [DOI] [PubMed] [Google Scholar]
- 27.Elliott RM. Bunyaviruses and climate change. Clin Microbiol Infect. 2009;15:510–7. doi: 10.1111/j.1469-0691.2009.02849.x. [DOI] [PubMed] [Google Scholar]
- 28.Goddard J, deShazo R. Bed bugs (Cimex lectularius) and clinical consequences of their bites. JAMA. 2009;301:1358–66. doi: 10.1001/jama.2009.405. [DOI] [PubMed] [Google Scholar]
- 29.Brustolin M, Talavera S, Santamaría C, et al. Culex pipiens and S tegomyia albopicta (= Aedes albopictus) populations as vectors for lineage 1 and 2 West Nile virus in Europe. Med Vet Entomol. 2016;30:166–73. doi: 10.1111/mve.12164. [DOI] [PubMed] [Google Scholar]
- 30.Martins VE, Alencar CH, Kamimura MT, et al. Occurrence of natural vertical transmission of dengue-2 and dengue-3 viruses in Aedes aegypti and Aedes albopictus in Fortaleza, Ceará, Brazil. PLoS One. 2012;7:e41386. doi: 10.1371/journal.pone.0041386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grunnill M, Boots M. How important is vertical transmission of dengue viruses by mosquitoes (Diptera: Culicidae)? J Med Entomol. 2016;53:1–9. doi: 10.1093/jme/tjv168. [DOI] [PubMed] [Google Scholar]
- 32.Chompoosri J, Thavara U, Tawatsin A, et al. Vertical transmission of Indian Ocean Lineage of chikungunya virus in Aedes aegypti and Aedes albopictus mosquitoes. Parasit Vectors. 2016;9:227. doi: 10.1186/s13071-016-1505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh RK, Dhama K, Malik YS, et al. Zika virus-emergence, evolution, pathology, diagnosis, and control: current global scenario and future perspectives-a comprehensive review. Vet Q. 2016;36:150–75. doi: 10.1080/01652176.2016.1188333. [DOI] [PubMed] [Google Scholar]
- 34.Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett. 2005;8:558–74. doi: 10.1111/j.1461-0248.2005.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pepin M, Bouloy M, Bird BH, Kemp A, Paweska J. Rift Valley fever virus (Bunyaviridae: Phlebovirus): an update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet Res. 2010;41:61. doi: 10.1051/vetres/2010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franz E, van Hoek AH, Wuite M, et al. Molecular hazard identification of non-O157 Shiga toxin-producing Escherichia coli (STEC) PLoS One. 2015;10:e0120353. doi: 10.1371/journal.pone.0120353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Labeaud AD, Bashir F, King CH. Measuring the burden of arboviral diseases: the spectrum of morbidity and mortality from four prevalent infections. Popul Health Metr. 2011;9:1. doi: 10.1186/1478-7954-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marimoutou C, Vivier E, Oliver M, Boutin JP, Simon F. Morbidity and impaired quality of life 30 months after chikungunya infection: comparative cohort of infected and uninfected French military policemen in Reunion Island. Medicine (Baltimore) 2012;91:212–9. doi: 10.1097/MD.0b013e318260b604. [DOI] [PubMed] [Google Scholar]
- 39.Sharp PM, Hahn BH. Origins of HIV and the AIDS pandemic. Cold Spring Harb Perspect Med. 2011;1:a006841. doi: 10.1101/cshperspect.a006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aborode AT, David KB, Uwishema O, et al. Fighting COVID-19 at the expense of malaria in Africa: the consequences and policy options. Am J Trop Med Hyg. 2021;104:26–9. doi: 10.4269/ajtmh.20-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]