Abstract
Introduction
Diarrheal diseases have existed since antiquity, resulting in high morbidity and mortality rates, particularly among children in developing countries. To eradicate these diseases, it is crucial to identify the pathogens that cause them and immediately initiate appropriate treatment. This retrospective study aims to investigate the incidence of childhood gastroenteritis and the epidemiological features of its causative agents.
Methods
During an 11-year period (2010–2020), as many as 51159 stool samples were obtained from children aged 0-17 years. These samples were examined for the presence of parasitic, bacterial, and/or viral gastroenteritis agents and evaluated retrospectively. The records obtained from the "ENLIL Hospital Information Management System Modules" were used to collect patient-related information.
Results
The most frequently observed pathogens were rotavirus (22.4%), adenovirus (2.2), Giardia lamblia (1.6%), and Campylobacter spp. (3.0%), considering the number of samples examined for each organism. The total incidence of viruses was about 25%, parasites 5% and the rate of pathogenic bacteria was 2%. In one-way ANOVA analysis, pathogen positivity was found to be significantly higher in children aged 3-5 years compared to those aged 15-17 and 0-2 [F (5, 51153, 17,588, p<0.001)]. The highest demand for the investigation of GE factors from stool samples was made in August, September and July. According to the number of samples examined, the highest pathogen positivity was in February, October, May, December and March, respectively. The most common pathogens involved in coinfections, occurring in 0.04% of the studied cases, were rotavirus and Giardia lamblia.
Conclusions
Parasitic, viral, and bacterial gastroenteritis maintain their current status with a high prevalence in children under 18 years of age, especially in children aged 0-4 years in Erzurum, Turkey.
Keywords: Children, diarrhea, intestinal pathogens, Turkey
Introduction
Although microbial gastrointestinal diseases affect people of all ages, they particularly increase morbidity and mortality in infants and children.1 The incidences of these diseases are high in developing countries, but their effects are also felt in developed countries.2 Diarrhea-related illnesses are reported to be the fifth leading cause of death in children.3 Reported to cause more than 200,000 child deaths worldwide each year, viruses are the most common among childhood gastroenteritis (GE) agents, followed by bacteria and then parasites.4 In Germany, for example, viral pathogens were reported to be responsible for 93% of GEs in children under five years of age.5 Bacterial enteric infections are usually transmitted to humans by food-borne origin. Research has found that the actual number of bacterial gastroenteritis cases in the community is several times higher than the number of recorded cases. Parasites, like bacteria, can invade the intestinal mucosa and cause inflammation of the mucosa and diarrhea.6 Poor sanitation and inadequate personal hygiene are the biggest factors behind the spread of intestinal parasites that cause GE.7 Most parasite-related infections and deaths occur in developing countries, and they result in significant economic loss.8
According to data from the Turkish Statistical Institute (TUIK), in 2019, the mortality rate from any cause in children under five years of age in Turkey was 11.2 per thousand; in Erzurum, the rate was 12.5 per thousand.9 TUIK reports that diarrheal diseases are the most common health problem after upper respiratory tract infections in children of all age groups in Turkey. The fact that child mortality in Erzurum is higher than Turkey’s average has been an important reason for conducting this study. In addition, it is important to determine the etiology of GE in order to determine the role of enteric pathogens in epidemics, develop necessary diagnostic methods, prevent diseases, and understand their epidemiological importance. Because of these things, this study looked at how often bacterial, viral, and parasitic agents caused GE in children in Erzurum and the surrounding areas over the last 11 years.
Methods
Ethics approval and study group
The study was approved by the Clinical Research Ethics Committee of Ataturk University Faculty of Medicine (approval number 469, letter dated November 5, 2020). In our retrospective study to investigate the agents of gastroenteritis, the records of Atatürk University Health Research and Application Center Research Hospital, which provides health services to approximately 4.5 million people living in Eastern and Northeastern Anatolian provinces, were evaluated. The hospital information of 51159 patients aged 0-17 years who came to our hospital with gastrointestinal complaints and entered the hospital system with at least one of the diagnostic codes for "certain infectious and parasitic diseases" (ICD-10 codes: A00-B99) in the 11-year period covering 2010-2020 was obtained. In our laboratories, samples that have been kept for more than two hours are not included in parasitological and bacteriological examinations. Except in the prediagnosis of toxic megacolon, solid stool samples and samples taken with a cotton swab, brought with toilet paper, or contaminated with detergent, disinfectant, water, or urine are not included in the parasitological examination in our laboratory.
Data collection
We obtained permission from the hospital administration to obtain patient data. The ENLIL Hospital Information Management System module was used to obtain demographic data such as the name, sex, and age of the patients; clinical data such as diagnosis and prediagnosis; and laboratory data such as the antigen, and parasite detected in stool for enteric pathogens and stool culture results. The retrieval of patient results from hospital records was completed in January 2021. These data were entered into Excel 2016.
Collecting stool samples and sending them to the laboratory
Stool samples are taken during active diarrhea and before antibiotic treatment in clinics where the patient is treated. In case of delay in examination, samples are stored at 4°C and microbiologically processed within 30 minutes.
Microbiological processes and evaluation of results
In our laboratories, conventional method protocols are used for the isolation and diagnosis of bacterial pathogens (Salmonella spp. and Shigella spp.) from stool. Molecular methods with commercial multiplex real-time PCR test kits changing over the years were used for the diagnosis of Campylobacter spp., Vibrio spp., Shiga toxin 1 and 2 (EHEC), and Y. enterocolitica. Immunochromatographic methods are used for the detection of rotavirus (RoV), norovirus (NoV) and Cryptosporidium antigens. In the parasitological examination of the stool, macroscopic observations are first made for the consistency of the stool and the presence of blood or mucus, and helminth adults. Then, using native-Lugol preparations, trophozoites, cysts, oocysts, or helminth eggs are examined microscopically. The cellophane tape method is used to search for E. vermicularis larvae or eggs.
Statistical analysis
Statistical analyses were performed to determine the differences and relationships between the variables. First, we transferred the patient information obtained from the "Hospital Information Management System Modules" to Microsoft Office Excel 2016 and then analyzed the variables with the SPSS software package version 24.0.0.0 (IBM Corp., USA). Bonferroni post hoc analysis and one-way ANOVA were used for between-group comparisons. We considered p<0.05 to be statistically significant.
Results
In this study, bacteriological, virological, and parasitological laboratory results of 51,159 pediatric patients aged 0-17 years who were admitted to our hospital with gastrointestinal complaints and/or were treated during the 11-year period covering 2010-2020 are presented. The mean age±SD of the cases was 5.41±0.04 years, and they were predominantly male (56.9%; n=29.089).
Hospital records show that the most requested examination in the 11-year period for the purpose of investigating GE agents was the parasite investigation (44,884 requests), followed by viral antigen search (3,107 requests for RoV and AdV; 82 requests for NoV) and stool culture (3,168 requests for Salmonella spp. and Shigella spp.; 413 requests for Campylobacter spp.). Vibrio spp., Shiga toxin 1 and 2 (EHEC), and Y. enterocolitica positivity were not found in hospital records. GE agents and detection rates are given in Table 1. Specimens with coinfection were considered positive for each etiologic agent separately. Based on the number of tests done, the most common agents of gastroenteritis in children were viruses (771/3137, or 24.6%), followed by parasites (2153/41.453, or 5.2%), and then bacteria (64/1423, or 4.5%).
Table 1.
Gastroenteritis agent positivity according to the number of samples tested
| Pathogens | No. of tested samples | Positive number | % |
|---|---|---|---|
| Viruses* | |||
| Rotavirus | 3107 | 697 | 22.4 |
| Adenovirus | 3107 | 69 | 2.2 |
| Parasites | |||
| E. histolytica/dispar | 44884 | 1357 | 3.0 |
| Giardia lamblia | 44884 | 711 | 1.6 |
| Blastocystis hominis | 44884 | 20 | 0.0 |
| Trichomonas hominis | 44884 | 5 | 0.0 |
| Chilomastix mesnili | 44884 | 4 | 0.0 |
| Balantidium coli | 44884 | 1 | 0.0 |
| Cryptosporidium spp. | 44884 | 1 | 0.0 |
| Dientamoeba fragilis | 44884 | 1 | 0.0 |
| Ascaris lumbricoides | 44884 | 21 | 0.0 |
| Taenia spp. | 44884 | 19 | 0.0 |
| Hymenolepis spp. | 44884 | 11 | 0.0 |
| E. vermicularis | 44884 | 2 | 0.0 |
| Bacteria | |||
| Salmonella spp. | 3168 | 33 | 1.0 |
| Shigella spp. | 3168 | 25 | 0.8 |
| Campylobacter spp. | 413 | 6 | 1.5 |
No information could be obtained from the hospital system for the viral pathogen norovirus, except for five positive records.
It was determined that 36.5% of the cases (18689/51159) were clustered at the age of 0-2 years, and the number of cases decreased dramatically as the age progressed. According to the results of one-way ANOVA analysis, pathogen positivity in age groups showed a significant difference [df (between groups) = 5, df (within groups) = 51153, F=17.59, p<0.001)]. According to the Bonferroni post hoc test results, the positivity observed in children aged 3-5 years was significantly higher than the other ages except 6-8 and 9-11 years; the positivity in children aged 15-17 years was found to be significantly lower than in children of all other ages (Table 2)
Table 2.
Pathogen positivity by age groups
| Total | Positive | Negative | |||
|---|---|---|---|---|---|
| Age group | n | n | % | n | % |
| 0-2 years | 18689 | 922 | 4.9 | 17767 | 95.1 |
| 3-5 years | 10373 | 737 | 7.1 | 9636 | 92.9 |
| 6-8 years | 9436 | 617 | 6.5 | 8819 | 93.5 |
| 9-11 years | 5713 | 364 | 6.4 | 5349 | 93.6 |
| 12-14 years | 4345 | 247 | 5.7 | 4098 | 94.3 |
| 15-17 years | 2603 | 101 | 3.9 | 2502 | 96.1 |
| Total | 51159 | 2988 | 5.8 | 48171 | 94.2 |
Dual-pathogen (coinfection) was detected in 20 (0.04%) of the cases with gastroenteritis. The most common pathogens in coinfections were RoV and Giardia lamblia. Table 3 shows how many different infections patients with gastroenteritis had at the same time.
Table 3.
Combined infections observed in patients with gastroenteritis
| Coinfections | n |
|---|---|
| Giardia lamblia + rotavirus | 8 |
| E. histolytica/dispar + rotavirus | 6 |
| Giardia lamblia + Hymenolepis nana | 3 |
| Giardia lamblia + Ascaris lumbricoides | 1 |
| Giardia lamblia + Blastocystis hominis | 1 |
| Hymenolepis nana + Ascaris lumbricoides | 1 |
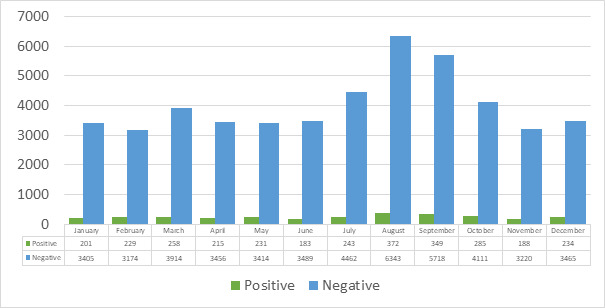
As seen in Figure 1, the highest number of requests from the microbiology laboratory were made July to September adjacent to this month for the investigation of GE agents from stool samples in our hospital, considering all years. However, considering the number of samples examined, the months with pathogen positivity were February (6.7%), October (6.5%), May (6.3%), December (6.3%), and March (6.2%).
Figure 1. The distribution of pathogen positive and negative stool samples by months.
Discussion
Gastroenteritis is one of the primary health problems that includes many notifiable infections and causes human and economic losses. Despite the developing health technologies in diagnosis and treatment, this problem continues to be a life-threatening disease group in children in the developing countries of the world, including Turkey. Most of the studies investigating the etiology of gastroenteritis in Turkey have focused on either viruses, bacteria, or parasites. In this retrospective study, we present data from Turkey on the incidence of all pathogen groups, the age of the cases, and the relationship with factors such as coinfections and season. It has been determined in the present study that the most common pathogen group in Erzurum is represented by viruses, with a rate of approximately 25%, and rotavirus has the highest prevalence among all pathogens. Parasites, mostly E. histolytica/dispar (with a rate of 3.0%) and Giardia lamblia (1.6%), took the second place.
The pathogen group in last place was bacteria, and the most frequently isolated bacterial agent was Campylobacter spp.
Considering all the months of all years, the highest demand from the microbiology laboratory for the investigation of GE agents in our hospital was July to September, which are on the right and left of this month. However, in Erzurum, which has a harsh continental climate, it was observed that the highest GE positivity was in February, October, May, December, and March, and the lowest positivity was in summer. We observed that GE positivity was partially concentrated in the spring. Our results were similar to most of the other local results, but the prevalence of the pathogens varied regionally. Gultepe et al. found RoV positivity to be higher than AdV in children with diarrhea in Van, Turkey, and reported both RoV and AdV positivity were 4.5% and viral antigen positivity was most common in the 2-year-old group, during autumn and winter months.10 We found coinfections in 0.04% of GE cases. The most common coinfection in Turkey occurs between RoV and Giardia lamblia.
Different results have been reported regarding the prevalence and demographic characteristics of GE in other countries. Calderaro et al. reported 84.6% of viruses, 70.7% of bacteria, and 61.4% of parasites were found in children under the age of five in Italy, and viruses were more common during winter.11 Khan stated the prevalence of GE in Pakistan was higher in children younger than six months and decreased in the 10-15 age group, and also acute GE cases increased between winter and spring.7 There is a similarity in the age, and seasonal results of pathogen positivity between Pakistan and Erzurum, Turkey. Aktas et al. found in children aged 0-5 years with acute diarrhea in Erzurum, the highest frequency of viral pathogens, RoV, was detected, followed by NoV, AdV, and astrovirus. 12 Coskun and Kasap found the RoV rate of Tokat was higher than that of AdV, especially during winter.13 Çiftçi and Maçin found RoV was mainly found in spring in Konya.14 Ozkan et al. found it was higher in the 3-5 age group during the winter.15 The high rate of pathogen positivity in outpatients in Turkey shows the rate of transmission of GEs in the community is high. Chung et al. found RoV positivity increased during the winter and spring months in children under five years of age with acute GE.16 Ozmen et al. detected viral acute GEs at a rate of 63%, the highest rate during winter.17 Calderaro et al. detected RoV most frequently in children under the age of five and in the spring season.11 RoV is one of the most frequently isolated GE agents in the world, and its infections increase, especially during the winter and spring months.
In our region, the highest GE pathogen positivity was observed in children aged 3-5 years; it was found to be low in children aged 15-17 years and 0-2 years, respectively. This result can be explained by the fact infants aged 0-2 years are breastfed and receive limited exposure to the external environment as well as the enhanced immunity of children aged 15-17. Chung et al. reported NoV was isolated at a rate of 17% of GE cases and was most commonly seen as etiological agent in children under the age of two.16 Yılmaz et al. reported NoV infections are common during December and January in Istanbul.18 AdV, which is the most common viral pathogen after RoV in our region, had a positivity rate of 2.3% and was most commonly isolated from cases aged 0-2 years. Unlike other viruses, AdVs were most commonly detected during the summer and autumn. AdV incidence and epidemiologic indicators vary from country to country. For example, in Italy, De Francesco et al. detected AdV in 7.1% of the cases; they reported it was frequent in children under two years old and peaked in April and June.19 In Shanghai, Lu et al. found the AdV positivity rate was about 3.5%, and infection was most common in children under the age of three and during the summer months. 20 In Bangladesh, Afrad et al. stated AdV virus infections are most common during the rainy season.21
Unlike viral acute GE agents, bacterial infections are more likely to be detected in hot weather. The most commonly isolated bacterial pathogen in Turkey is Salmonella, followed by Shigella and Campylobacter strains. Calderaro et al. isolated bacterial agents at a rate of 55.1% in Italy.11 They found Salmonella infections are more common in children aged 0-5 years. Tseng et al. found Salmonella infection was more common in Taiwanese children under five years of age.22 Mahmoudi et al. detected Salmonella infection in 42% of Iranians and reported that Salmonella strains were more common in males and during the summer months.23 Chung et al. reported that Salmonella was isolated at a rate of 21.8% and was most common in children under two years of age, peaking in July.16 Almost half of all Salmonella infections in Turkey occur during the summer months. These results are similar to the results of the study mentioned above. Shigella infections, which we have identified in second place among bacteria, are mostly seen in developing countries and in children under five years old.
Soofi et al. reported shigellosis is prominent during the hot months of the year and in children under five years old in Asian countries.24 Mahmoudi et al. determined Shigella gastroenteritis is more common in autumn.23 Shigella positivity was found to be higher in autumn and in children 3-11 years in our region. Five of the six Campylobacter strains we isolated from GE cases were obtained during the autumn and summer months. Soofi et al. reported campylobacteriosis is common in children under two years of age, especially during the summer months in six Asian countries.24
Parasitic GE pathogens are one of the main research topics in Turkey. In one of these studies, Ozmen et al. reported the rate of parasites in Kahramanmaraş was 36%, and parasites were common during the summer months.17 E. histolytica/dispar, Giardia lamblia, Blastocystis hominis, Ascaris lumbricoides, Taenia spp., and Hymenolepis spp. are the most commonly detected parasites in Erzurum, Turkey.
The most important limitation of this study was the inability to assess regularly over the years an infectious agent such as norovirus. Accordingly, it is understood that the viral pathogen positivity would be slightly higher than the rate we reported, but there most likely will be no change in the incidence order of viral, parasitic, and bacterial pathogens.
Conclusions
The results of this study, in which more than 50,000 cases were evaluated, can be used as a reference for comparison in future studies. In particular, viral gastrointestinal infections continue to be common in our region. These infections cause not only health problems but also economic losses, unnecessary hospitalizations, and the risk of nosocomial infections. To prevent the spread of GEs in the society, it is necessary to inform the public about these diseases and their transmission routes as well as to improve the municipal services and infrastructure systems in the settlements. Identifying the source of GE and monitoring its epidemiology will play a key role in developing appropriate strategies to prevent these infections.
Footnotes
Authors’ contributions statement: OA and BC put forward the research question. OA and BC designed the study. BC carried out data collection. OA did the statistical analysis, writing, and critical review of the article. OA and BC read and approved the final version of the article.
Conflicts of interest: All authors – none to declare.
Funding: None to declare.
Availability of data: The data supporting the findings of this study are available from the corresponding author, upon reasonable request.
Ethical approval and informed consent: The ethics committee at the numbered /469 approved this study, dated 05 November 2020, of the Clinical Research Ethics Committee of Ataturk University Faculty of Medicine. Since our study was a retrospective study, informed consent statement was not applied.
References
- 1.Guarino A, Aguilar J, Berkley J, et al. Acute gastroenteritis in children of the world: what needs to be done? J Pediatr Gastroenterol Nutr. 2020;70:694–701. doi: 10.1097/MPG.0000000000002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nycz BT, Pretty K, Gomez-Trujillo A, Sanchez B, Dominguez SR. Description of enteropathic Escherichia coli species in pediatric patients at a quaternary children’s hospital. J Pediatr Infect Dis Soc. 2020;9:573–9. doi: 10.1093/jpids/piz081. [DOI] [PubMed] [Google Scholar]
- 3.Hartman S, Brown E, Loomis E, Russell HA. Gastroenteritis in children. Am Fam Physician. 2019;99:732. [PubMed] [Google Scholar]
- 4.Stuempfig ND, Seroy J. StatPearls. Treasure Island (FL): StatPearls Publishing; 2021. Jan, Viral gastroenteritis. (Updated 2021 Jun 24) Available from: https://www.ncbi.nlm.nih.gov/books/NBK518995/ [Google Scholar]
- 5.Posovszky C, Buderus S, Classen M, Lawrenz B, Keller KM, Koletzko S. Acute infectious gastroenteritis in infancy and childhood. Dtsch Arztebl Int. 2020;117:615–24. doi: 10.3238/arztebl.2020.0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huston CD. Parasite and host contributions to the pathogenesis of amebic colitis. Trends Parasitol. 2004;20:23–6. doi: 10.1016/j.pt.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Khan MA. Epidemiological studies on gastroenteritis in children in the Bannu district, Khyber Pakhtunkhwa, Pakistan. Z Gesundh Wiss. 2021:1–8. doi: 10.1007/s10389-021-01592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan U, Paparini A, Oskam C. New technologies for detection of enteric parasites. Trends Parasitol. 2017;33:532–46. doi: 10.1016/j.pt.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Turkish Statistical Institute. 2020. Statistics on Child. 2020. [Accessed 28 June 2021]. Available at: https://www.tuik.gov.tr/media/announcements/istatistiklerle_cocuk_2020.pdf.
- 10.Gültepe B, Güdücüoğlu H, Çıkman A, Parlak M, Berktaş M. Prevalence of rotavirus and adenovirus gastroenteritis observed around the Van. Sakarya Tıp Derg. 2013;3:131–4. doi: 10.5505/sakaryamj.2013.85547. [DOI] [Google Scholar]
- 11.Calderaro A, Martinelli M, Buttrini M, et al. Contribution of the FilmArray(®) Gastrointestinal Panel in the laboratory diagnosis of gastroenteritis in a cohort of children: a two-year prospective study. Int J Med Microbiol. 2018;308:514–21. doi: 10.1016/j.ijmm.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Aktaş O, Aydin H, Timurkan MO. A molecular study on the prevalence and coinfections of rotavirus, norovirus, astrovirus and adenovirus in children with gastroenteritis. Minerva Pediatr. 2019;71:431–7. doi: 10.23736/S0026-4946.16.04304-X. [DOI] [PubMed] [Google Scholar]
- 13.Şay Coşkun US, Kasap T. [Frequency of rotavirus and adenovirus in pediatric patients with acute gastroenteritis] Çağdaş Tıp Dergisi. 2019;9:85–8. doi: 10.16899/gopctd.459823. [DOI] [Google Scholar]
- 14.Çiftçi N, Maçin S. [Investigation of gastroenteritis frequency caused by adenovirus and rotavirus. Sağlık Akademisi Kastamonu] 2021;6:43–51. doi: 10.25279/sak.644024. [DOI] [Google Scholar]
- 15.Akyüz Özkan E, Yeşilyurt E, Çilsal Z, Yımaz N, Öztürk O, Sadigov A. [Frequency of rotavirus and enteric adenovirus infection in children with acute gastroenteritis admitted to children outpatient clinic] Bozok Tıp Dergisi. 2020;10:61–4. doi: 10.16919/bozoktip.697037. [DOI] [Google Scholar]
- 16.Chung N, Wang SM, Shen CF, et al. Clinical and epidemiological characteristics in hospitalized young children with acute gastroenteritis in southern Taiwan: according to major pathogens. J Microbiol Immunol Infect. 2017;50:915–22. doi: 10.1016/j.jmii.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Özmen Ş, Acıpayam C, Güneş H, Akkeçeci BNS, Orak F. Comparison of Clinical and Laboratory Findings According to the Agents in Children with Acute Gastroenteritis. STED. 2020;29:161–7. doi: 10.17942/sted.665414. [DOI] [Google Scholar]
- 18.Yılmaz İ, Salman N, Sütçü M, et al. The incidence of norovirus, rotavirus and adenovirus in children with acute gastroenteritis. J Child. 2015;15:51–5. doi: 10.5222/j.child.2015.051. [DOI] [Google Scholar]
- 19.De Francesco MA, Lorenzin G, Meini A, Schumacher RF, Caruso A. Nonenteric adenoviruses associated with gastroenteritis in hospitalized children. Microbiol Spectr. 2021;9:e0030021. doi: 10.1128/Spectrum.00300-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu L, Zhong H, Xu M, et al. Molecular and epidemiological characterization of human adenovirus and classic human astrovirus in children with acute diarrhea in Shanghai, 2017-2018. BMC Infect Dis. 2021;21:713. doi: 10.1186/s12879-021-06403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Afrad MH, Avzun T, Haque J, et al. Detection of enteric- and non-enteric adenoviruses in gastroenteritis patients, Bangladesh, 2012-2015. J Med Virol. 2018;90:677–84. doi: 10.1002/jmv.25008. [DOI] [PubMed] [Google Scholar]
- 22.Tseng CF, Chiu NC, Huang CY, et al. The epidemiology of non-typhoidal Salmonella gastroenteritis and Campylobacter gastroenteritis in pediatric inpatients in northern Taiwan. J Microbiol Immunol Infect. 2019;52:449–55. doi: 10.1016/j.jmii.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 23.Mahmoudi S, Pourakbari B, Moradzadeh M, et al. Prevalence and antimicrobial susceptibility of Salmonella and Shigella spp. among children with gastroenteritis in an Iranian referral hospital. Microb Pathog. 2017;109:45–8. doi: 10.1016/j.micpath.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 24.Soofi SB, Habib MA, von Seidlein L, et al. A comparison of disease caused by Shigella and Campylobacter species: 24 months community based surveillance in 4 slums of Karachi, Pakistan. J Infect Public Health. 2011;4:12–21. doi: 10.1016/j.jiph.2010.10.001. [DOI] [PubMed] [Google Scholar]


