Abstract
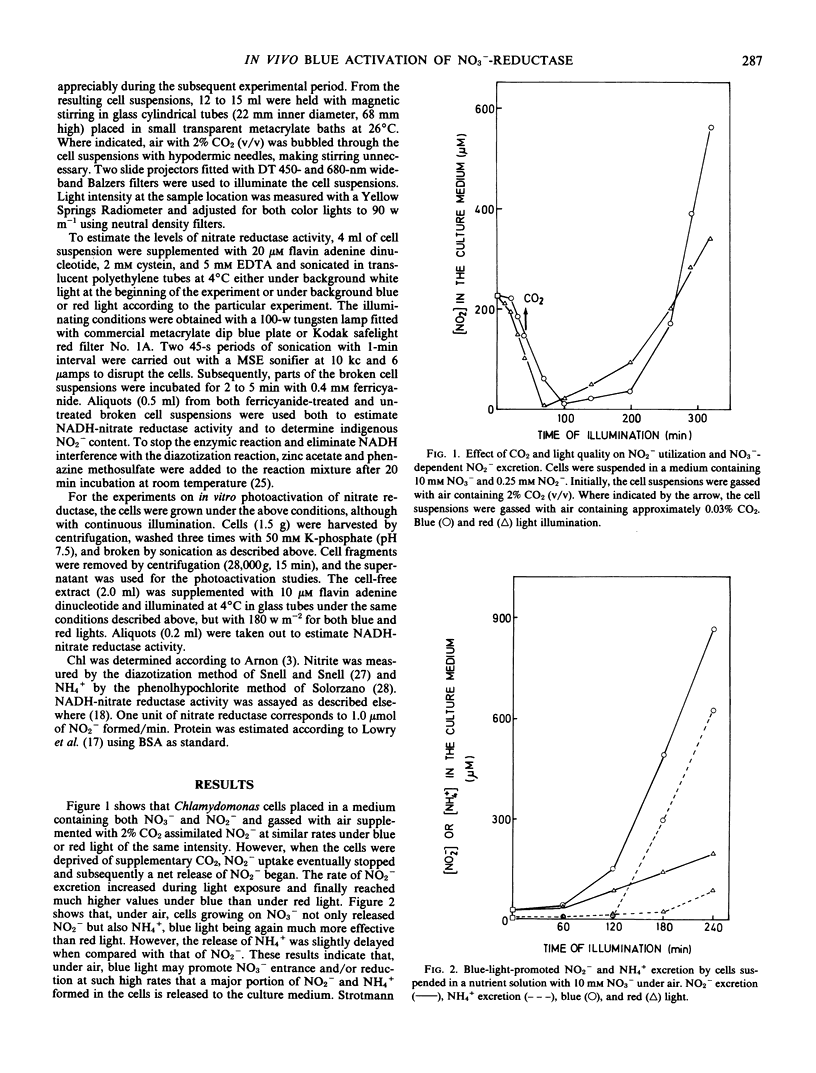
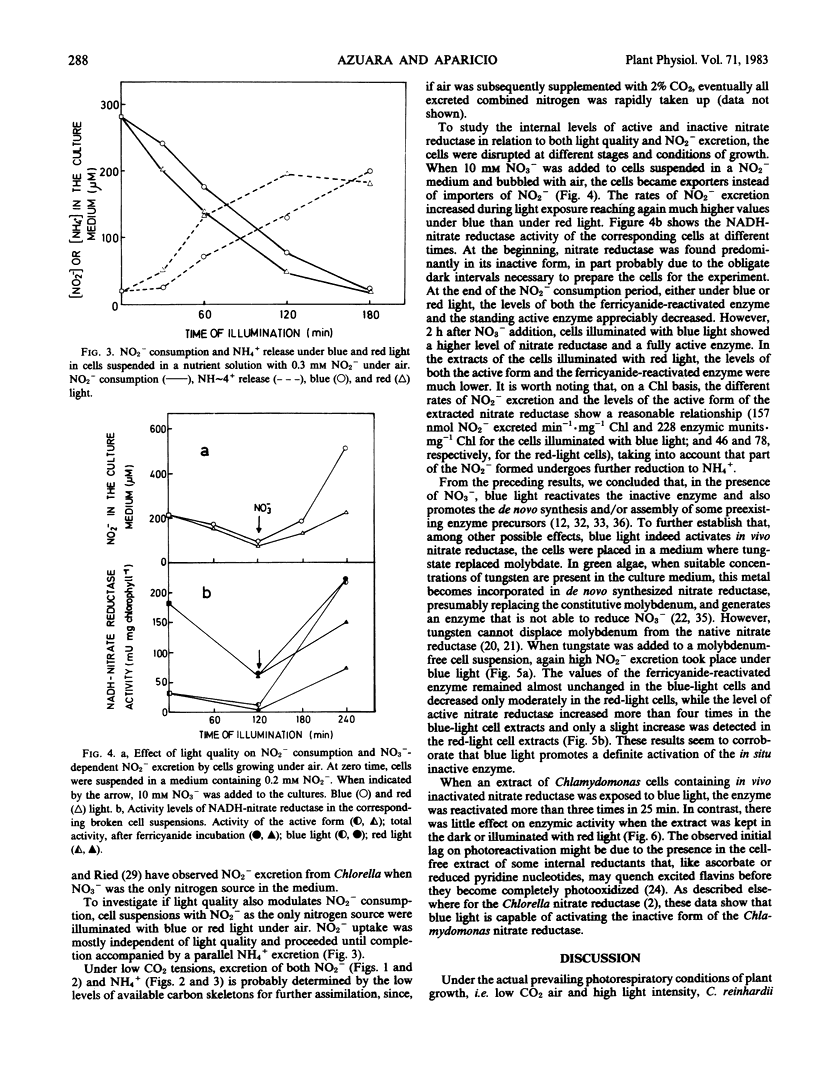
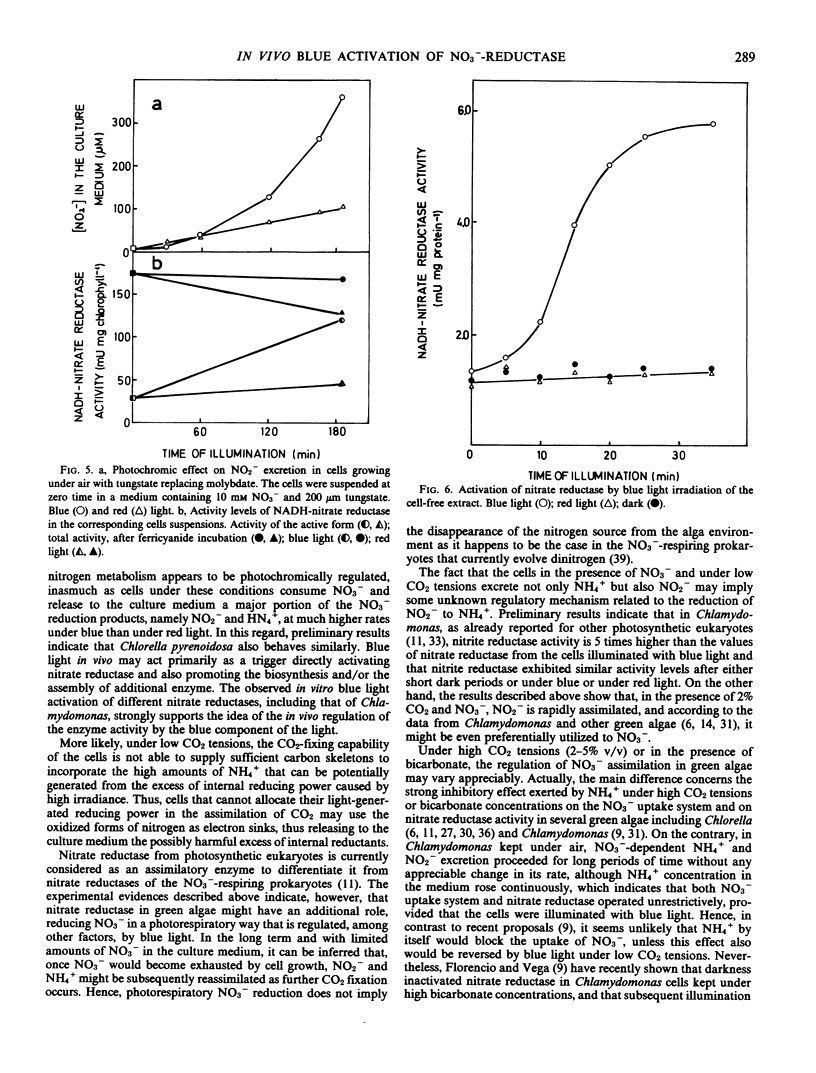
Chlamydomonas reinhardii cells, growing photoautotrophically under air, excreted to the culture medium much higher amounts of NO2− and NH4+ under blue than under red light. Under similar conditions, but with NO2− as the only nitrogen source, the cells consumed NO2− and excreted NH4+ at similar rates under blue and red light. In the presence of NO3− and air with 2% CO2 (v/v), no excretion of NO2− and NH4+ occurred and, moreover, if the bubbling air of the cells that were currently excreting NO2− and NH4+ was enriched with 2% CO2 (v/v), the previously excreted reduced nitrogen ions were rapidly reassimilated. The levels of total nitrate reductase and active nitrate reductase increased several times in the blue-light-irradiated cells growing on NO3− under air. When tungstate replaced molybdate in the medium (conditions that do not allow the formation of functional nitrate reductase), blue light activated most of the preformed inactive enzyme of the cells. Furthermore, nitrate reductase extracted from the cells in its inactive form was readily activated in vitro by blue light. It appears that under high irradiance (90 w m−2) and low CO2 tensions, cells growing on NO3− or NO2− may not have sufficient carbon skeletons to incorporate all the photogenerated NH4+. Because these cells should have high levels of reducing power, they might use NO3− or, in its absence, NO2− as terminal electron acceptors. The excretion of the products of NO2− and NH4+ to the medium may provide a mechanism to control reductant level in the cells. Blue light is suggested as an important regulatory factor of this photorespiratory consumption of NO3− and possibly of the whole nitrogen metabolism in green algae.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aparicio P. J., Roldan J. M., Calero F. Blue light photoreactivation of nitrate reductase from green algae and higher plants. Biochem Biophys Res Commun. 1976 Jun 21;70(4):1071–1077. doi: 10.1016/0006-291x(76)91011-1. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Rosa M. A., Vega J. M., Zumft W. G. Composition and structure of assimilatory nitrate reductase from Ankistrodesmus braunii. J Biol Chem. 1981 Jun 10;256(11):5814–5819. [PubMed] [Google Scholar]
- Johnson J. L., Hainline B. E., Rajagopalan K. V. Characterization of the molybdenum cofactor of sulfite oxidase, xanthine, oxidase, and nitrate reductase. Identification of a pteridine as a structural component. J Biol Chem. 1980 Mar 10;255(5):1783–1786. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Notton B. A., Hewitt E. J. The role of tungsten in the inhibition of nitrate reductase activity in spinach (spinacea oleracea L.) leaves. Biochem Biophys Res Commun. 1971 Aug 6;44(3):702–710. doi: 10.1016/s0006-291x(71)80140-7. [DOI] [PubMed] [Google Scholar]
- Scholl R. L., Harper J. E., Hageman R. H. Improvements of the nitrite color development in assays of nitrate reductase by phenazine methosulfate and zinc acetate. Plant Physiol. 1974 Jun;53(6):825–828. doi: 10.1104/pp.53.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischner R. Light-mediated Activation of Nitrate Reductase in Synchronous Chlorella. Plant Physiol. 1978 Aug;62(2):284–286. doi: 10.1104/pp.62.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega J. M., Herrera J., Aparicio P. J., Paneque A., Losada M. Role of molybdenum in nitrate reduction by chlorella. Plant Physiol. 1971 Sep;48(3):294–299. doi: 10.1104/pp.48.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]


