Key Points
Question
What is the association between fetal exposure to antiseizure medications (ASMs) and subsequent adaptive, behavioral or emotional, and neurodevelopmental disorder outcomes?
Findings
This cohort study found that adaptive functioning did not differ between children of women with epilepsy and children of women without epilepsy; however, a significant decrease in functioning was seen with increasing third-trimester ASM blood concentrations. Secondary analysis revealed that this association was evident for levetiracetam and lamotrigine, the ASMs with sample sizes large enough for analysis.
Meaning
This study suggests that these exposure-dependent associations warrant psychiatric or psychological screening and referral of women with epilepsy and their offspring when appropriate.
Abstract
Importance
The association of fetal exposure to antiseizure medications (ASMs) with outcomes in childhood are not well delineated.
Objective
To examine the association of fetal ASM exposure with subsequent adaptive, behavioral or emotional, and neurodevelopmental disorder outcomes at 2, 3, and 4.5 years of age.
Design, Setting, and Participants
The Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study is a prospective, observational cohort study conducted at 20 epilepsy centers in the US. A total of 456 pregnant women with epilepsy or without epilepsy were enrolled from December 19, 2012, to January 13, 2016. Children of enrolled women were followed up with formal assessments at 2, 3, 4.5, and 6 years of age. Statistical analysis took place from August 2022 to May 2023.
Exposures
Exposures included mother’s epilepsy status as well as mother’s ASM blood concentration in the third trimester (for children of women with epilepsy). Women with epilepsy were enrolled regardless of ASM regimen.
Main Outcomes and Measures
The primary outcome was the Adaptive Behavior Assessment System, Third Edition (ABAS-3) General Adaptive Composite (GAC) score among children at 4.5 years of age. Children of women with epilepsy and children of women without epilepsy were compared, and the associations of ASM exposures with outcomes among exposed children were assessed. Secondary outcomes involved similar analyses of other related measures.
Results
Primary analysis included 302 children of women with epilepsy (143 boys [47.4%]) and 84 children of women without epilepsy (45 boys [53.6%]). Overall adaptive functioning (ABAS-3 GAC score at 4.5 years) did not significantly differ between children of women with epilepsy and children of women without epilepsy (parameter estimate [PE], 0.4 [95% CI, −2.5 to 3.4]; P = .77). However, in adjusted analyses, a significant decrease in functioning was seen with increasing third-trimester maximum ASM blood concentrations (PE, −7.8 [95% CI, −12.6 to −3.1]; P = .001). This decrease in functioning was evident for levetiracetam (PE, −18.9 [95% CI, −26.8 to −10.9]; P < .001) and lamotrigine (PE, −12.0 [95% CI, −23.7 to −0.3]; P = .04), the ASMs with sample sizes large enough for analysis. Results were similar with third-trimester maximum daily dose.
Conclusions and Relevance
This study suggests that adaptive functioning of children of women with epilepsy taking commonly used ASMs did not significantly differ from that of children of women without epilepsy, but there was an exposure-dependent association of ASMs with functioning. Thus, psychiatric or psychological screening and referral of women with epilepsy and their offspring are recommended when appropriate. Additional research is needed to confirm these findings.
This cohort study examines the association of fetal exposure to antiseizure medications with subsequent adaptive, behavioral or emotional, and neurodevelopmental disorder outcomes at 2, 3, and 4.5 years of age.
Introduction
Although knowledge of antiseizure medication (ASM)–related teratogenesis has increased over the past 2 decades, the risks of fetal exposure are known for only a few ASMs.1,2 Previous studies have reported a greater cognitive neurodevelopmental risk for valproate compared with carbamazepine, lamotrigine, or phenytoin,3,4,5 with decreased adaptive functioning and greater risk of attention-deficit/hyperactivity disorder.6,7 However, ASM prescription practices have changed,8 and the cognitive and behavioral risks remain uncertain for most ASMs1,2 except for levetiracetam, which appears to have less risks than valproate.9,10,11
Teratogenicity risks are dose dependent, and understanding higher-exposure risks will affect dose management. Dose-dependent associations with malformations are seen across ASMs.12 Neuropsychological teratogenicity is likely dose dependent but has only been demonstrated in humans for valproate. Variable changes in clearance occur during pregnancy for several ASMs,13,14,15 which could obscure dose-dependent associations.
Previous studies have reported 2- and 3-year-old cognitive outcomes for children with fetal exposure to ASMs commonly used during pregnancy in the US.16,17 Cognitive outcomes among children born to women with epilepsy did not differ significantly from children born to women without epilepsy. Women with epilepsy had more symptoms of anxiety and depression, but this was not associated with the cognitive outcomes among their children. Although verbal abilities were not associated with third-trimester ASM blood concentrations, secondary analyses revealed exposure-dependent associations were present on multiple measures, which were more apparent for levetiracetam. Because ASM-exposed children did not differ from children of women without epilepsy, we concluded that levetiracetam can be safely used for women of childbearing potential with consideration of dosing during pregnancy similar to other ASMs. In this report, we describe the adaptive and emotional or behavioral outcomes, as well as the proportions of neurodevelopmental disorders, in this same cohort at 2, 3, and 4.5 years of age.
Methods
Design
The Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drug (MONEAD) study is a National Institutes of Health–funded, prospective, observational cohort investigation in the US enrolling pregnant women with epilepsy and pregnant women without epilepsy at 20 specialty epilepsy centers (eAppendix 1 in Supplement 1). MONEAD is a continuation of the Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study, enrolling a new cohort representative of the ASMs currently being used at tertiary epilepsy centers. MONEAD evaluates both maternal and child outcomes, and the primary neurodevelopmental aims are to compare long-term outcomes (at 6 years of age) among children of women with epilepsy vs children of women without epilepsy and evaluate whether there is an ASM fetal exposure–dependent association with cognitive and behavioral outcomes. Local institutional review boards approved the study at each epilepsy center (eAppendix 1 in Supplement 1), and written and oral informed consent was obtained from all adult participants. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Participants
Enrollment occurred from December 19, 2012, through January 13, 2016 (eFigure in Supplement 1). Pregnant women with epilepsy and pregnant women without epilepsy were recruited from the epilepsy centers, by referral from obstetricians and other physicians, and by self-referral. Inclusion criteria for women with epilepsy were as follows: aged 14 to 45 years and less than 20 weeks’ gestational age. Exclusion criteria included a history of psychogenic nonepileptic spells, an expected IQ of less than 70, other major medical illness, progressive cerebral disease, and switching ASMs during pregnancy prior to enrollment. MONEAD enrolled women with epilepsy regardless of ASM regimen to provide a more representative sample. A total of 456 pregnant women with epilepsy or without epilepsy were enrolled. Children born to women with epilepsy and children born to women without epilepsy, their fathers, and maternal relatives were also enrolled. Data on seizures and ASMs were collected using a daily electronic diary and were verified at study visits and via medical records.
Outcome Measures
Adaptive functioning was assessed by the Adaptive Behavior Assessment System, Third Edition (ABAS-3),18 completed by the mother or father of the child at 3 and 4.5 years of age. Emotional or behavioral functioning was measured by the Behavior Assessment System for Children, Second Edition (BASC-2),19 and the level of stress in the parent-child relationship was assessed by the Parenting Stress Index, Fourth Edition (PSI-4),20 completed by the mother or father of the child at 2, 3, and 4.5 years of age. Autism spectrum disorder screening was conducted using the Modified Checklist for Autism in Toddlers (M-CHAT)21 at 2 years; the Gilliam Autism Rating Scale, Third Edition (GARS-3)22 at 3 years; and the Social Responsiveness Scale, Second Edition (SRS-2)23 at 4.5 years. Completion rates varied, and scores were not significantly associated with the COVID-19 shutdown (eTables 1 and 2 in Supplement 1). Rating scales were scored by assessors blinded to the child study group.
The primary outcome was the ABAS-3 General Adaptive Composite (GAC) score when the child was 4.5 years of age. Secondary outcomes included the GAC score when the child was 3 years of age; the Social, Practical, and Conceptual Domain scores of the ABAS-3 when the child was 3 and 4.5 years of age; the scale scores from the BASC-2 when the child was 2, 3, and 4.5 years of age; and the proportion of at-risk scores (>90th percentile) from the PSI-4 Parent and Child Domains and the subdomains. Based on the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition),24 a diagnosis of intellectual disability required both the Differential Ability Scale-2 General Conceptual Ability score when the child was 3 years of age and a GAC standard score of 70 or less when the child was 4.5 years of age. Expressive language disorder was defined as an expressive language index (ELI) score of 77 or less when the child was 3 years of age, and receptive language disorder was defined as a receptive language index (RLI) score of 77 or less when the child was 3 years of age. Mixed expressive and receptive language disorder was defined as both an ELI score and RLI score of 77 or less when the child was 3 years of age. Risk for attention-deficit/hyperactivity disorder inattentive presentation was defined as a BASC-2 Attention Problems T score of 65 or more and risk for attention-deficit/hyperactivity disorder combined presentation as both BASC-2 Attention Problems and Hyperactivity T scores of 65 or more. Autism spectrum risk was defined as an M-CHAT total score of 3 or more when the child was 2 years of age or an M-CHAT Critical score of 2 or more when the child was 2 years of age, a GARS-3 Autism Index score of 85 or more when the child was 3 years of age, and an SRS-2 Total Score T score of 70 or more when the child was 4.5 years of age.
Risk Factors and Confounding Variables
Potential confounding variables or covariates independently associated with behavior outcomes included mother’s age at enrollment; IQ score25; weeks’ gestational age at enrollment; educational level; employment status; household income; planned or welcomed pregnancy; major depressive episode during pregnancy; smoking, alcohol, and substance use during pregnancy; problematic family functioning (McMaster Family Assessment Device)26,27 at the child’s 2-year-old visit; and lifetime history of mood and anxiety disorders, as well as child’s race and ethnicity; sex; weeks’ gestational age at birth; birth weight; small for gestational age; and major congenital malformations. Additional variables for children of women with epilepsy included mother’s epilepsy type, number of seizures during pregnancy, and mother having more than 5 seizures during pregnancy.28
Potential risk factors and confounding variables included breastfeeding status, periconceptional folate use, folate dose (none, >0-0.4 mg, >0.4-1.0 mg, >1.0-4.0 mg, and >4.0 mg), average maternal anxiety (Beck Anxiety Inventory score),29 depression (Beck Depression Inventory–2 score),30 perceived stress (Perceived Stress Scale–14 score)31 during pregnancy and/or after birth through the child’s 2-year-old visit, and average maternal sleep quality (Pittsburgh Sleep Quality Index score)32 during pregnancy and/or post partum. Additional risk factors for children of women with epilepsy taking ASMs included ASM group (monotherapy vs polytherapy), ASM category (lamotrigine monotherapy, levetiracetam monotherapy, other monotherapy, lamotrigine plus levetiracetam polytherapy, and other polytherapy), and third-trimester ASM dose.
Statistical Analysis
Primary Analyses
Statistical analysis took place from August 2022 to May 2023. Primary objectives were (1) GAC score at 4.5 years of age of children of women with epilepsy vs children of women without epilepsy, and (2) the association between the child’s GAC score at 4.5 years of age and the mother’s third-trimester ASM blood concentration in the children of women with epilepsy. No adjustment of the type I error rate was applied because the primary outcomes address different research questions. To standardize ASM values, the ratio of ASM blood concentration (hereafter referred to as the ratio ASM concentration) and the ratio of defined daily dosages (hereafter referred to as the ratio dose) were calculated using the same methods as in analysis at 3 years of age.17 The maximum value during the third trimester was used for analysis.
Primary analyses used an imputation population, including children with nonmissing GAC scores at 3 or 4.5 years of age. Markov chain Monte Carlo methods33,34 using a fully conditional specification were used to impute missing GAC scores at 4.5 years of age from available scores at 3 years of age, including all risk factors and additional variables associated with the outcome or likelihood of missing data. Separate imputation models were run for each primary analysis. The imputation model for primary analysis 2 was restricted to children of women with epilepsy with available third-trimester ASM blood concentrations. Imputation generated 50 imputed data sets, and the Rubin rule35 was used to combine estimates across data sets. Unadjusted and adjusted linear regression models were used to compare GAC scores at 4.5 years of age between children of women with epilepsy and children of women without epilepsy, reported as least-squares mean values, and to assess their association with the maximum observed third-trimester ratio ASM concentrations.
Covariates were selected using a stepwise selection algorithm for completers (children with GAC scores at 4.5 years of age). Mother’s IQ and either study group or ratio ASM concentration were included in the models a priori. The Akaike information criterion was used to compare models. The significance level for covariate entry was P = .10, and to remain in the model, it was P = .15. Variables with more than 5% missing data were excluded from consideration. All P values were from 2-sided tests, and results were deemed statistically significant at P ≤ .05.
Secondary Analyses
All secondary analyses used the completers population and were adjusted for the same covariates. Associations with risk factors were assessed using unadjusted and adjusted linear regression models. Interaction models were run to assess the associations between maximum third-trimester ratio ASM concentration or dose and the GAC score at 4.5 years of age by ASM group or ASM category.
Analyses comparing children of women with epilepsy vs children of women without epilepsy and the association with the mother’s third-trimester ratio ASM concentration and ratio dose were performed on all continuous behavior measures collected at 2, 3, and 4.5 years of age using unadjusted and adjusted longitudinal generalized linear models with generalized estimating equations36 and an unstructured covariance structure. An age by study group, age by ratio ASM concentration, or age by ratio dose interaction term was included. The Fisher exact test was used to test differences in categorical behavioral outcomes between children of women with epilepsy and children of women without epilepsy. Due to low counts, associations with the ratio ASM concentrations and ratio dose were not performed, and proportions of categorical behavioral outcomes were summarized by ASM category.
Sensitivity Analyses
Risk factors, potential covariates, and outcomes between children enrolled in the study who were included in the primary analyses and those who were excluded from primary analyses were evaluated to assess the potential associations of missing data. Primary analysis 1 was rerun excluding children of women with epilepsy not taking ASMs during pregnancy. Both primary analyses were repeated using a completers population, imputing missing values for all enrolled children and excluding birth-related covariates from the adjusted model. Additional details are provided in eAppendix 2 and eAppendix 3 in Supplement 1.
Results
Of 345 children born to women with epilepsy and 106 children born to women without epilepsy, 302 children of women with epilepsy (143 boys [47.4%]) and 84 children of women without epilepsy (45 boys [53.6%]) were included in primary analysis 1. The reasons for exclusion from analysis populations are summarized in the eFigure in Supplement 1. The third-trimester ASM regimen for mothers with epilepsy included 74.8% (226 of 302) receiving monotherapy, 20.5% (62 of 302) receiving polytherapy, 4.3% (13 of 302) receiving no ASMs throughout pregnancy, and 0.3% (1 of 302) with no third-trimester exposure due to delivery in the second trimester (eTable 3 in Supplement 1). Lamotrigine and levetiracetam were the most common monotherapies (43.8% [99 of 226] and 34.5% [78 of 226], respectively, of all monotherapies), and lamotrigine plus levetiracetam was the most common polytherapy treatment (43.5% of polytherapies [27 of 62]). Primary analysis 2 included 271 children of women with epilepsy with third-trimester ASM blood concentrations. Demographic characteristics, baseline characteristics, and risk factors for children of women with epilepsy vs those of women without epilepsy for primary analysis 1 are listed in Table 1 and Table 2 and in eTables 4 and 5 in Supplement 1. Demographic characteristics, baseline characteristics, and risk factors for children of women with epilepsy in primary analysis 2 are listed in eTables 6 and 7 in Supplement 1.
Table 1. Demographic Characteristics, Baseline Characteristics, and Risk Factors for Children of Women With Epilepsy vs Women Without Epilepsy in Primary Analysis 1: Categorical Variablesa.
| Categorical variable | No. (%) | P valueb | |
|---|---|---|---|
| Children of women with epilepsy (n = 302) | Children of women without epilepsy (n = 84) | ||
| Child’s sex: male | 143 (47.4) | 45 (53.6) | .33 |
| Small for gestational agec | 17 (5.8) | 7 (8.4) | .44 |
| Any breastfeedingd | 232 (77.1) | 75 (89.3) | .01 |
| Periconceptional folate use | 268 (88.7) | 56 (66.7) | <.001 |
| Periconceptional folate dose, mge | |||
| 0 | 34 (11.4) | 28 (34.1) | <.001 |
| >0-0.4 | 25 (8.4) | 13 (15.9) | |
| >0.4-1 | 58 (19.4) | 32 (39.0) | |
| >1-4 | 160 (53.5) | 9 (11.0) | |
| >4 | 22 (7.4) | 0 | |
| Major depressive episode during pregnancyf | 12 (4.0) | 1 (1.2) | .31 |
| Mother’s educational level | |||
| College degree (advanced) | 82 (27.2) | 30 (35.7) | .28 |
| College degree (not advanced) | 137 (45.4) | 32 (38.1) | |
| No college degree | 83 (27.5) | 22 (26.2) | |
| Smokingg | 19 (6.3) | 5 (6.0) | ≥.99 |
| Illicit substance useg,h | 9 (3.0) | 4 (4.8) | .49 |
| McMaster FAD problematic family functioning at child’s 2-year visiti | 53 (18.5) | 12 (14.6) | .51 |
Abbreviation: FAD, Family Assessment Device.
Missing values not used in calculation of percentages or in P values. Table only includes risk factors and covariates included in primary analysis models. Complete tables are eTables 4 and 5 in Supplement 1.
From the Fisher exact test.
Missing for 8 children of women with epilepsy and 1 child of women without epilepsy.
Missing for 1 child of women with epilepsy.
Missing for 3 children of women with epilepsy and 2 children of women without epilepsy.
Depression based on Structured Clinical Interview for DSM Disorders Mood Module at any depression assessment during pregnancy.
Self-reported smoking or illicit substance use (including marijuana) at any time during pregnancy.
Missing for 1 child of women with epilepsy and 1 child of women without epilepsy.
Missing for 16 children of women with epilepsy and 2 children of women without epilepsy.
Table 2. Demographic Characteristics, Baseline Characteristics, and Risk Factors for Children of Women With Epilepsy vs Women Without Epilepsy in Primary Analysis 1: Continuous Variablesa.
| Continuous variable | Children of women with epilepsy (n = 302) | Children of women without epilepsy (n = 84) | P valueb | ||||
|---|---|---|---|---|---|---|---|
| No. | Mean (95% CI) | Median (range) | No. | Mean (95% CI) | Median (range) | ||
| Birth weight, kg | 295 | 3.2 (3.2-3.3) | 3.2 (0.6-4.9) | 84 | 3.3 (3.1-3.4) | 3.3 (1.1-5.0) | .61 |
| Mother’s age at enrollment, y | 302 | 30.8 (30.3-31.4) | 31 (18-46) | 84 | 29.8 (28.7-30.9) | 31 (15-38) | .09 |
| Mother’s IQ | 302 | 98.3 (96.8-99.7) | 100 (58-124) | 84 | 104.6 (101.7-107.5) | 106 (72-131) | <.001 |
| Beck Depression Inventory–2c | |||||||
| Enrollment through 2-y visit | 301 | 6.3 (5.7-6.9) | 5.3 (0.0-36.8) | 84 | 5.1 (4.3-6.0) | 4.7 (0.0-24.0) | .09 |
| Pregnancy | 302 | 7.1 (6.5-7.7) | 5.7 (0.0-38.7) | 84 | 5.9 (5.0-6.8) | 5.0 (0.0-17.7) | .11 |
| After birth | 301 | 5.8 (5.2-6.5) | 4.5 (0.0-35.6) | 84 | 4.5 (3.6-5.5) | 3.7 (0.0-29.0) | .06 |
| Beck Anxiety Inventoryc | |||||||
| Enrollment through 2-y visit | 301 | 5.2 (4.7-5.7) | 3.8 (0.0-34.1) | 84 | 3.5 (2.7-4.4) | 2.7 (0.0-26.0) | <.001 |
| Pregnancy | 302 | 6.4 (5.8-7.1) | 4.8 (0.0-38.3) | 84 | 4.9 (3.9-5.9) | 3.4 (0.0-29.3) | .03 |
| After birth | 301 | 4.4 (3.9-5.0) | 3.0 (0.0-31.6) | 84 | 2.6 (1.8-3.5) | 1.5 (0.0-23.5) | <.001 |
| Perceived Stress Scale–14c | |||||||
| Enrollment through 2-y visit | 301 | 17.9 (17.1-18.6) | 17.6 (3.3-40.6) | 84 | 16.9 (15.8-18.0) | 17.2 (6.4-29.1) | .32 |
| Pregnancy | 302 | 18.0 (17.2-18.8) | 17.7 (2.7-40.0) | 84 | 16.3 (15.0-17.6) | 15.7 (3.3-30.5) | .09 |
| After birth | 301 | 17.8 (17.0-18.6) | 17.6 (2.0-41.0) | 84 | 17.2 (16.0-18.4) | 16.9 (6.2-33.0) | .54 |
| Pittsburgh Sleep Quality Indexd,e | |||||||
| Pregnancy and post partum | 237 | 5.7 (5.4-6.1) | 5.6 (0.1-13.7) | 64 | 5.3 (4.7-5.9) | 5.0 (1.0-12.3) | .17 |
| Pregnancy | 276 | 5.8 (5.4-6.1) | 5.3 (0.0-15.5) | 75 | 4.7 (4.1-5.3) | 4.3 (1.0-11.8) | .005 |
| Post partum | 240 | 5.7 (5.4-6.0) | 5.4 (0.0-13.6) | 64 | 5.6 (4.9-6.3) | 5.8 (0.3-12.5) | .89 |
Missing values not used in calculation of P values. Table only includes risk factors and covariates included in primary analysis models. Complete tables are eTables 4 and 5 in Supplement 1.
From the t test (birth weight, age, IQ, and Pittsburgh Sleep Quality Index scores) and Wilcoxon rank sum test (Beck Depression Inventory–2, Beck Anxiety Inventory, and Perceived Stress Scale–14 scores).
Mean of all assessments completed during time period. Pregnancy: enrollment through day of delivery; after birth: after delivery through 2-year visit.
Pittsburgh Sleep Quality Index scores not assessed for inclusion in imputation model due to extensive missing data.
Weighted monthly mean of Pittsburgh Sleep Quality Index assessments completed during time period. At most, 1 Pittsburgh Sleep Quality Index score per day was used in the calculation. Pregnancy: enrollment through the day before delivery; post partum: 6 weeks through 9 months after delivery; and pregnancy and post partum: assessments completed during either period (only women with scores in both periods included).
Primary Analysis 1
There were no statistically significant differences between GAC scores at 4.5 years of age for children of women with epilepsy and those for children of women without epilepsy (unadjusted least-squares mean, 101.4 [95% CI, 99.9-102.9] vs 101.1 [95% CI, 98.4-103.8]; adjusted least-squares mean, 101.4 [95% CI, 100.0-102.8] vs 101.0 [95% CI, 98.4-103.6]; and parameter estimate [PE], 0.4 [95% CI, −2.5 to 3.4]; P = .77) (Figure 1A and Table 3). Variables significantly associated with lower GAC scores at 4.5 years of age in the fully adjusted model included mother’s higher postbirth perceived stress, lower educational level, major depressive episode during pregnancy, older age, and child’s male sex (Table 3).
Figure 1. Adaptive Behavior Assessment System, Third Edition (ABAS-3), General Adaptive Composite (GAC) Scores at 4.5 Years of Age for Children of Women With Epilepsy (WWE) vs Women Without Epilepsy (WWoE), and ABAS-3 GAC Scores at 4.5 Years of Age vs Third-Trimester Maximum Observed Ratio Antiseizure Medication (ASM) Concentrations for Children of WWE.
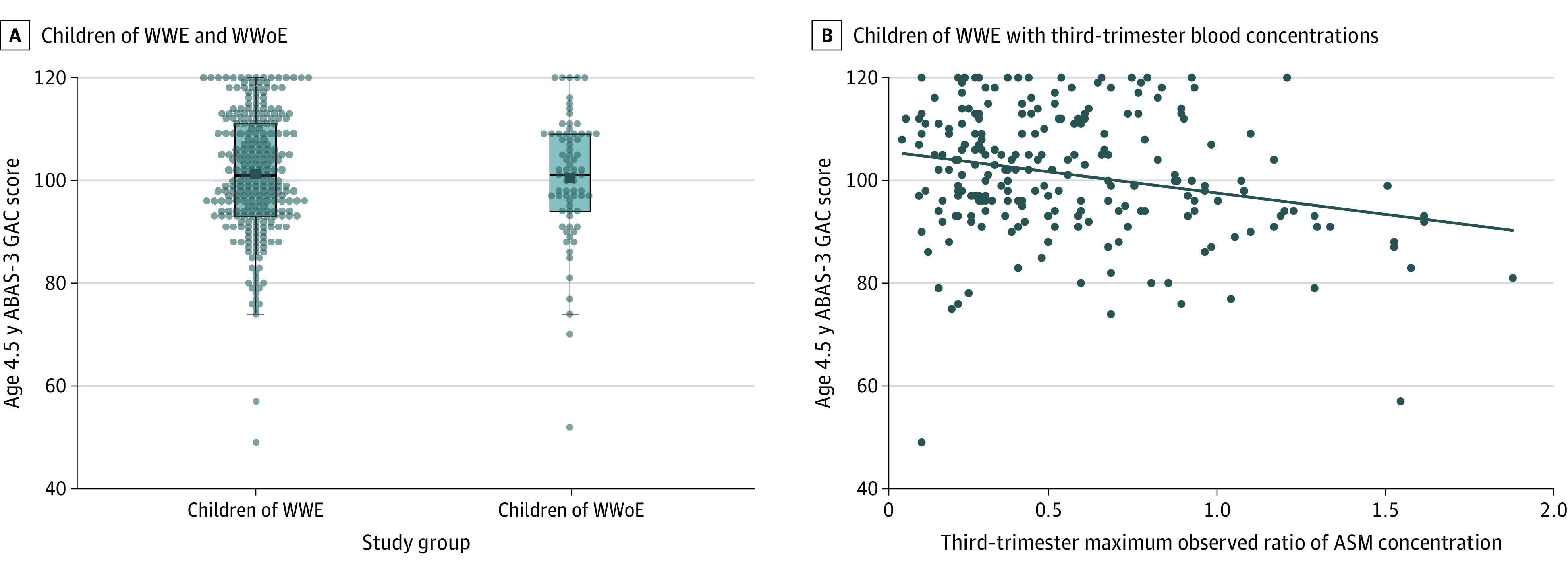
A, Box plots for children’s ABAS-3 GAC scores at 4.5 years of age for children of WWE and WWoE. Children with imputed scores were excluded (imputation population, n = 326). B, Scatterplot with fitted linear regression line for ABAS-3 GAC scores at 4.5 years of age vs third-trimester maximum observed ratio of ASM concentration for children of WWE with third-trimester blood levels. Children with imputed scores were excluded (imputation population, n = 229). Sample sizes for individual ASM regimens included in the full imputation population (n = 271) include the following: lamotrigine = 98, levetiracetam = 77, oxcarbazepine = 14, carbamazepine = 10, zonisamide = 10, topiramate = 4, lacosamide = 2, valproate = 2, gabapentin = 1, phenobarbital = 1, lamotrigine plus levetiracetam = 25, lacosamide plus levetiracetam = 5, and other polytherapy = 22 (see eTable 3 in Supplement 1 for a list of other polytherapy regimens with ≤3 children with mothers taking a regimen).
Table 3. Full Model Summaries for ABAS-3 GAC Score at 4.5 Years of Age for Children of Women With Epilepsy vs Women Without Epilepsy and for Children of Women With Epilepsy as a Function of Third-Trimester ASM Blood Concentrationsa.
| Model parameter | Parameter estimate (95% CI) | P value |
|---|---|---|
| Children of women with epilepsy and women without epilepsy, imputation analysis (n = 386) | ||
| Mother’s study group: women with epilepsy vs women without epilepsy | 0.4 (−2.5 to 3.4) | .77 |
| Mother’s IQ | 0.0 (−0.1 to 0.1) | .92 |
| Mother’s educational level | <.001 | |
| College degree (advanced) | Reference | NA |
| No college degree | −7.1 (−11.1 to −3.1) | <.001 |
| College degree (not advanced) | −1.5 (−4.5 to 1.5) | .32 |
| Mother’s postbirth mean PSS score (through child’s 2-y visit) | −0.4 (−0.6 to −0.2) | <.001 |
| Mother’s major depressive episode during pregnancy | 8.1 (0.9 to 15.3) | .03 |
| Mother’s age at enrollment | −0.3 (−0.5 to 0.0) | .047 |
| Child’s sex: male vs female | −4.1 (−6.4 to −1.7) | <.001 |
| Mother’s illicit substance use during pregnancy | −6.8 (−13.7 to 0.0) | .05 |
| Child’s birth weight, kg | 2.1 (−0.1 to 4.3) | .06 |
| Child small for gestational age | −4.7 (−9.8 to 0.4) | .07 |
| Children of women with epilepsy with third-trimester blood concentrations, imputation analysis (n = 271) | ||
| Mother’s third-trimester maximum observed ratio of ASM concentrationb | −7.8 (−12.6 to −3.1) | .001 |
| Mother’s ASM group: monotherapy vs polytherapyc | −4.9 (−9.3 to −0.5) | .03 |
| Mother’s IQ | 0.0 (−0.1 to 0.2) | .51 |
| Mother’s educational level | .01 | |
| College degree (advanced) | Reference | NA |
| No college degree | −6.2 (−11.0 to −1.5) | .01 |
| College degree (not advanced) | −0.8 (−4.3 to 2.6) | .64 |
| Mother’s postbirth mean BAI score (through child’s 2-y visit) | −0.4 (−0.7 to −0.1) | .006 |
| Mother’s age at enrollment | −0.3 (−0.6 to 0.0) | .03 |
| McMaster FAD problematic family functioning at child’s 2-y visit | −5.4 (−9.1 to −1.7) | .004 |
| Child small for gestational age | −5.9 (−11.8 to 0.0) | .049 |
| Child’s sex: male vs female | −2.8 (−5.7 to 0.1) | .05 |
| Mother’s smoking during pregnancy | −6.4 (−13.2 to 0.3) | .06 |
Abbreviations: ABAS-3, Adaptive Behavior Assessment System, Third Edition; ASM, antiseizure medication; BAI, Beck Anxiety Inventory; FAD, Family Assessment Device; GAC, General Adaptive Composite; NA, not applicable; PSS, Perceived Stress Scale–14.
Variables selected into model using a stepwise selection algorithm with the completers population. IQ and mother’s study group or mother’s third-trimester ratio of ASM concentration were included in the model a priori. The Akaike information criterion was used to compare models. The significance level for covariate entry was set to P = .10, and the significance level to remain in the model was set to P = .15.
Ratio of ASM concentration calculated as the ratio of the upper limit for therapeutic range. For mothers receiving polytherapy, the ratio of ASM concentration was calculated by summing the ratio of ASM concentration for each ASM. The maximum observed value was recorded during the third trimester, including the day of delivery.
Mother’s ASM group at time of third-trimester maximum ratio of ASM concentration.
Primary Analysis 2
Higher third-trimester ratio ASM concentrations were associated with lower GAC scores at 4.5 years of age in unadjusted analyses (PE, −7.4 [95% CI, −11.6 to −3.3]; P < .001) and adjusted analyses (PE, −7.8 [95% CI, −12.6 to −3.1]; P = .001) (Figure 1B and Table 3). Additional variables significant in the fully adjusted model included higher mother’s postbirth anxiety level, lower educational level, older age, ASM monotherapy, problematic family functioning at child’s 2-year-old visit, and born small for gestational age (Table 3).
Secondary Analyses
For study completers, the third-trimester ratio ASM concentrations and GAC scores at 4.5 years of age were negatively associated in adjusted analyses with levetiracetam monotherapy (PE, −18.9 [95% CI, −26.8 to −10.9]; P < .001) and lamotrigine monotherapy (PE, −12.0 [95% CI, −23.7 to −0.3]; P = .04) when stratified by ASM category and separately for all monotherapy (PE, −10.7 [95% CI, −16.6 to −4.8]; P < .001) when stratified by ASM group (Figure 2; eTable 8 and eTable 9 in Supplement 1). Similar findings were observed with the maximum third-trimester ratio dose (eTables 10-12 in Supplement 1).
Figure 2. Association Between Adaptive Behavior Assessment System, Third Edition (ABAS-3) General Adaptive Composite (GAC) Scores and Third-Trimester Maximum Observed Ratio of Antiseizure Medication (ASM) Concentration for Children of Women With Epilepsy by Mother’s ASM Category.

Scatterplot with fitted linear regression line for ABAS-3 GAC score at 4.5 years of age vs third-trimester maximum observed ratio of ASM concentration for children of women with epilepsy with third-trimester blood levels for lamotrigine (A), levetiracetam (B), other monotherapy (C), lamotrigine plus levetiracetam (D), and other polytherapy (E). The completers population (n = 229) included sample sizes, parameter estimates (PEs), 95% CIs, and P values from a multiple linear regression model that included the mother’s third-trimester maximum observed ratio of ASM concentration, ASM category, and their interaction, adjusting for mother’s IQ, educational level, age at enrollment, smoking during pregnancy, postbirth mean anxiety score, McMaster Family Assessment Device problematic family functioning at child’s 2-year visit, and child’s sex and small-for-gestational-age status. A total of 12 children were excluded from the adjusted analysis due to missing covariates.
Breastfeeding, folate use and dose, and sleep quality were not associated with GAC scores at 4.5 years of age in adjusted analyses (eTables 13-16 in Supplement 1). Higher maternal anxiety and perceived stress during pregnancy and postbirth periods for all children and for children of women with epilepsy analyzed separately and higher maternal depression scores during the postbirth period for all children were associated with lower GAC scores at 4.5 years of age (eTables 17 and 18 in Supplement 1).
No notable differences were observed for children of women with epilepsy vs those of women without epilepsy among the additional measures of child behavior (eTable 19 in Supplement 1). In adjusted analyses, significant associations between higher third-trimester ratio ASM concentrations with poorer adaptive and behavior outcomes were seen for ABAS-3 Social and Practical composite scores at 4.5 years of age, BASC-2 aggression and social skills scores at 4.5 years of age, and BASC-2 hyperactivity scores at 2 years of age (eTable 20 in Supplement 1). All 4 ABAS-3 Composite scores at 3 and 4.5 years of age were associated with a higher third-trimester ratio dose (eTable 21 in Supplement 1). Higher third-trimester ratio ASM concentrations for levetiracetam were associated with all 4 ABAS-3 Composite scores at 4.5 years of age and BASC-2 hyperactivity, aggression, atypicality, attention problems, and social skills scores at 4.5 years of age (eTable 22 in Supplement 1). Except for increasing aggression, similar findings were evident with analysis of the third-trimester ratio dose (eTable 23 in Supplement 1). Proportions of neurodevelopmental disorders were low and did not differ between children of women with epilepsy and children of women without epilepsy (eTable 24 in Supplement 1) and were similar across ASM categories (eTable 25 in Supplement 1).
Sensitivity Analyses
Comparisons of children included and children excluded from primary analyses are summarized in eTables 26, 27, 28, 29, 30, and 31 in Supplement 1. In sensitivity analyses of the primary analyses, results were similar to the main results (eTables 32-35 in Supplement 1).
Discussion
Although parents’ ratings of their child’s overall adaptive functioning (ABAS-3 GAC score) did not significantly differ between women with epilepsy and women without epilepsy (with adjusted mean standard scores in the average range), a significant decrease in functioning was seen in parents’ ratings with increasing third-trimester ASM blood concentrations. This decrease in functioning was evident for levetiracetam and lamotrigine when children were 4.5 years of age but not 3 years of age. A significant decrease in functioning was also observed when analyzing third-trimester dose. This decrease was evident for levetiracetam and lamotrigine when children were 4.5 years of age and only for lamotrigine when children were 3 years of age. Analysis of the other ABAS-3 composite scores revealed that the decreases predominantly involved social skills and personal or health-related self-help skill development. Factors associated with decreased adaptive functioning were mother’s age at enrollment, educational level, her level of perceived stress from birth through the child’s 2-year visit, occurrence of a major depressive episode during pregnancy, and male sex. For third-trimester ASM blood concentrations, significant factors associated with decreased adaptive functioning were mother’s age at enrollment, educational level, maternal anxiety level from birth through the child’s 2-year visit, the presence of problematic family functioning at the child’s 2-year-old visit, monotherapy, and child being born small for gestational age.
Analysis of parent ratings of their child’s emotional or behavioral functioning (BASC-2) that persisted at age 4.5 years revealed that adjusted mean T scores were all in the average range (higher scores represent increased difficulty for clinical scales, and lower scores represent increased difficulty for the adaptive scales). However, children of women with epilepsy demonstrated increased aggression and social skill deficits at 4.5 years of age with increasing third-trimester ASM blood concentrations. Increasing social skill deficits at 4.5 years of age were also observed with increasing third-trimester dose. Children of mothers who were prescribed levetiracetam exhibited increased hyperactivity, aggression, atypicality, attention problems, and social skill deficits at 4.5 years of age with increasing third-trimester ASM blood concentrations. Except for increasing aggression, similar findings were evident with analysis of third-trimester dose.
Analysis of the proportion of children at risk for neurodevelopmental disorder indicated that the percentages of children of women with epilepsy and children of women without epilepsy did not differ significantly with regard to the presence of intellectual disability, language disorder, risk for attention-deficit/hyperactivity disorder, or risk for autism spectrum disorder at 2, 3, or 4.5 years of age, nor were there differences by ASM category.
Decreasing adaptive functioning among children of women with epilepsy by 4.5 years of age was associated with increasing third-trimester ASM blood concentrations, especially for levetiracetam exposure and, to a lesser degree, lamotrigine exposure. Sample sizes for other ASMs were inadequate to assess individually. Because ASM concentrations were within therapeutic ranges for most women, exposure-dependent associations cannot be explained by excessively high exposures. All ASMs are potential teratogens known to act in an exposure-dependent manner,37 and in utero exposure-dependent associations should not be surprising. However, these findings need to be replicated in a separate cohort. Furthermore, maternal anxiety, parental stress, major depressive episodes, and problematic family functioning among these women can be associated with difficulties in adaptive functioning among their children. Clinicians need to balance seizure control during pregnancy with unnecessary ASM overexposure to the developing fetus that may be associated with long-term neurodevelopment.2,38 Thus, routine screening of these women and their offspring, along with referral for psychiatric and/or psychological intervention when necessary, are further supported.10,39
The absence of an association between adaptive functioning and breastfeeding while taking ASMs is similar to previous findings among children at 3 years of age17 and to findings of an independent Norwegian study,40 although beneficial associations were found at 6 years of age in a prior cohort.41 In this cohort, we have shown that ASM blood concentrations are generally low in children who are breastfeeding.42 Given the multiple benefits associated with breastfeeding for both the mother and the child, we encourage women with epilepsy to breastfeed. Furthermore, we did not find a significant association of periconceptual folate with adaptive functioning.
Strengths and Limitations
This study has some strengths, including its prospective design with control for potentially confounding factors with formal objective assessment of adaptive and emotional or behavioral functioning in children. To our knowledge, MONEAD is the first investigation to assess the association of ASM exposure with child outcomes using ASM blood concentrations.
This study also has some limitations, including the lack of ASM randomization due to ethical and practical issues in investigations of pregnant women with epilepsy. Like all observational studies, there may be residual and unmeasured confounding factors, which thus require replication across studies. Neuropsychological assessments at 2, 3, and 4.5 years of age may not detect associations seen at older ages. Beyond lamotrigine and levetiracetam, sample sizes for many ASMs were small, precluding individual ASM evaluations. MONEAD enrolled pregnant women with epilepsy irrespective of ASM, so the ASM distribution likely reflects current prescribing patterns at US epilepsy centers but may not reflect ASM use among the general population. However, the 2 most common ASMs in MONEAD were the same as in a recent large US database study.43
Conclusions
This cohort study suggests that adaptive functioning of children of women with epilepsy taking commonly used ASMs did not significantly differ from that of children of women without epilepsy, but there was an exposure-dependent association of ASMs with functioning. Thus, psychiatric or psychological screening and referral of women with epilepsy and their offspring are recommended when appropriate. The use of ASMs for nonepilepsy indications has expanded and now includes the majority of ASM exposures in pregnancy. The teratogenic effects of most ASMs remain unknown,1 which is an important area for future research along with delineation of genotypic risk factors for ASM teratogenicity.
eAppendix 1. MONEAD Clinical Sites and Investigators
eAppendix 2. Additional Details of Outcomes
eAppendix 3. Additional Details of Statistical Analyses
eFigure. Flowchart of Enrollment and Exclusions
eTable 1. Children With Non-Missing Assessments at Ages 2, 3, 4.5
eTable 2. ABAS-3 General Adaptive Composite Scores at Age 4.5, Before vs After COVID-19 Shutdown, Completers Population, N = 326
eTable 3. Summary of Mother's 3rd Trimester ASM
eTable 4. Demographic, Baseline Characteristics, and Risk Factors for Children of WWE vs WWoE, Categorical Variables, Children in Primary Analysis 1, N = 386
eTable 5. Demographic, Baseline Characteristics, and Risk Factors for Children of WWE vs WWoE, Continuous Variables, Children in Primary Analysis 1, N = 386
eTable 6. Demographic, Baseline Characteristics, and Risk Factors for Children of WWE and Mothers With Max Observed Ratio ASM Concentration in 3rd Trimester, Categorical Variables, Children in Primary Analysis 2, N = 271
eTable 7. Demographic, Baseline Characteristics, and Risk Factors for Children of WWE and Mothers With Max Observed Ratio ASM Concentration in 3rd Trimester, Continuous Variables, Children in Primary Analysis 2, N = 271
eTable 8. Association Between Age 4.5 ABAS-3 General Adaptive Composite Score and Maximum Observed Ratio ASM Concentrationa During 3rd Trimester by ASM Category, Children of WWE With 3rd Trimester Blood Levels, Completers Population
eTable 9. Association Between Age 4.5 ABAS-3 General Adaptive Composite Score and Maximum Observed Ratio ASM Concentration During 3rd Trimester by ASM Group, Children of WWE With 3rd Trimester Blood Levels, Completers Population
eTable 10. Association Between Age 4.5 ABAS-3 General Adaptive Composite Score and Mother's Maximum Ratio DDD During 3rd Trimester, Children of WWE on ASM in 3rd Trimester, Completers Population
eTable 11. Association Between Age 4.5 ABAS-3 General Adaptive Composite Score and Mother’s Maximum Ratio DDD During 3rd Trimester by ASM Category, Children of WWE on ASM in 3rd Trimester, Completers Population
eTable 12. Association Between Age 4.5 ABAS-3 General Adaptive Composite Score and Mother's Maximum Ratio DDD During 3rd Trimester by ASM Group, Children of WWE on ASM in 3rd Trimester, Completers Population
eTable 13. Age 4.5 ABAS-3 General Adaptive Composite Score by Breastfeeding Status, Folate Use, and Folate Dose, Children of WWE and WWoE, Completers Population
eTable 14. Association Between Age 4.5 ABAS-3 General Adaptive Composite Score and Mother's Sleep Quality (PSQI), Children of WWE and WWoE, Completers Population
eTable 15. Age 4.5 ABAS-3 General Adaptive Composite Score by Breastfeeding Status, Folate Use, and Folate Dose, Children of WWE on ASM in 3rd Trimester, Completers Population
eTable 16. Association Between Age 4.5 ABAS-3 General Adaptive Composite Score and Maternal Sleep Quality (PSQI), Children of WWE on ASM in 3rd Trimester, Completers Population
eTable 17. Association Between Age 4.5 ABAS-3 General Adaptive Composite Score and Maternal Psychiatric Risk Factors (BDI, BAI, PSS), Children of WWE and WWoE, Completers Population
eTable 18. Association Between Age 4.5 ABAS-3 General Adaptive Composite Score and Mother Psychiatric Risk Factors (BDI, BAI, PSS), Children of WWE on ASM in 3rd Trimester, Completers Population
eTable 19. Continuous Behavior Scores by Mother’s Study Group and Child’s Age, Children of WWE vs WWoE, Completers Population
eTable 20. Association Between Continuous Behavior Scores and Maximum Observed Ratio ASM Concentration in 3rd Trimester by Child’s Age, Children of WWE With 3rd Trimester Blood Levels, Completers Population
eTable 21. Association Between Continuous Behavior Scores and Maximum Ratio DDD in 3rd Trimester by Child’s Age, Children of WWE on ASM in 3rd Trimester, Completers Population
eTable 22. Association Between Continuous Behavior Scores and Maximum Observed Ratio ASM Concentration in 3rd Trimester by ASM Category and Child’s Age, Children of WWE with 3rd Trimester Blood Levels, Completers Population
eTable 23. Association Between Continuous Behavior Scores and Maximum Ratio DDD in 3rd Trimester by ASM Category and Child's Age, Children of WWE With 3rd Trimester Blood Levels, Completers Population
eTable 24. Categorical Behavior Scores by Mother’s Study Group and Child’s Age, Children of WWE vs WWoE, Completers Population
eTable 25. Categorical Behavior Scores by Mother's ASM Category, Children of WWE With 3rd Trimester Blood Levels, Completers Population
eTable 26. Demographic, Baseline Characteristics, and Risk Factors for Children Included vs Excluded From Primary Analysis 1, Categorical Variables, Children in Primary Analysis 1, N = 451
eTable 27. Demographic, Baseline Characteristics, and Risk Factors for Children Included vs Excluded From Primary Analysis 1, Continuous Variables, Children in Primary Analysis 1, N = 451
eTable 28. Outcomes for Children Included vs Excluded From Primary Analysis 1, All Children, N = 451
eTable 29. Demographic, Baseline Characteristics, and Risk Factors for Children Included vs Excluded From Primary Analysis 2, Categorical Variables, Children of WWE on ASM in 3rd Trimester, N = 329
eTable 30. Demographic, Baseline Characteristics, and Risk Factors for Children Included vs Excluded From Primary Analysis 2, Continuous Variables, Children of WWE on ASM in 3rd Trimester, N = 329
eTable 31. Outcomes for Children Included vs Excluded From Primary Analysis 2, Children of WWE on ASM in 3rd Trimester, N = 329
eTable 32. Age 4.5 ABAS-3 General Adaptive Composite Score by Mother’s Study Group – Sensitivity Analysis, Children of WWE and WWoE
eTable 33. Association Between Age 4.5 ABAS-3 General Adaptive Composite Score and Maximum Observed Ratio ASM Concentration During 3rd Trimester – Sensitivity Analysis, Children of WWE on ASM During the 3rd Trimester
eTable 34. Summary of Age 4.5 ABAS-3 General Adaptive Composite Score by Mother’s Study Group and Pregnancy ASM Status, Children of WWE and WWoE, Completers Population, N = 326
eTable 35. Age 4.5 ABAS-3 General Adaptive Composite Score by Mother's Study Group - Sensitivity Analysis Excluding Children of WWE Not on ASM During Pregnancy, Children of WWE on ASM During Pregnancy and WWoE, Completers Population, N = 313
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Meador KJ, Loring DW. Developmental effects of antiepileptic drugs and the need for improved regulations. Neurology. 2016;86(3):297-306. doi: 10.1212/WNL.0000000000002119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomson T, Battino D, Bromley R, et al. Management of epilepsy in pregnancy: a report from the International League Against Epilepsy Task Force on Women and Pregnancy. Epileptic Disord. 2019;21(6):497-517. doi: 10.1111/epi.16395 [DOI] [PubMed] [Google Scholar]
- 3.Meador KJ, Baker GA, Browning N, et al. ; NEAD Study Group . Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med. 2009;360(16):1597-1605. doi: 10.1056/NEJMoa0803531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meador KJ, Baker GA, Browning N, et al. ; NEAD Study Group . Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12(3):244-252. doi: 10.1016/S1474-4422(12)70323-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen MJ, Meador KJ, May R, et al. ; NEAD Study Group . Fetal antiepileptic drug exposure and learning and memory functioning at 6 years of age: the NEAD prospective observational study. Epilepsy Behav. 2019;92:154-164. doi: 10.1016/j.yebeh.2018.12.031 [DOI] [PubMed] [Google Scholar]
- 6.Cohen MJ, Meador KJ, Browning N, et al. Fetal antiepileptic drug exposure: motor, adaptive, and emotional/behavioral functioning at age 3 years. Epilepsy Behav. 2011;22(2):240-246. doi: 10.1016/j.yebeh.2011.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen MJ, Meador KJ, Browning N, et al. ; NEAD Study Group . Fetal antiepileptic drug exposure: adaptive and emotional/behavioral functioning at age 6 years. Epilepsy Behav. 2013;29(2):308-315. doi: 10.1016/j.yebeh.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meador KJ, Pennell PB, May RC, et al. ; MONEAD Investigator Group . Changes in antiepileptic drug-prescribing patterns in pregnant women with epilepsy. Epilepsy Behav. 2018;84:10-14. doi: 10.1016/j.yebeh.2018.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bromley RL, Calderbank R, Cheyne CP, et al. ; UK Epilepsy and Pregnancy Register . Cognition in school-age children exposed to levetiracetam, topiramate, or sodium valproate. Neurology. 2016;87(18):1943-1953. doi: 10.1212/WNL.0000000000003157 [DOI] [PubMed] [Google Scholar]
- 10.Huber-Mollema Y, Oort FJ, Lindhout D, Rodenburg R. Behavioral problems in children of mothers with epilepsy prenatally exposed to valproate, carbamazepine, lamotrigine, or levetiracetam monotherapy. Epilepsia. 2019;60(6):1069-1082. doi: 10.1111/epi.15968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huber-Mollema Y, van Iterson L, Oort FJ, Lindhout D, Rodenburg R. Neurocognition after prenatal levetiracetam, lamotrigine, carbamazepine or valproate exposure. J Neurol. 2020;267(6):1724-1736. doi: 10.1007/s00415-020-09764-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blotière PO, Miranda S, Weill A, et al. Risk of early neurodevelopmental outcomes associated with prenatal exposure to the antiepileptic drugs most commonly used during pregnancy: a French nationwide population-based cohort study. BMJ Open. 2020;10(6):e034829. doi: 10.1136/bmjopen-2019-034829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomson T, Battino D, Bonizzoni E, et al. ; EURAP Study Group . Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP Epilepsy and Pregnancy Registry. Lancet Neurol. 2011;10(7):609-617. doi: 10.1016/S1474-4422(11)70107-7 [DOI] [PubMed] [Google Scholar]
- 14.Reisinger TL, Newman M, Loring DW, Pennell PB, Meador KJ. Antiepileptic drug clearance and seizure frequency during pregnancy in women with epilepsy. Epilepsy Behav. 2013;29(1):13-18. doi: 10.1016/j.yebeh.2013.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voinescu PE, Park S, Chen LQ, et al. Antiepileptic drug clearances during pregnancy and clinical implications for women with epilepsy. Neurology. 2018;91(13):e1228-e1236. doi: 10.1212/WNL.0000000000006240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meador KJ, Cohen MJ, Loring DW, et al. ; Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs Investigator Group . Two-year-old cognitive outcomes in children of pregnant women with epilepsy in the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs Study. JAMA Neurol. 2021;78(8):927-936. doi: 10.1001/jamaneurol.2021.1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meador KJ, Cohen MJ, Loring DW, et al. ; MONEAD Investigator Group . Cognitive outcomes at age 3 years in children with fetal exposure to antiseizure medications (MONEAD study) in the USA: a prospective, observational cohort study. Lancet Neurol. 2023;22(8):712-722. doi: 10.1016/S1474-4422(23)00199-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison PL, Oakland T. Adaptive Behavior Assessment System. 3rd ed. Western Psychological Services; 2015. [Google Scholar]
- 19.Reynolds C, Kamphaus RW. Behavior Assessment System for Children. 2nd ed. American Guidance Service; 2004. [Google Scholar]
- 20.Abidin RR. Parenting Stress Index. 4th ed. Psychological Assessment Resources, Inc; 2007. [Google Scholar]
- 21.Instructions for Taking and Scoring the M-CHAT-R autism test. M-CHAT-R (Modified Checklist for Autism in Toddlers, Revised). Austism Speaks. Accessed October 11, 2023. https://www.autismspeaks.org/screen-your-child
- 22.Gilliam JE. Gilliam Autism Rating Scale. 3rd ed. Pro-Ed; 2014. [Google Scholar]
- 23.Constantino JN, Gruber CP. The Social Responsiveness Scale. 2nd ed. Western Psychological Services; 2012. [Google Scholar]
- 24.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013. [Google Scholar]
- 25.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI). Psychological Corporation; 1999. [Google Scholar]
- 26.Epstein NB, Baldwin LM, Bishop DS. The McMaster Family Assessment Device. J Marital Fam Ther. 1983;9(2):171-180. doi: 10.1111/j.1752-0606.1983.tb01497.x [DOI] [Google Scholar]
- 27.Ryan CE, Epstein NB, Keitner GI. Evaluating and Treating Families: The McMaster Approach. Taylor & Francis; 2005. [Google Scholar]
- 28.Adab N, Kini U, Vinten J, et al. The longer term outcome of children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry. 2004;75(11):1575-1583. doi: 10.1136/jnnp.2003.029132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beck AT. Beck Anxiety Inventory (BAI). The Psychological Corporation; 1990. [Google Scholar]
- 30.Beck AT, Steer RA. Beck Depression Inventory Manual–Revised. The Psychological Corporation; 1996. [Google Scholar]
- 31.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396. doi: 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- 32.Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737-740. doi: 10.1016/S0022-3999(02)00330-6 [DOI] [PubMed] [Google Scholar]
- 33.Li KH. Imputation using Markov chains. J Stat Comput Simul. 1988;30:57-79. doi: 10.1080/00949658808811085 [DOI] [Google Scholar]
- 34.Little RJA, Rubin DB. Statistical Analysis With Missing Data. 2nd ed. John Wiley and Sons; 2002. [Google Scholar]
- 35.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13-22. doi: 10.1093/biomet/73.1.13 [DOI] [Google Scholar]
- 36.Ikonomidou C, Bittigau P, Ishimaru MJ, et al. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287(5455):1056-1060. doi: 10.1126/science.287.5455.1056 [DOI] [PubMed] [Google Scholar]
- 37.Tomson T, Battino D, Bonizzoni E, et al. ; EURAP Study Group . Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP Registry. Lancet Neurol. 2018;17(6):530-538. doi: 10.1016/S1474-4422(18)30107-8 [DOI] [PubMed] [Google Scholar]
- 38.Pennell PB, French JA, May RC, et al. ; MONEAD Study Group . Changes in seizure frequency and antiepileptic therapy during pregnancy. N Engl J Med. 2020;383(26):2547-2556. doi: 10.1056/NEJMoa2008663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huber-Mollema Y, Oort FJ, Lindhout D, Rodenburg R. Maternal epilepsy and behavioral development of the child: family factors do matter. Epilepsy Behav. 2019;94:222-232. doi: 10.1016/j.yebeh.2019.03.030 [DOI] [PubMed] [Google Scholar]
- 40.Veiby G, Engelsen BA, Gilhus NE. Early child development and exposure to antiepileptic drugs prenatally and through breastfeeding: a prospective cohort study on children of women with epilepsy. JAMA Neurol. 2013;70(11):1367-1374. doi: 10.1001/jamaneurol.2013.4290 [DOI] [PubMed] [Google Scholar]
- 41.Meador KJ, Baker GA, Browning N, et al. ; Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) Study Group . Breastfeeding in children of women taking antiepileptic drugs: cognitive outcomes at age 6 years. JAMA Pediatr. 2014;168(8):729-736. doi: 10.1001/jamapediatrics.2014.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Birnbaum AK, Meador KJ, Karanam A, et al. ; MONEAD Investigator Group . Antiepileptic drug exposure in infants of breastfeeding mothers with epilepsy. JAMA Neurol. 2020;77(4):441-450. doi: 10.1001/jamaneurol.2019.4443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim H, Faught E, Thurman DJ, Fishman J, Kalilani L. Antiepileptic drug treatment patterns in women of childbearing age with epilepsy. JAMA Neurol. 2019;76(7):783-790. doi: 10.1001/jamaneurol.2019.0447 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. MONEAD Clinical Sites and Investigators
eAppendix 2. Additional Details of Outcomes
eAppendix 3. Additional Details of Statistical Analyses
eFigure. Flowchart of Enrollment and Exclusions
eTable 1. Children With Non-Missing Assessments at Ages 2, 3, 4.5
eTable 2. ABAS-3 General Adaptive Composite Scores at Age 4.5, Before vs After COVID-19 Shutdown, Completers Population, N = 326
eTable 3. Summary of Mother's 3rd Trimester ASM
eTable 4. Demographic, Baseline Characteristics, and Risk Factors for Children of WWE vs WWoE, Categorical Variables, Children in Primary Analysis 1, N = 386
eTable 5. Demographic, Baseline Characteristics, and Risk Factors for Children of WWE vs WWoE, Continuous Variables, Children in Primary Analysis 1, N = 386
eTable 6. Demographic, Baseline Characteristics, and Risk Factors for Children of WWE and Mothers With Max Observed Ratio ASM Concentration in 3rd Trimester, Categorical Variables, Children in Primary Analysis 2, N = 271
eTable 7. Demographic, Baseline Characteristics, and Risk Factors for Children of WWE and Mothers With Max Observed Ratio ASM Concentration in 3rd Trimester, Continuous Variables, Children in Primary Analysis 2, N = 271
eTable 8. Association Between Age 4.5 ABAS-3 General Adaptive Composite Score and Maximum Observed Ratio ASM Concentrationa During 3rd Trimester by ASM Category, Children of WWE With 3rd Trimester Blood Levels, Completers Population
eTable 9. Association Between Age 4.5 ABAS-3 General Adaptive Composite Score and Maximum Observed Ratio ASM Concentration During 3rd Trimester by ASM Group, Children of WWE With 3rd Trimester Blood Levels, Completers Population
eTable 10. Association Between Age 4.5 ABAS-3 General Adaptive Composite Score and Mother's Maximum Ratio DDD During 3rd Trimester, Children of WWE on ASM in 3rd Trimester, Completers Population
eTable 11. Association Between Age 4.5 ABAS-3 General Adaptive Composite Score and Mother’s Maximum Ratio DDD During 3rd Trimester by ASM Category, Children of WWE on ASM in 3rd Trimester, Completers Population
eTable 12. Association Between Age 4.5 ABAS-3 General Adaptive Composite Score and Mother's Maximum Ratio DDD During 3rd Trimester by ASM Group, Children of WWE on ASM in 3rd Trimester, Completers Population
eTable 13. Age 4.5 ABAS-3 General Adaptive Composite Score by Breastfeeding Status, Folate Use, and Folate Dose, Children of WWE and WWoE, Completers Population
eTable 14. Association Between Age 4.5 ABAS-3 General Adaptive Composite Score and Mother's Sleep Quality (PSQI), Children of WWE and WWoE, Completers Population
eTable 15. Age 4.5 ABAS-3 General Adaptive Composite Score by Breastfeeding Status, Folate Use, and Folate Dose, Children of WWE on ASM in 3rd Trimester, Completers Population
eTable 16. Association Between Age 4.5 ABAS-3 General Adaptive Composite Score and Maternal Sleep Quality (PSQI), Children of WWE on ASM in 3rd Trimester, Completers Population
eTable 17. Association Between Age 4.5 ABAS-3 General Adaptive Composite Score and Maternal Psychiatric Risk Factors (BDI, BAI, PSS), Children of WWE and WWoE, Completers Population
eTable 18. Association Between Age 4.5 ABAS-3 General Adaptive Composite Score and Mother Psychiatric Risk Factors (BDI, BAI, PSS), Children of WWE on ASM in 3rd Trimester, Completers Population
eTable 19. Continuous Behavior Scores by Mother’s Study Group and Child’s Age, Children of WWE vs WWoE, Completers Population
eTable 20. Association Between Continuous Behavior Scores and Maximum Observed Ratio ASM Concentration in 3rd Trimester by Child’s Age, Children of WWE With 3rd Trimester Blood Levels, Completers Population
eTable 21. Association Between Continuous Behavior Scores and Maximum Ratio DDD in 3rd Trimester by Child’s Age, Children of WWE on ASM in 3rd Trimester, Completers Population
eTable 22. Association Between Continuous Behavior Scores and Maximum Observed Ratio ASM Concentration in 3rd Trimester by ASM Category and Child’s Age, Children of WWE with 3rd Trimester Blood Levels, Completers Population
eTable 23. Association Between Continuous Behavior Scores and Maximum Ratio DDD in 3rd Trimester by ASM Category and Child's Age, Children of WWE With 3rd Trimester Blood Levels, Completers Population
eTable 24. Categorical Behavior Scores by Mother’s Study Group and Child’s Age, Children of WWE vs WWoE, Completers Population
eTable 25. Categorical Behavior Scores by Mother's ASM Category, Children of WWE With 3rd Trimester Blood Levels, Completers Population
eTable 26. Demographic, Baseline Characteristics, and Risk Factors for Children Included vs Excluded From Primary Analysis 1, Categorical Variables, Children in Primary Analysis 1, N = 451
eTable 27. Demographic, Baseline Characteristics, and Risk Factors for Children Included vs Excluded From Primary Analysis 1, Continuous Variables, Children in Primary Analysis 1, N = 451
eTable 28. Outcomes for Children Included vs Excluded From Primary Analysis 1, All Children, N = 451
eTable 29. Demographic, Baseline Characteristics, and Risk Factors for Children Included vs Excluded From Primary Analysis 2, Categorical Variables, Children of WWE on ASM in 3rd Trimester, N = 329
eTable 30. Demographic, Baseline Characteristics, and Risk Factors for Children Included vs Excluded From Primary Analysis 2, Continuous Variables, Children of WWE on ASM in 3rd Trimester, N = 329
eTable 31. Outcomes for Children Included vs Excluded From Primary Analysis 2, Children of WWE on ASM in 3rd Trimester, N = 329
eTable 32. Age 4.5 ABAS-3 General Adaptive Composite Score by Mother’s Study Group – Sensitivity Analysis, Children of WWE and WWoE
eTable 33. Association Between Age 4.5 ABAS-3 General Adaptive Composite Score and Maximum Observed Ratio ASM Concentration During 3rd Trimester – Sensitivity Analysis, Children of WWE on ASM During the 3rd Trimester
eTable 34. Summary of Age 4.5 ABAS-3 General Adaptive Composite Score by Mother’s Study Group and Pregnancy ASM Status, Children of WWE and WWoE, Completers Population, N = 326
eTable 35. Age 4.5 ABAS-3 General Adaptive Composite Score by Mother's Study Group - Sensitivity Analysis Excluding Children of WWE Not on ASM During Pregnancy, Children of WWE on ASM During Pregnancy and WWoE, Completers Population, N = 313
Nonauthor Collaborators
Data Sharing Statement


