Abstract
Streptococcus iniae was isolated from diseased wild fish collected near a mariculture facility where gilthead sea bream and European sea bass exhibited a similar infection. Species-specific PCR and ribotyping confirmed that wild and cultured fish were infected by a single S. iniae clone. Wild fish are therefore potential amplifiers of pathogenic S. iniae strains.
Streptococcus iniae (8) infections of fish were reported in salmonids (Onchorynchus mykiss, Onchorynchus kisutch) and tilapines (Oreochromis spp.) cultured in Israel (2) and in the Far East (6). The recent isolation of S. iniae from tilapines and hybrid striped bass (Morone saxatilis × Morone chrysops) farmed in the United States (3, 7) indicates that infections due to S. iniae are an expanding threat to aquaculture. S. iniae is also a zoonotic agent (10). Restriction fragment length polymorphism (RFLP) ribotyping of S. iniae was the only tool available to characterize strains during various outbreaks, demonstrating the existence of different clones (ribotypes) specific to each country (4).
In the autumn of 1996, populations of gilthead sea bream (Sparus aurata) and European sea bass (Dicentrarchus labrax) in a mariculture facility along the Israeli Mediterranean shore suffered mortalities. At the same time, mortalities were observed among wild fish (spine foot; Siganus rivulatus) living in the proximity of the facility. Pure colonies of beta-hemolytic gram-positive cocci were isolated from brains and kidneys of diseased fish (10 sea bream, 10 sea bass, and 10 wild spine foot) on Columbia (Difco) agar plates supplemented with 5% (vol/vol) sheep blood. API 20 STREP and API 50CH kits (bioMerieux, Marcy l’Etoile, France), incubated at 24°C, characterized all the isolates as S. iniae (3). This is the first report of S. iniae infection in wild fish, in gilthead sea bream, and in European sea bass.
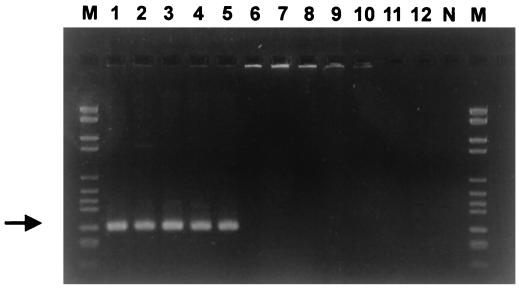
Definitive identification of the isolates was achieved by PCR amplification of the S. iniae DNA sequence with species-specific primers. Sin-1 [5′-CTAGAGTACACATGTACT(AGCT)AAG-3′] and Sin-2 (5′-GGATTTTCCACTCCCATTAC-3′) were selected by comparing the 16S rRNA gene sequence of S. iniae with those of other bacteria by use of the Genetics Computer Group (GCG) (version 9.0, UNIX) FastA program. PCRs were carried out in a final volume of 50 μl containing 1 U of Vent DNA polymerase (New England Biolabs), 1× buffer (provided with the Vent DNA polymerase by New England Biolabs), nucleotides (final concentration for each nucleotide, 0.25 mmol/liter), bovine serum albumin (0.1 mg/ml), and primers (8 ng/ml each). Bacterial DNA was obtained from cells by touching colonies, grown on blood agar medium, with a sterile syringe needle which was then dipped into the PCR mixture. Typical cycling parameters were 1 min of denaturation (94°C), 1 min of annealing (55°C), and 90 s of extension (72°C) for 35 cycles. The reaction was started by a denaturation step (3 min at 94°C) and ended by a 10-min extension step at 72°C. The PCR-amplified samples were subjected to electrophoresis (90 min, 90 V) in a 3% agarose gel (FMC) with 1× TAE buffer (40 mM Tris-acetate, 1 mM EDTA, pH 8) and stained by ethidium bromide. The DNA molecular weight marker VI (Boehringer) was used to estimate the size of the amplified fragment. The PCR assay resulted in the amplification of a 300-bp band detected among all the strains tested, including the marine fish isolates and the reference strains S. iniae ATCC 29178T and S. iniae ND 2-16 (CIP 103769) (Fig. 1, lanes 1 to 5). No specific band was amplified when other fish pathogens were used, thus demonstrating the specificity of the PCR assay (Fig. 1, lanes 6 to 12).
FIG. 1.
Specificity of the S. iniae PCR assay. Agarose gel (3%)-resolved ethidium bromide-stained PCR products from template DNA of the following needle-touched bacterial colonies. Lane 1, S. iniae ATCC 29178T; lane 2, S. iniae ND 2-16; lane 3, isolate KFP 109 (sea bream); lane 4, isolate KFP 101 (European sea bass); lane 5, isolate KFP 110 (wild spine foot); lane 6, S. agalactiae ATCC 13813; lane 7, Lactococcus garvieae ATCC 43921T; lane 8, Vagococcus salmoninarum NCFB 2777T; lane 9, L. lactis NCFB 604T; lane 10, S. parauberis ATCC 13387; lane 11, L. piscium NCFB 2778T; lane 12, Aeromonas salmonicida 3173/86; N, negative control (no DNA); M, DNA molecular weight marker VI. The arrow indicates the expected length of 300 bp.
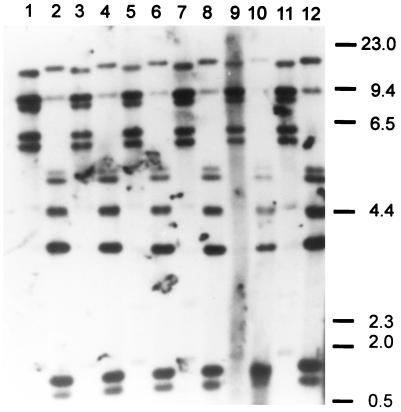
Six isolates from the different fish species were randomly selected for RFLP ribotyping. DNAs were extracted with phenol-chloroform as described previously (3). Five micrograms of genomic DNA from each isolate was completely digested (16 h at 37°C) with 50 U each of the restriction enzymes HindIII and EcoRI (Promega, Madison, Wis.) and separated by electrophoresis on a 20-cm-long 1% agarose gel. Southern blotting and all other procedures, including hybridization to a digoxigenin (Boehringer)-labeled 7.5-kb BamHI fragment of pKK3535 comprising the E. coli rRNA B operon (1), were performed as described previously (4). Chemiluminescence detection (Boehringer) revealed that all of the isolates clustered in a single ribotype (Fig. 2). EcoRI digests of DNAs revealed five restriction fragments of 5 to 14 kb (Fig. 2, lanes 1, 3, 5, 7, 9, and 11), identical to the American EcoRI type a ribotype (4). Ribotyping of HindIII digests (Fig. 2, lanes 2, 4, 6, 8, 10, and 12) resulted in seven restriction fragments, invariably identical to the American HindIII type a ribotype (4).
FIG. 2.
RFLP ribotyping of S. iniae isolates. Lanes 1 and 2, sea bream isolate KFP 109 (EcoRI and HindIII digests); lanes 3 and 4, sea bream isolate KFP 111 (EcoRI and HindIII digests); lanes 5 and 6, sea bass isolate KFP 101 (EcoRI and HindIII digests); lanes 7 and 8, sea bass isolate KFP 112 (EcoRI and HindIII digests); lanes 9 and 10, spine foot isolate KFP 103 (EcoRI and HindIII digests); lanes 11 and 12, spine foot isolate KFP 110 (EcoRI and HindIII digests).
Since, epidemiologically, all fish were infected by descendants of a single clone, it is likely that they acquired the infection from the same source. Mortality in wild spine foot chronologically preceded that observed in the cultured fish. The pathology seen in the wild fish was more severe than that in the cultured fish. In the former, pathology was expressed as systemic disease with diffuse visceral hemorrhages versus, in the latter, exudative meningitis accompanied by panophthalmitis. These data indicate that wild spine foot are more susceptible to the infection than are cultured fish. Wild spine foot are therefore natural indicators of the disease. Furthermore, the close epidemiological relationships between strains suggest that a cross-infection occurred between wild fish and cultured fish. However, the nature of the reservoir of this pathogen is still unknown. It has been proposed that organic matter, mud, and even seawater might be the reservoir of bacteria pathogenic for fish (5). Romalde et al. (9) have shown that Atlantic salmon (Salmo salar) are carriers of Enterococcus pathogenic to turbot (Scophthalmus maximus) and transmit the bacteria to cultured turbot. Since these investigators did not carry out a molecular epidemiological study, no definite conclusions can be drawn. The finding that, under experimental conditions, horizontal and oral-fecal routes are the modes of transmission in turbot is of only limited significance. Cultured fish live in an environment in which food, feces, and water are practically inseparable,and it is obvious that infection is spread from the surroundings. The high pathogenicity of S. iniae for wild fish shows a poor evolutionary adaptation to this species and suggests that the cultured fish might have been the source of infection even if they died later.
The presence of wild spine foot, although playing a negative role by disseminating the disease, might be helpful in limiting the severity of the event. Sudden death of wild fish in the proximity of a farm could be used as an alert signal and should prompt measures to limit losses. The epidemiological puzzle concerning the nature of the natural reservoir of S. iniae remains unsolved.
Acknowledgments
This work was supported by a joint American-Israeli grant (BARD IS-2727-96R).
REFERENCES
- 1.Brosius J, Ulrich A, Raker M A, Gray A, Dull T J, Gutell R R, Noller H F. Construction and fine mapping of recombinant plasmids containing the rRNB ribosomal RNA operon of E. coli. Plasmid. 1981;6:112–118. doi: 10.1016/0147-619x(81)90058-5. [DOI] [PubMed] [Google Scholar]
- 2.Eldar A, Bejerano Y, Livoff A, Horovitz A, Bercovier H. Experimental streptococcal meningo-encephalitis in cultured fish. Vet Microbiol. 1995;43:33–40. doi: 10.1016/0378-1135(94)00052-x. [DOI] [PubMed] [Google Scholar]
- 3.Eldar A, Frelier P F, Asanta L, Varner P W, Lawhon S, Bercovier H. Streptococcus shiloi, the name for an agent causing septicemic infection in fish, is a junior synonym of Streptococcus iniae. Int J Syst Bacteriol. 1995;45:840–842. doi: 10.1099/00207713-45-4-840. [DOI] [PubMed] [Google Scholar]
- 4.Eldar A, Lawhon S, Frelier P F, Asanta L, Simpson B R, Varner P W, Bercovier H. DNA restriction length polymorphisms of 16S rRNA and of whole rRNA genes (ribotyping) of S. iniae isolates from the United States and Israel. FEMS Microbiol Lett. 1997;151:155–162. doi: 10.1111/j.1574-6968.1997.tb12564.x. [DOI] [PubMed] [Google Scholar]
- 5.Kitao T, Aoki T, Iwata K. Epidemiologic study on streptococcosis of cultured yellowtail (Seriola quinqueradiata). I. Distribution of Streptococcus sp. in seawater and muds around yellowtail farms. Bull Jpn Soc Sci Fish. 1979;45:567–572. [Google Scholar]
- 6.Kitao T. Streptococcal infections. In: Inglis V, Roberts R J, Bromage N R, editors. Bacterial diseases of fish. Oxford, United Kingdom: Blackwell; 1993. pp. 196–210. [Google Scholar]
- 7.Perera R P, Johnson S K, Collins M D, Lewis D H. Streptococcus iniae associated with mortality of Tilapia nilotica × T. aurea hybrids. J Aquat Anim Health. 1995;6:335–340. [Google Scholar]
- 8.Pier G B, Madin S H. Streptococcus iniae sp. nov., a beta hemolytic streptococcus isolated from an Amazon freshwater dolphin, Inia geoffrensis. Int J Syst Bacteriol. 1976;26:545–553. [Google Scholar]
- 9.Romalde J L, Magariños B, Nuñez S, Barja J L, Toranzo A E. Host range susceptibility of Enterococcus sp. strains isolated from diseased turbot: possible routes of infection. Appl Environ Microbiol. 1996;62:607–611. doi: 10.1128/aem.62.2.607-611.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinstein M R, Litt M, Kertesz D A, Wyper P, Rose D, Coulter M, NcGeer A, Facklam R, Ostach D, Willey B M, Borczyk A, Low D E. Invasive infections due to a fish pathogen, Streptococcus iniae. New Engl J Med. 1997;337:589–594. doi: 10.1056/NEJM199708283370902. [DOI] [PubMed] [Google Scholar]