Abstract
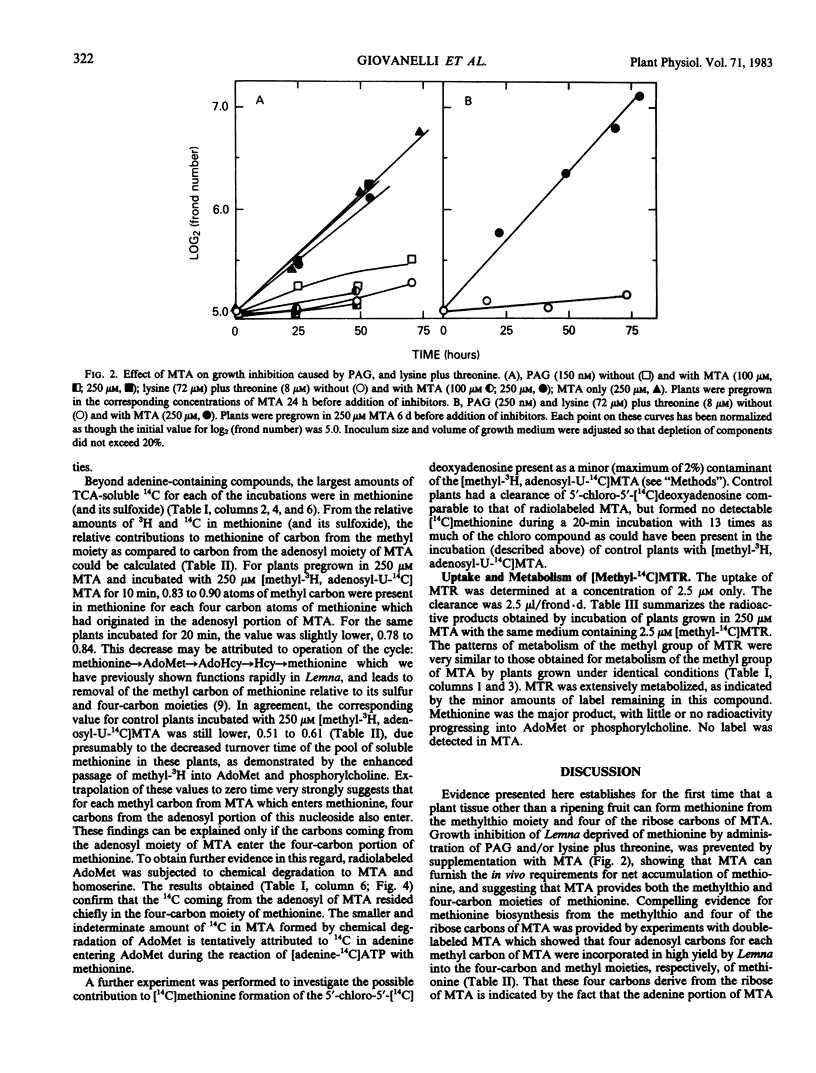
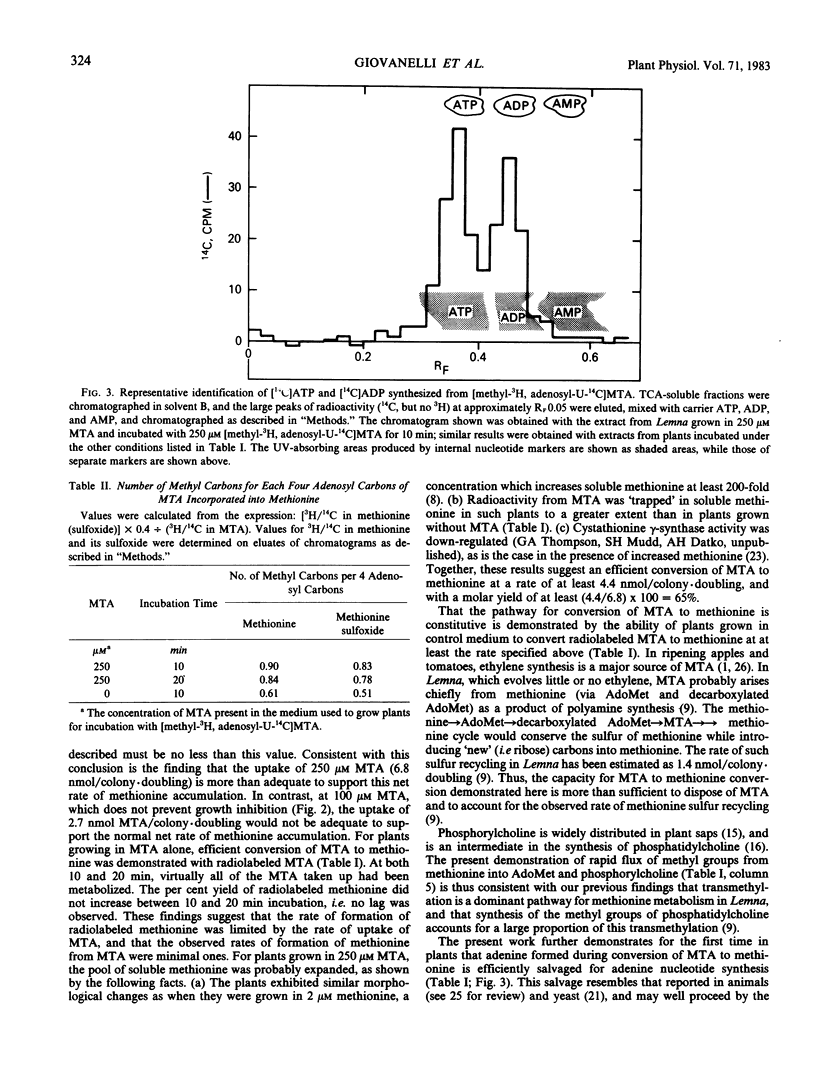
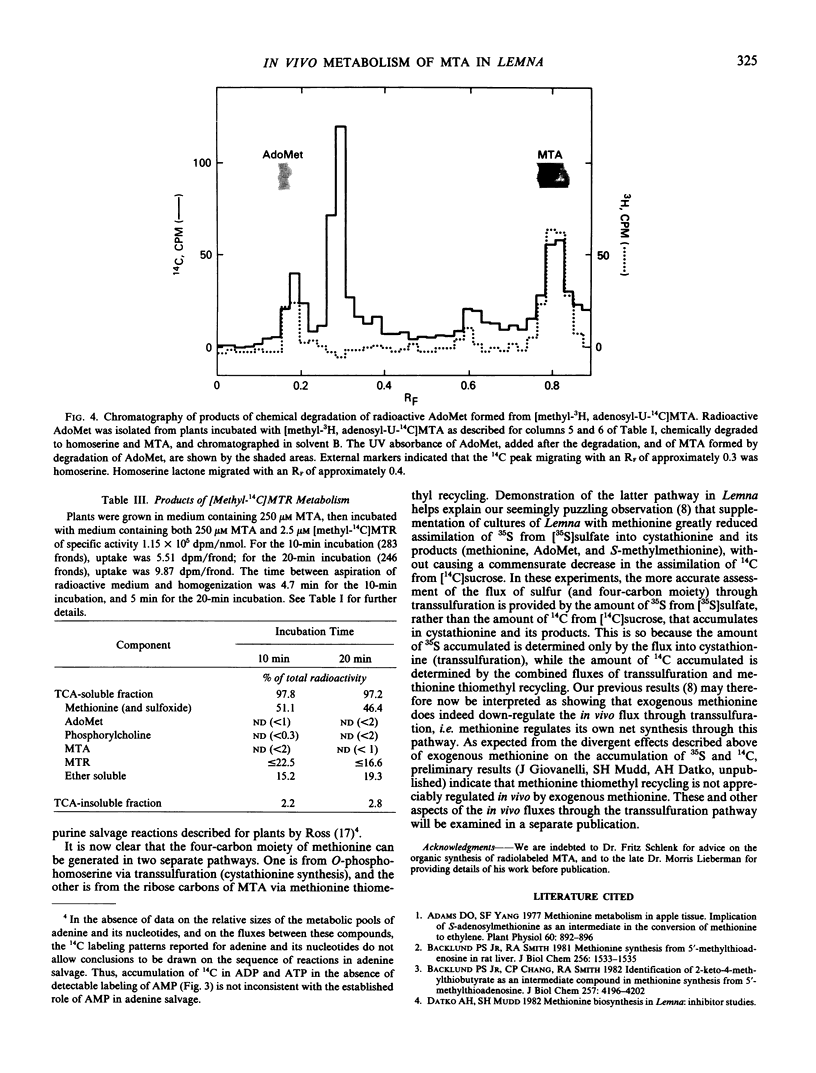
Evidence is presented that Lemna converts 5′-methylthioadenosine (MTA) to methionine. The methylthio moiety and four of the ribose carbons of the nucleoside contribute the methylthio and the four-carbon moieties of methionine. Plants grown in the presence of inhibitors which block methionine biosynthesis convert MTA to methionine at a rate sufficient to sustain normal growth (at least 4.4 nanomoles per colony per doubling with a molar yield of at least 65%). The pathway for conversion is shown to be constitutive in plants grown in standard medium and to function at a rate sufficient to dispose of MTA arising as a result of polyamine synthesis, and to explain the observed rate (1.4 nanomoles per colony per doubling) of preferential recycling of methionine sulfur (Giovanelli, Mudd, Datko 1981 Biochem Biophys Res Commun 100: 831-839). Rapid entry of methionine methyl into S-adenosylmethionine and phosphorylcholine was observed for plants grown in standard medium. Adenine generated during this cycle is efficiently salvaged into ADP and ATP.
Conversion of MTA to methionine completes the steps in methionine thiomethyl recycling (Giovanelli, Mudd, Datko 1981 Biochem Biophys Res Commun 100: 831-839) in which the sulfur of methionine is retained while the four-carbon moiety is not. The findings further show that the four-carbon moiety of methionine can be derived via the ribose moiety of MTA in addition to the established route from O-phosphohomoserine via transsulfuration. Previous observations (Giovanelli, Mudd, Datko 1980 Biochemistry of Plants pp 453-505) can now be interpreted as establishing that exogenous methionine down-regulates its own net synthesis via the transsulfuration pathway.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Yang S. F. Methionine metabolism in apple tissue: implication of s-adenosylmethionine as an intermediate in the conversion of methionine to ethylene. Plant Physiol. 1977 Dec;60(6):892–896. doi: 10.1104/pp.60.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backlund P. S., Jr, Chang C. P., Smith R. A. Identification of 2-keto-4-methylthiobutyrate as an intermediate compound in methionine synthesis from 5'-methylthioadenosine. J Biol Chem. 1982 Apr 25;257(8):4196–4202. [PubMed] [Google Scholar]
- Backlund P. S., Jr, Smith R. A. Methionine synthesis from 5'-methylthioadenosine in rat liver. J Biol Chem. 1981 Feb 25;256(4):1533–1535. [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H., Giovanelli J. Lemna paucicostata Hegelm. 6746: DEVELOPMENT OF STANDARDIZED GROWTH CONDITIONS SUITABLE FOR BIOCHEMICAL EXPERIMENTATION. Plant Physiol. 1980 May;65(5):906–912. doi: 10.1104/pp.65.5.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H., Datko A. H. Recycling of methionine sulfur in a higher plant by two pathways characterized by either loss or retention of the 4-carbon moiety. Biochem Biophys Res Commun. 1981 May 29;100(2):831–839. doi: 10.1016/s0006-291x(81)80249-5. [DOI] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H. Sulfuration of O-acetylhomoserine and O-acetylserine by two enzyme fractions from spinach. Biochem Biophys Res Commun. 1968 Apr 19;31(2):275–280. doi: 10.1016/0006-291x(68)90742-0. [DOI] [PubMed] [Google Scholar]
- Giovanelli J., Owens L. D., Mudd S. H. beta-Cystathionase In Vivo Inactivation by Rhizobitoxine and Role of the Enzyme in Methionine Biosynthesis in Corn Seedlings. Plant Physiol. 1973 Mar;51(3):492–503. doi: 10.1104/pp.51.3.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guranowski A. B., Chiang P. K., Cantoni G. L. 5'-Methylthioadenosine nucleosidase. Purification and characterization of the enzyme from Lupinus luteus seeds. Eur J Biochem. 1981 Feb;114(2):293–299. doi: 10.1111/j.1432-1033.1981.tb05148.x. [DOI] [PubMed] [Google Scholar]
- Guranowski A., Paszewski A. Metabolism of 5'-methylthioadenosine in Aspergillus nidulans. An alternative pathway for methionine synthesis via utilization of the nucleoside methylthio group. Biochim Biophys Acta. 1982 Aug 6;717(2):289–294. doi: 10.1016/0304-4165(82)90181-7. [DOI] [PubMed] [Google Scholar]
- Lipton S. H., Bodwell C. E. A rapid method for detecting chemical alteration of methionine. J Agric Food Chem. 1977 Sep-Oct;25(5):1214–1216. doi: 10.1021/jf60213a041. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Benson A. A., Tolbert N. E. Identification of Phosphoryl Choline as an Important Constituent of Plant Sap. Plant Physiol. 1956 Sep;31(5):407–408. doi: 10.1104/pp.31.5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenk F., Zydek-Cwick C. R., Dainko J. L. 5'-Methylthioadenosine and related compounds as precursors of S-adenosylmethionine in yeast. Biochim Biophys Acta. 1973 Sep 14;320(2):357–362. doi: 10.1016/0304-4165(73)90316-4. [DOI] [PubMed] [Google Scholar]
- Shapiro S. K., Barrett A. 5-Methylthioribose as a precursor of the carbon chain of methionine. Biochem Biophys Res Commun. 1981 Sep 16;102(1):302–307. doi: 10.1016/0006-291x(81)91521-7. [DOI] [PubMed] [Google Scholar]
- Shapiro S. K., Schlenk F. Conversion of 5'-methylthioadenosine into S-adenosylmethionine by yeast cells. Biochim Biophys Acta. 1980 Dec 1;633(2):176–180. doi: 10.1016/0304-4165(80)90403-1. [DOI] [PubMed] [Google Scholar]
- Thompson G. A., Datko A. H., Mudd S. H., Giovanelli J. Methionine Biosynthesis in Lemna: STUDIES ON THE REGULATION OF CYSTATHIONINE gamma-SYNTHASE, O-PHOSPHOHOMOSERINE SULFHYDRYLASE, AND O-ACETYLSERINE SULFHYDRYLASE. Plant Physiol. 1982 May;69(5):1077–1083. doi: 10.1104/pp.69.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson G. A., Datko A. H., Mudd S. H. Methionine Synthesis in Lemna: Inhibition of Cystathionine gamma-Synthase by Propargylglycine. Plant Physiol. 1982 Nov;70(5):1347–1352. doi: 10.1104/pp.70.5.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Y., Adams D. O., Lieberman M. Recycling of 5'-methylthioadenosine-ribose carbon atoms into methionine in tomato tissue in relation to ethylene production. Plant Physiol. 1982 Jul;70(1):117–121. doi: 10.1104/pp.70.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Seidenfeld J., Galletti P. Trends in the biochemical pharmacology of 5'-deoxy-5'-methylthioadenosine. Biochem Pharmacol. 1982 Feb 1;31(3):277–288. doi: 10.1016/0006-2952(82)90171-x. [DOI] [PubMed] [Google Scholar]
- Yu Y. B., Adams D. O., Yang S. F. 1-Aminocyclopropanecarboxylate synthase, a key enzyme in ethylene biosynthesis. Arch Biochem Biophys. 1979 Nov;198(1):280–286. doi: 10.1016/0003-9861(79)90420-x. [DOI] [PubMed] [Google Scholar]
- Yung K. H., Yang S. F., Schlenk F. Methionine synthesis from 3-methylthioribose in apple tissue. Biochem Biophys Res Commun. 1982 Jan 29;104(2):771–777. doi: 10.1016/0006-291x(82)90704-5. [DOI] [PubMed] [Google Scholar]


