Abstract
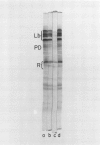
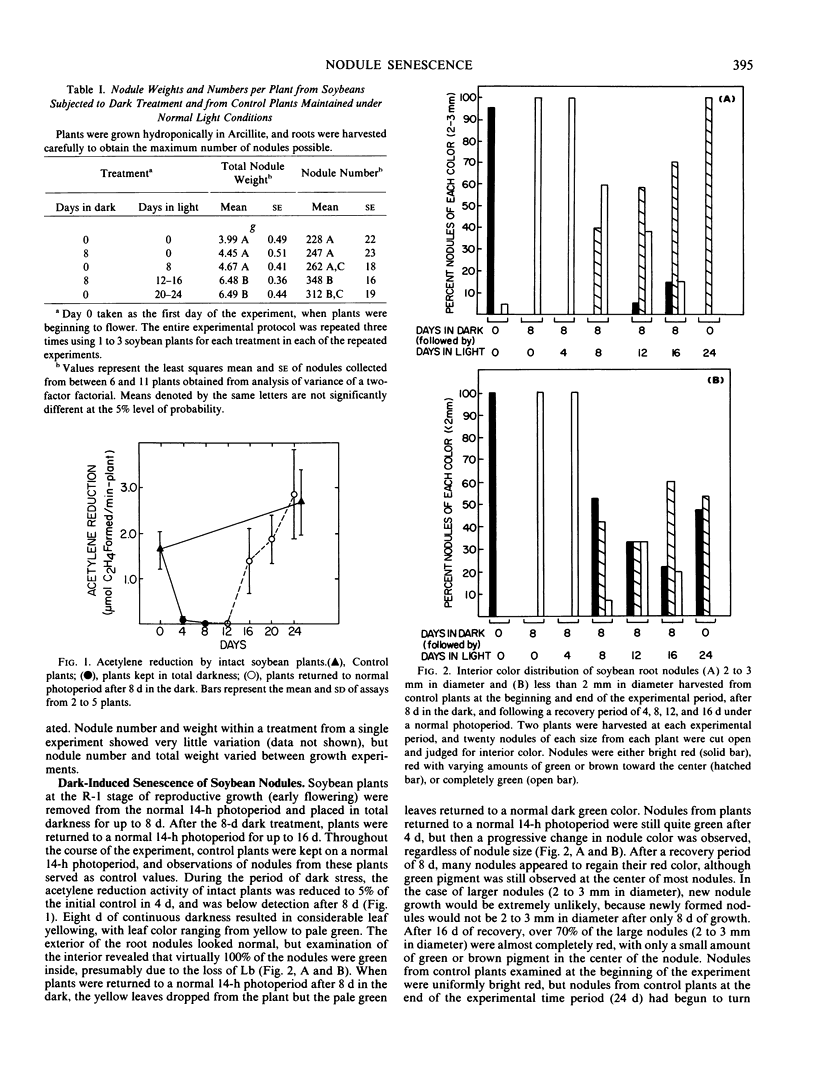
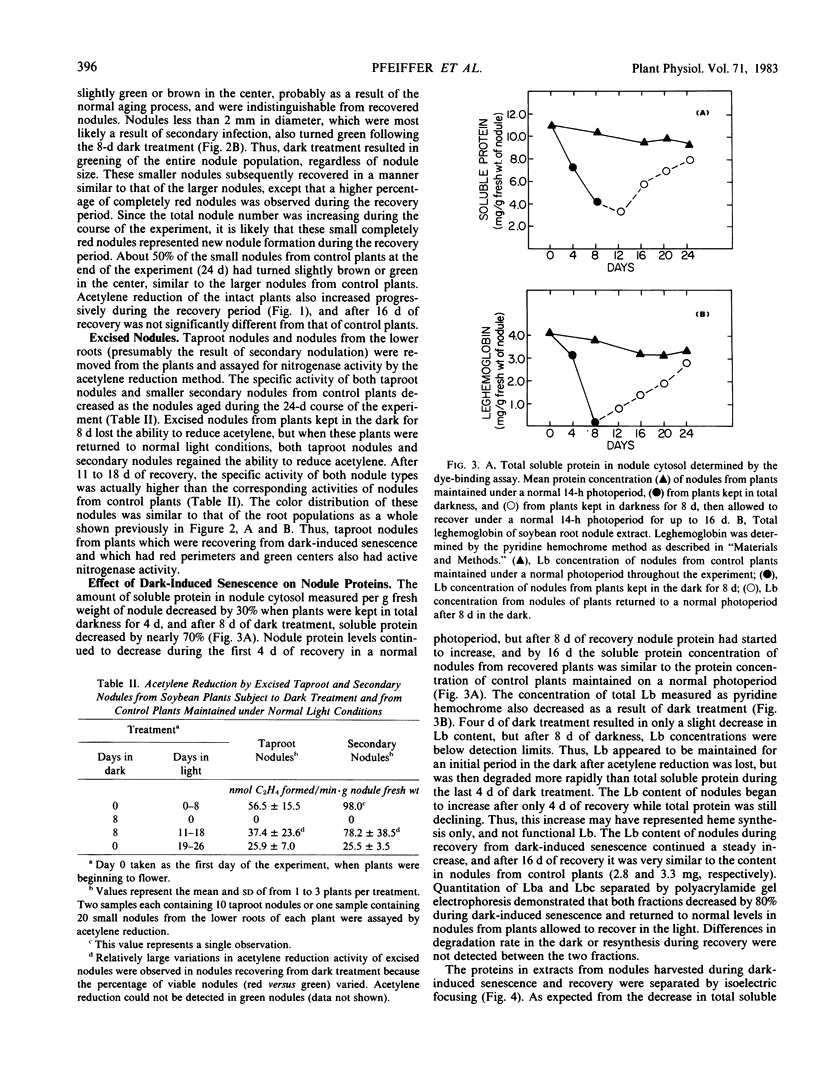
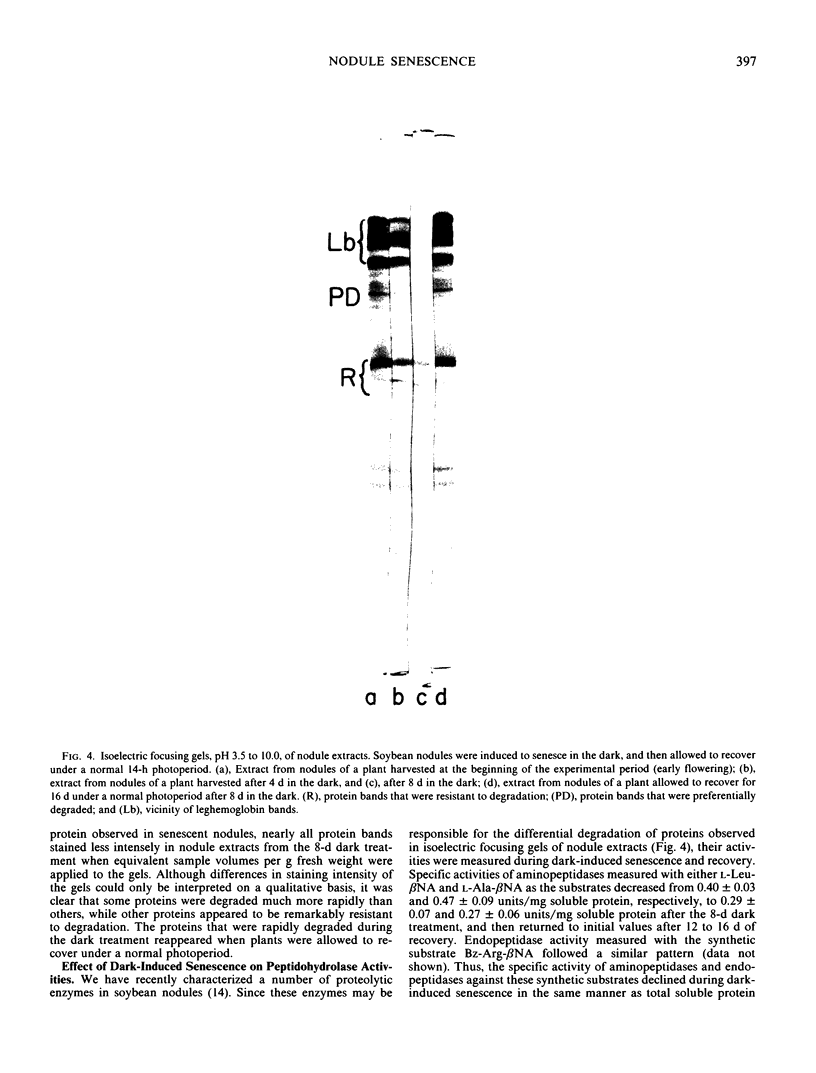
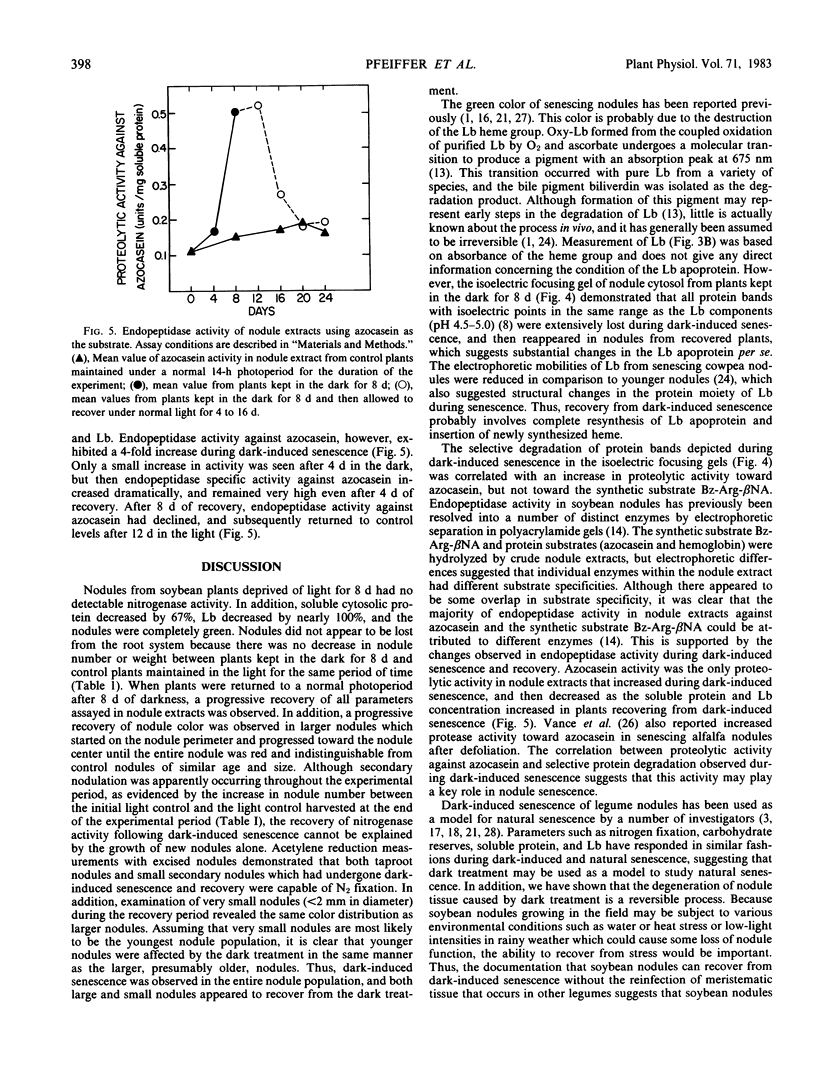
Nodule senescence was induced in intact soybean [Glycine max. (L.) Merr., cv Woodworth] plants by an 8-day dark treatment. Dark-induced senescence resulted in the complete loss of acetylene reduction activity, a 67% loss of total soluble protein, and an almost complete loss in total leghemoglobin of nodule extracts. Isoelectric focusing gels demonstrated a preferential loss of certain proteins, which was correlated with an increase in endoprotease specific activity toward azocasein. Nodules were completely green after the 8-day dark treatment. If plants were returned to a normal photoperiod after 8 days in the dark, nodules recovered from the dark treatment in 12 to 16 days. Acetylene reduction activity returned to normal, and both total soluble protein and leghemoglobin were resynthesized while protease activity against azocasein decreased to the level of control nodules. The nodule population that had turned green after 8 days in the dark exhibited a progressive increase in red color starting nearest the exterior of the nodule, and after 16 days of recovery nodules were indistinguishable from control nodules maintained under a normal photoperiod.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ching T. M., Hedtke S., Russell S. A., Evans H. J. Energy State and Dinitrogen Fixation in Soybean Nodules of Dark-grown Plants. Plant Physiol. 1975 Apr;55(4):796–798. doi: 10.1104/pp.55.4.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cralle H. T. Nitrogen fixation and vegetative regrowth of alfalfa and birdsfoot trefoil after successive harvests or floral debudding. Plant Physiol. 1981 May;67(5):898–905. doi: 10.1104/pp.67.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchsman W. H., Appleby C. A. Separation and determination of the relative concentrations of the homogeneous components of soybean leghemoglobin by isoelectric focusing. Biochim Biophys Acta. 1979 Aug 28;579(2):314–324. doi: 10.1016/0005-2795(79)90059-x. [DOI] [PubMed] [Google Scholar]
- Klucas R. V. Studies on soybean nodule senescence. Plant Physiol. 1974 Oct;54(4):612–616. doi: 10.1104/pp.54.4.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lehtovaara P., Perttilä U. Bile-pigment formation from different leghaemoglobins. Methine-bridge specificity of coupled oxidation. Biochem J. 1978 Nov 15;176(2):359–364. doi: 10.1042/bj1760359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik N. S., Pfeiffer N. E., Williams D. R., Wagner F. W. Peptidohydrolases of Soybean Root Nodules : IDENTIFICATION, SEPARATION, AND PARTIAL CHARACTERIZATION OF ENZYMES FROM BACTEROID-FREE EXTRACTS. Plant Physiol. 1981 Aug;68(2):386–392. doi: 10.1104/pp.68.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mederski H. J., Streeter J. G. Continuous, automated acetylene reduction assays using intact plants. Plant Physiol. 1977 Jun;59(6):1076–1081. doi: 10.1104/pp.59.6.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paau A. S., Cowles J. R. Development of Bacteroids in Alfalfa (Medicago sativa) Nodules. Plant Physiol. 1978 Oct;62(4):526–530. doi: 10.1104/pp.62.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. P., Evans H. J. Poly-beta-hydroxybutyrate Utilization by Soybean (Glycine max Merr.) Nodules and Assessment of Its Role in Maintenance of Nitrogenase Activity. Plant Physiol. 1971 Jun;47(6):750–755. doi: 10.1104/pp.47.6.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley C. W. Analytical fractionation of plant and animal proteins by gel electrofocusing. J Chromatogr. 1968 Aug 27;36(3):362–365. doi: 10.1016/s0021-9673(01)92959-0. [DOI] [PubMed] [Google Scholar]