Abstract
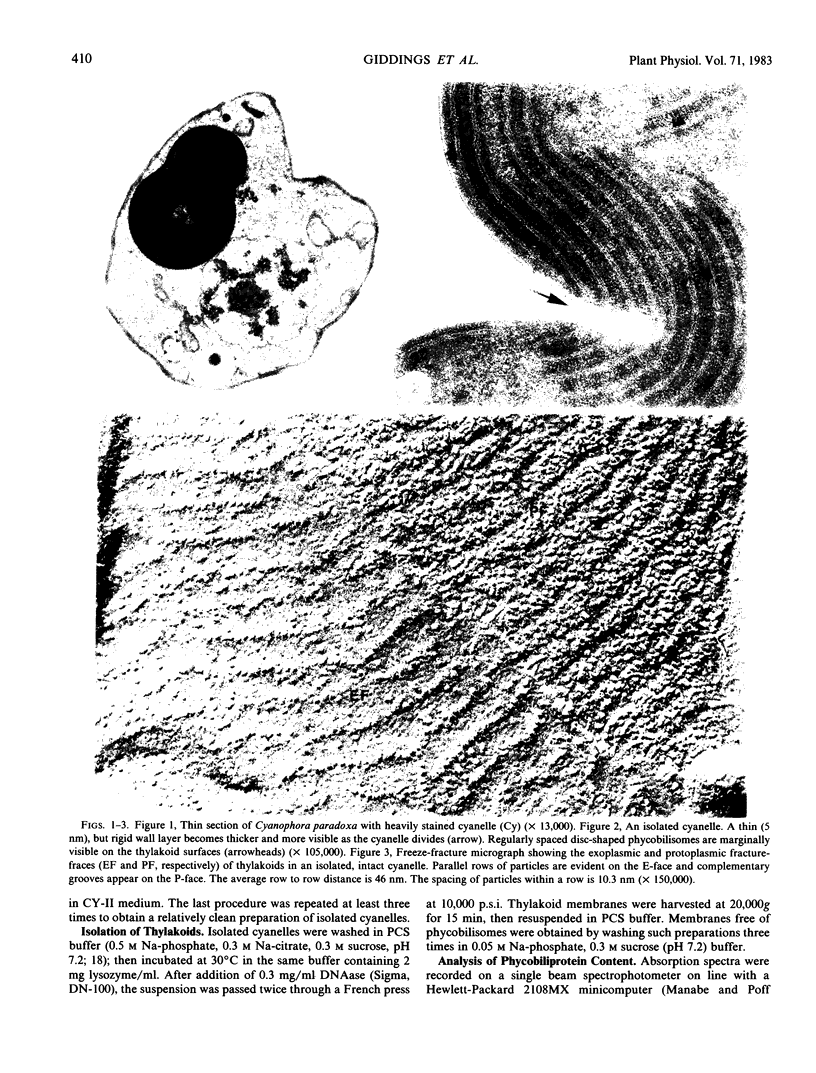
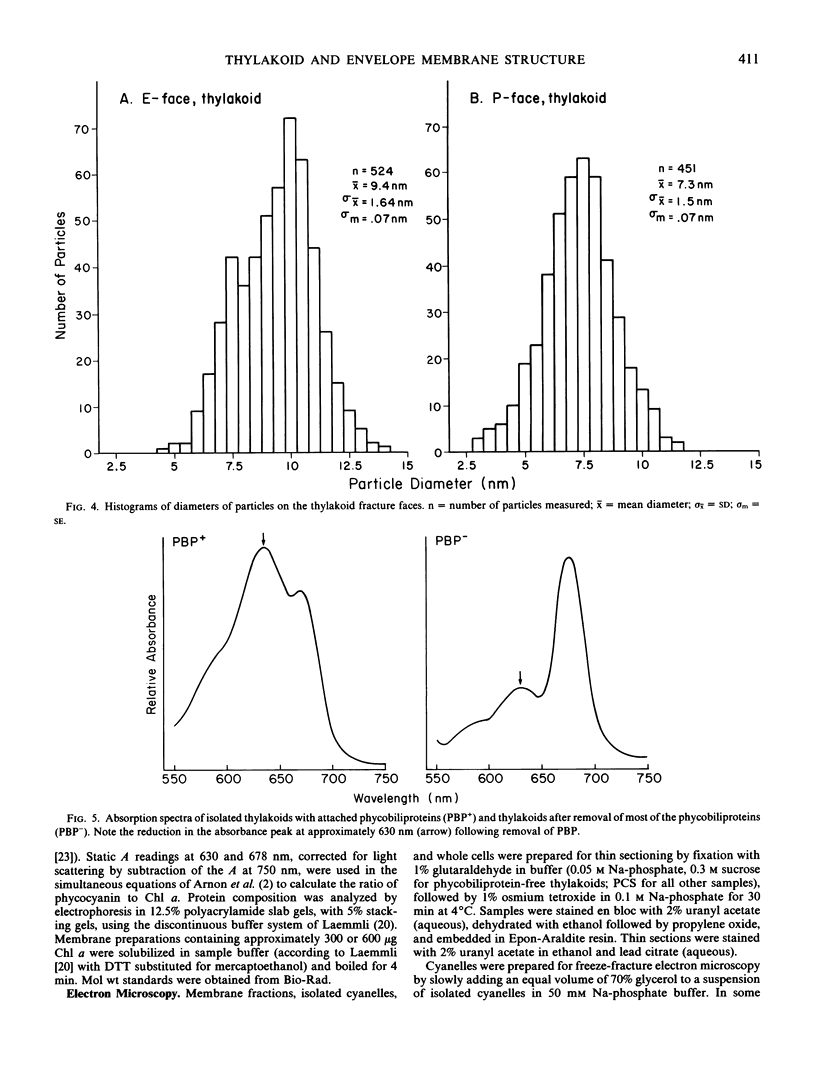
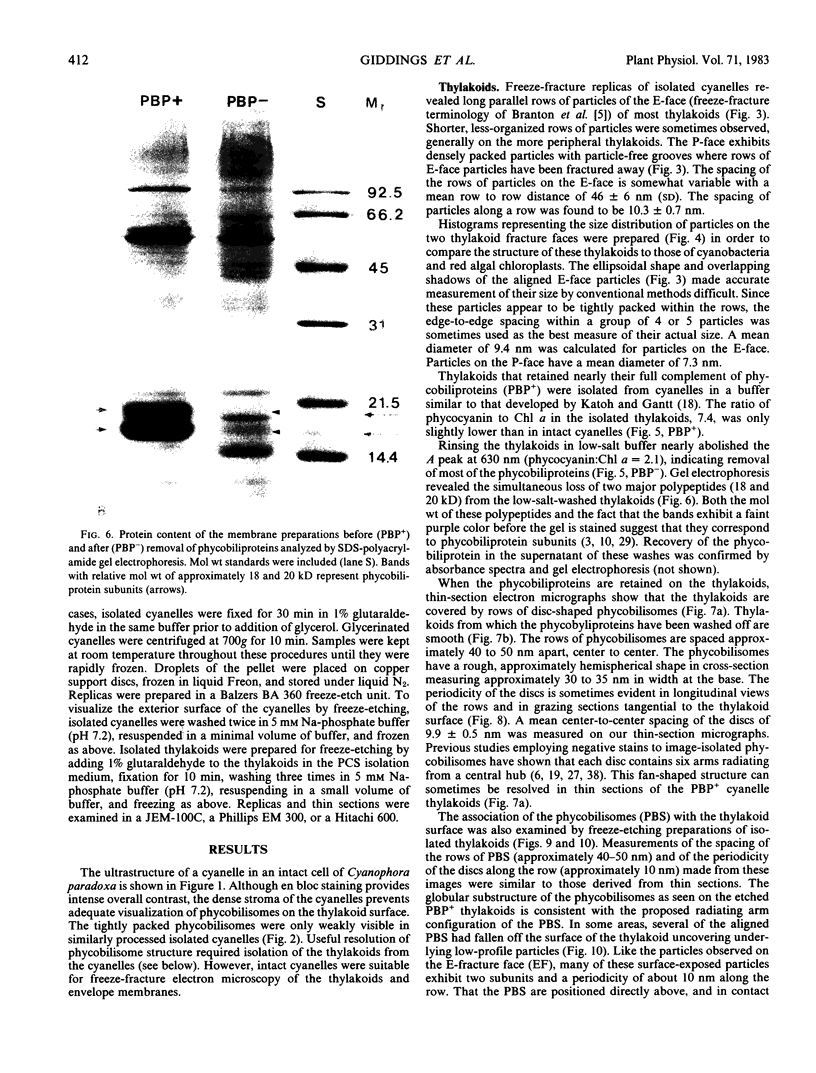
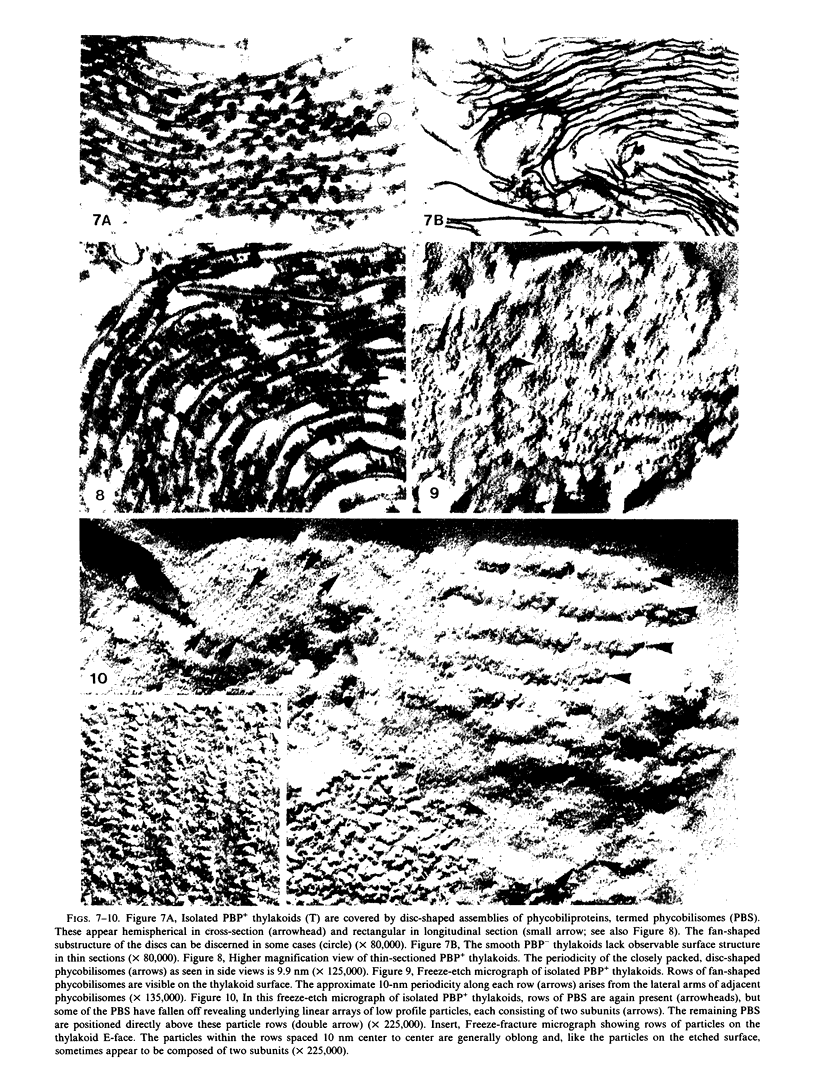
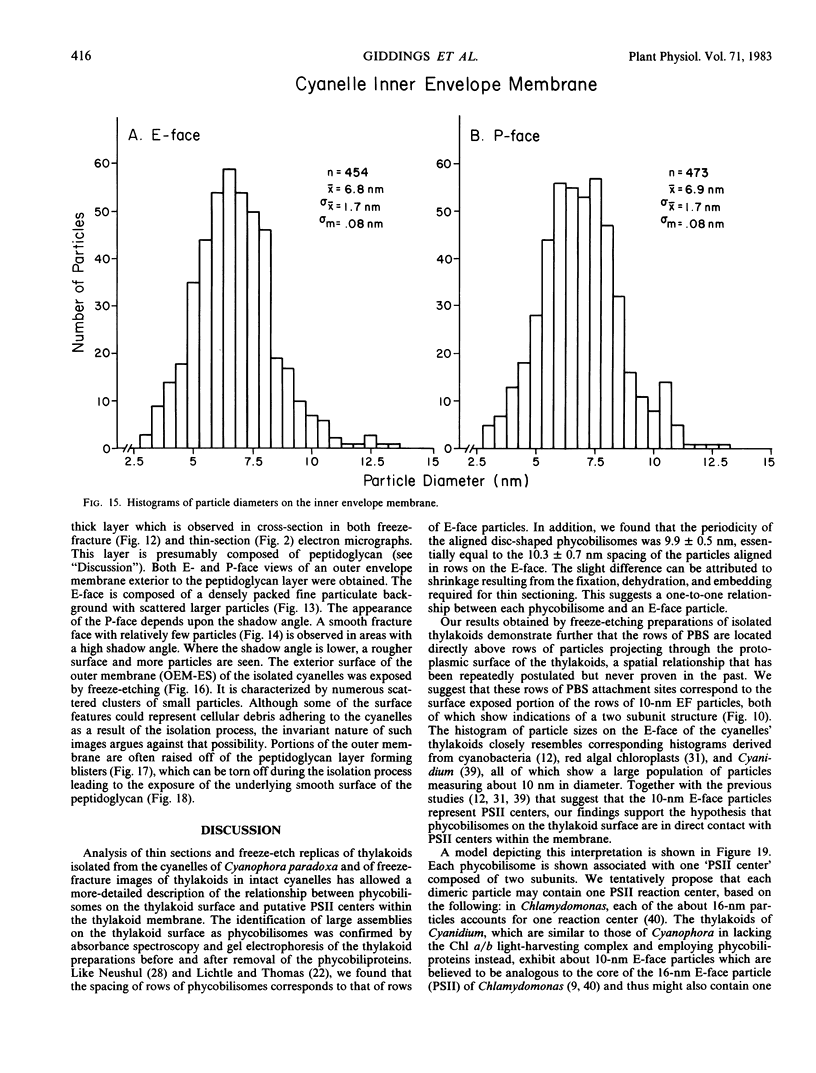
The cyanelles of Cyanophora paradoxa Korsch. are photosynthetically active obligate endosymbionts in which phycobiliproteins serve as the major accessory pigments. Freeze-fracture electron micrographs of thylakoids in isolated cyanelles reveal long parallel rows of particles covering most of the E-face, while a more random particle arrangement is evident in some areas. The center-to-center spacing of particles within these rows is about 10 nanometers. Their mean diameter was measured at 9.4 nanometers. The particles on the P-face have a mean diameter of 7.2 nanometers. Thylakoids that retained nearly the full complement of phycobiliproteins (determined spectrophotometrically and by gel electrophoresis) were isolated from the cyanelles. In thin sections of these preparations, rows of disc-shaped phycobilisomes are evident on the surface of the thylakoids. The spacing of the rows of phycobilisomes corresponds to that of the rows of E-face particles (approximately 45 nanometers, center to center). The periodicity of the disc-shaped phycobilisomes within a row is 10 nanometers suggesting a one-to-one association between phycobilisomes and E-face particles.
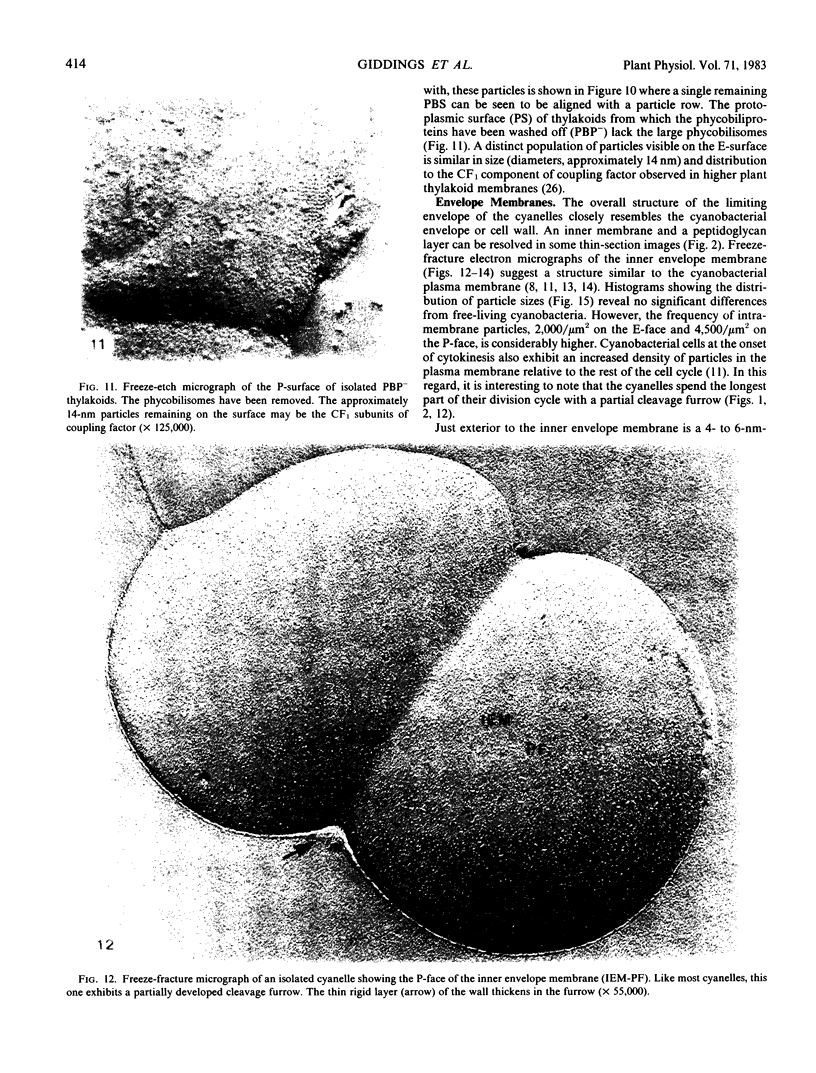
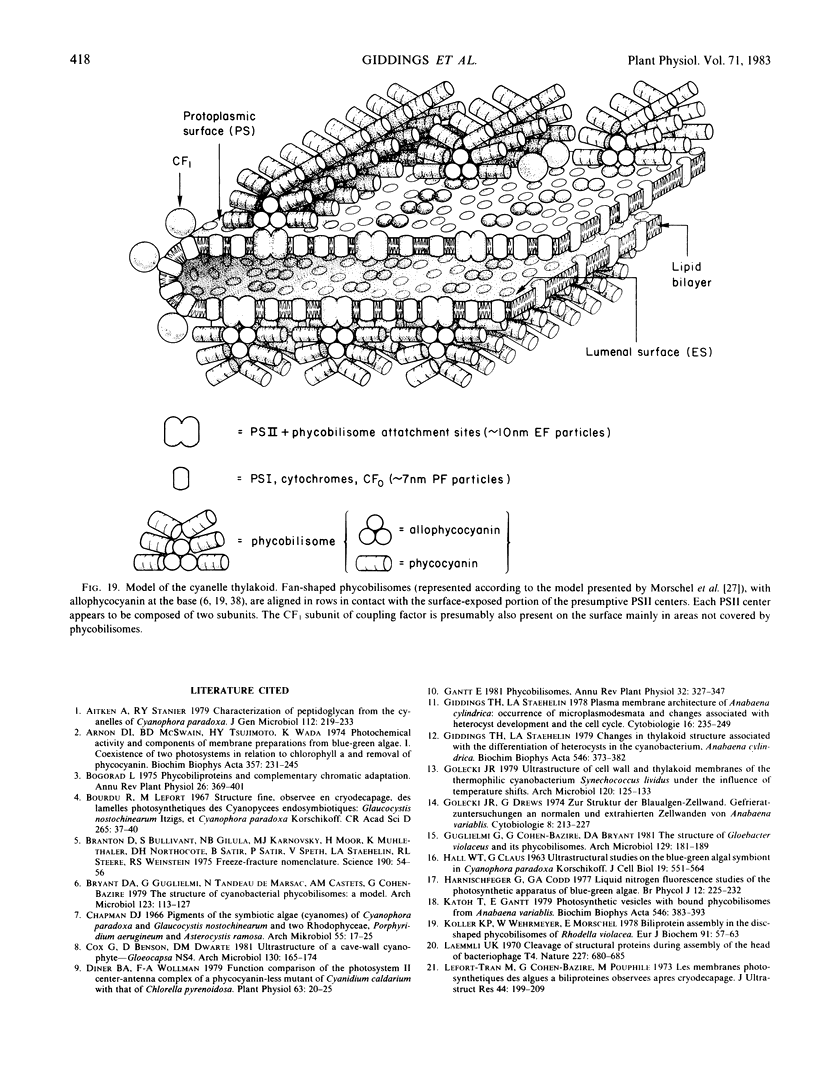
In addition, visualization of the protoplasmic surface (PS) of isolated thylakoids by freeze-etch electron microscopy shows that rows of disc-shaped phycobilisomes are aligned directly above rows of particles exhibiting two subunits, presumably the P-surface projections of the 10-nanometer intramembrane particles. These observations, together with earlier studies indicating that the 10-nanometer E-face particles probably represent photosystem II (PSII) complexes, suggest that phycobilisomes are positioned on the thylakoid surface in direct contact with PSII centers within the thylakoid membrane.
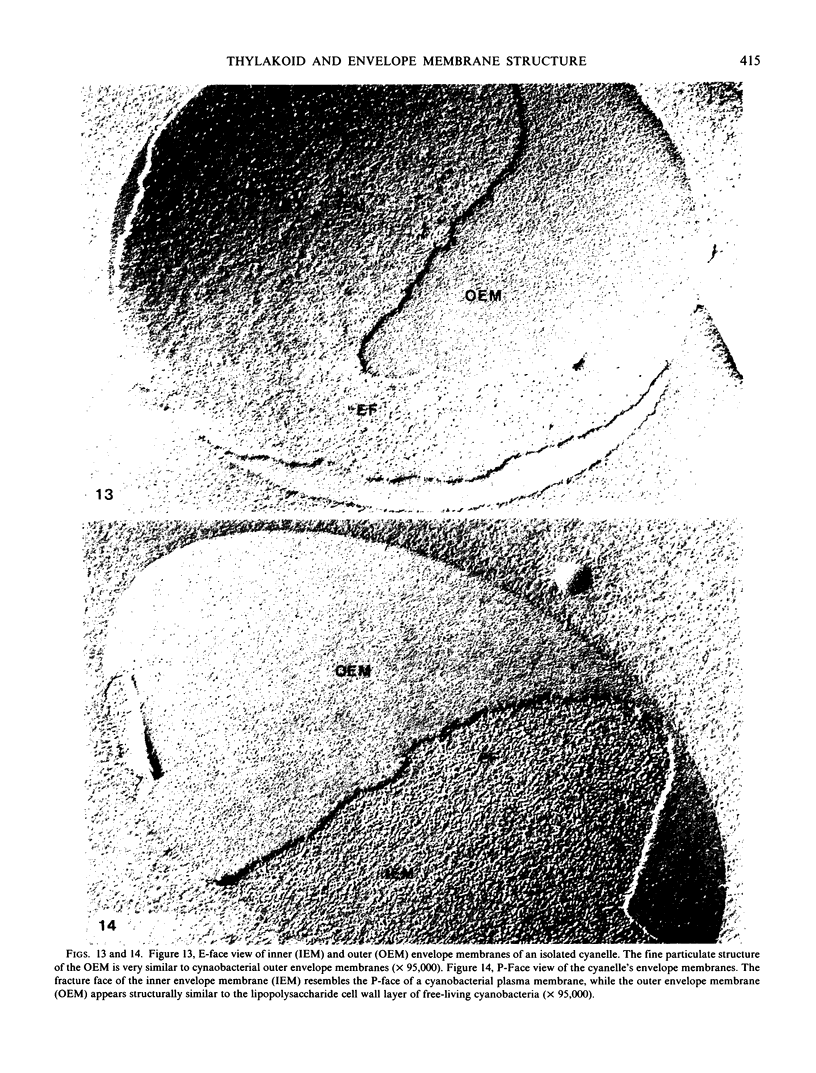
The inner envelope membrane of the cyanelles, observed in freeze-fracture replicas, resembles cyanobacterial plasma membranes and is dissimilar to the chloroplast envelope membranes of red or green algae. The envelope of isolated cyanelles exhibits two additional layers: (a) a 5- to 7-nanometer-thick layer that lies adjacent to the inner membrane and which seems to correspond to the peptidoglycan layer of cyanobacteria; and (b) a layer external to the purported peptidoglycan layer that exhibits fracture faces similar to those of the lipopolysaccharide layer of gram negative bacteria. Our findings indicate that the supramolecular architecture of cyanelles differs only slightly from free-living cyanobacteria to which they are presumably related.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I., McSwain B. D., Tsujimoto H. Y., Wada K. Photochemical activity and components of membrane preparations from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochim Biophys Acta. 1974 Aug 23;357(2):231–245. doi: 10.1016/0005-2728(74)90063-2. [DOI] [PubMed] [Google Scholar]
- Branton D., Bullivant S., Gilula N. B., Karnovsky M. J., Moor H., Mühlethaler K., Northcote D. H., Packer L., Satir B., Satir P. Freeze-etching nomenclature. Science. 1975 Oct 3;190(4209):54–56. doi: 10.1126/science.1166299. [DOI] [PubMed] [Google Scholar]
- Diner B. A., Wollman F. A. Functional Comparison of the Photosystem II Center-Antenna Complex of a Phycocyanin-less Mutant of Cyanidium caldarium with That of Chlorella pyrenoidosa. Plant Physiol. 1979 Jan;63(1):20–25. doi: 10.1104/pp.63.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddings T. H., Jr, Staehelin L. A. Changes in thylakoid structure associated with the differentiation of heterocysts in the cyanobacterium, Anabaena cylindrica. Biochim Biophys Acta. 1979 Jun 5;546(3):373–382. doi: 10.1016/0005-2728(79)90074-4. [DOI] [PubMed] [Google Scholar]
- HALL W. T., CLAUS G. ULTRASTRUCTURAL STUDIES ON THE BLUE-GREEN ALGAL SYMBIONT IN CYANOPHORA PARADOXA KORSCHIKOFF. J Cell Biol. 1963 Dec;19:551–563. doi: 10.1083/jcb.19.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh T., Gantt E. Photosynthetic vesicles with bound phycobilisomes from Anabaena variabilis. Biochim Biophys Acta. 1979 Jun 5;546(3):383–393. doi: 10.1016/0005-2728(79)90075-6. [DOI] [PubMed] [Google Scholar]
- Koller K. P., Wehrmeyer W., Mörschel E. Biliprotein assemble in the disc-shaped phycobilisomes of Rhodella violacea. On the molecular composition of energy-transfering complexes (tripartite units) forming the periphery of the phycobilisome. Eur J Biochem. 1978 Nov 2;91(1):57–63. doi: 10.1111/j.1432-1033.1978.tb20936.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lefort-Tran M., Cohen-Bazire G., Pouphile M. Les membranes photosynthétiques des algues à biliproteines observées apres cryodécapage. J Ultrastruct Res. 1973 Aug;44(3):199–209. doi: 10.1016/s0022-5320(73)80056-5. [DOI] [PubMed] [Google Scholar]
- Manabe K., Poff K. L. Purification and Characterization of the Photoreducible b-type Cytochrome from Dictyostelium discoideum. Plant Physiol. 1978 Jun;61(6):961–966. doi: 10.1104/pp.61.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulis L. Genetic and evolutionary consequences of symbiosis. Exp Parasitol. 1976 Apr;39(2):277–349. doi: 10.1016/0014-4894(76)90127-2. [DOI] [PubMed] [Google Scholar]
- Miller K. R., Staehelin L. A. Analysis of the thylakoid outer surface. Coupling factor is limited to unstacked membrane regions. J Cell Biol. 1976 Jan;68(1):30–47. doi: 10.1083/jcb.68.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neushul M. Uniformity of thylakoid structure in a red, a brown, and two blue-green algae. J Ultrastruct Res. 1971 Dec;37(5):532–543. doi: 10.1016/s0022-5320(71)80023-0. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Cohen-Bazire G. Phototrophic prokaryotes: the cyanobacteria. Annu Rev Microbiol. 1977;31:225–274. doi: 10.1146/annurev.mi.31.100177.001301. [DOI] [PubMed] [Google Scholar]
- Tel-or E., Malkin S. The photochemical and fluorescence properties of whole cells, spheroplasts and spheroplast particles from the blue-green alga Phormidium luridum. Biochim Biophys Acta. 1977 Feb 7;459(2):157–174. doi: 10.1016/0005-2728(77)90019-6. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Gingrich J. C., Glazer A. N. Cyanobacterial phycobilisomes. Particles from Synechocystis 6701 and two pigment mutants. J Cell Biol. 1980 Jun;85(3):558–566. doi: 10.1083/jcb.85.3.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman F. A., Olive J., Bennoun P., Recouvreur M. Organization of the photosystem II centers and their associated antennae in the thylakoid membranes: a comparative ultrastructural, biochemical, and biophysical study of Chlamydomonas wild type and mutants lacking in photosystem II reaction centers. J Cell Biol. 1980 Dec;87(3 Pt 1):728–735. doi: 10.1083/jcb.87.3.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman F. A. Ultrastructural Comparison of Cyanidium caldarium Wild Type and III-C Mutant Lacking Phycobilisomes. Plant Physiol. 1979 Feb;63(2):375–381. doi: 10.1104/pp.63.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gool A. P., Nanninga N. Fracture faces in the cell envelope of Escherichia coli. J Bacteriol. 1971 Oct;108(1):474–481. doi: 10.1128/jb.108.1.474-481.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]