Abstract
Background: Solid neoplasms have a heterogeneous incidence worldwide and in Brazil. Thus, the region delimited by the Legal Amazon has a distinct epidemiological profile. In Pará, Ophir Loyola Cancer Hospital(OLCH) accounts for 71.11% of hospital visits in the state. Methods: This was an ecological, exploratory, and mixed descriptive studythat investigated the epidemiological profile of patients with cancer treated at OLCH from January to December 2020. Sociodemographic data at admission were the primary variables, which were analyzed according to spatial distribution. Results: In this study, the data of 2952 patients were analyzed, with the majority being between the ages of 50 and 79 years (62.47%), female (59.49%), and diagnosed but without previous treatment (87.30%). The most common cancers were breast (16.50%), cervical (13.40%), stomach (8.98%), and prostate (7.72%). Of the 12 integration regions, Guajará had the highest number of referrals (49.86%), followed by Guamá (12.94%) and Caeté River (8.98%). Conclusion: The profile of care at OLCH showed a high incidence of solid malignancies compared to that in other regions of Brazil, indicating environmental and sociocultural influences on the carcinogenic profile present in the eastern Amazon.
Keywords: cancer, epidemiology, incidence, spatial analysis
1. Introduction
The geographical distribution of cancer is a major concern in global public health [1]. Associated with this reality, the susceptibility of a population is closely linked to risk factors and carcinogenic profiles in different groups, which explain the heterogeneous prevalence and incidences among countries [2,3].
Brazil, compared to other regions of the world, has a distinct epidemiological profile, with a high prevalence of breast cancer and a low survival rate compared to those for countries with a higher human development index [4,5]. In addition, the North and Northeast regions account for more than half of the cases of penile cancer, with a high mortality rate [6], as well as prostate cancer, which is the second most commonly diagnosed cancer in men over 40 years of age [7,8].
Regarding the proportion of cases, in the North Region, the incidence of stomach cancer ranks second among men and third among women [9], with mortality rates greater than 11.2 and 2.4 per 100 thousand inhabitants among men and among women, respectively, aged 50–69 years [10].
In an attempt to explain this profile among Brazilian regions, miscegenation is a relevant characteristic of the population of the State of Pará. Miscegenation occurs when populations that have remained isolated for hundreds of generations come together in a geographic space, and individuals from different populations of origin marry and reproduce [11]. Studies have indicated that the genetic aggregation of Asian and Caucasian population groups with the Amerindian population residing in Brazil, which over the years initiated this process of miscegenation, potentiated the introduction of genotypes favorable to certain malignancies [12,13].
In Brazil, highly complex units and centers of the Unified Health System are responsible for the majority of care for cancer patients [14]. In this context, the Ophir Loyola Cancer Hospital (OLCH) is considered a high-complexity oncology center (CACON) because it has the technical conditions, physical facilities, equipment, and human resources adequate to provide highly complex specialized care for the diagnosis and treatment of all types of cancer.
Due to the specific incidence and prevalence profile of cancers in the North Region, compared to those for the national scenario, the need to understand the regional epidemiological characteristics is associated with, as a priority, the identification of the main solid tumors. Thus, the objective of this study was to describe the epidemiological profile of patients treated in the CACON of OLCH who had confirmed diagnoses of solid neoplasms in 2020.
2. Method
This was an ecological, exploratory, and mixed descriptive study for which the reported and registered data of 2952 patients in the state of Pará and other states who were diagnosed and treated at the CACON of OLCH were analyzed. Data for sociodemographic, diagnostic, and region of origin variables were obtained from the hospital cancer registries (HCR), available in the information system maintained by the National Cancer Institute (NCI).
The data were grouped by integration region (IR) in Pará: Guajará, Guamá, Marajó, Caeté River, Capim River, Tocantins, Araguaia, Tucuruí Lake, Carajás, Xingu, Lower Amazon, and Tapajós. To illustrate the incidence and prevalence of different cancers in the integration regions of Pará, maps containing the regional distribution of registered cases were constructed.
The analysis and grouping of primary tumors wereconducted with reference to the International Statistical Classification of Diseases and Related Health Problems (ICD), 11th Revision, from code C00 to C97, and the International Classification of Diseases for Oncology (ICD-O), 3rd Edition. The identified, recorded, and organized information underwent descriptive statistical analyses, i.e., measures of central tendency (mean, mode, and median), variance and standard deviation, and absolute and relative frequencies, using IBM SPSS Statistics 27 and GraphPad Prism 6 software. A p-value ≤ 0.05 was considered significant.
3. Results
In 2020, there were 2952 patient admissions for cancer at OLCH, which is located in the Guajará integration region. Of this total, 1196 (40.5%) were men, and 1756 (59.5%) were women; 62.47% were between 50 and 79 years of age, and 31.06% were between 20 and 49 years of age. Furthermore, around 2577 (87.30%) patients began follow-up with the diagnosis, but without starting the first therapeutic modality. Table 1 shows the number of cases by age group, sex, education level, presence or absence of diagnosis and treatment prior to follow-up, and race or ethnicity.
Table 1.
Epidemiological description of patients with solid tumors in Pará treated at OLCH in 2020.
| Variables | n (%) |
|---|---|
| Age group | |
| ≤19 years | 12 (0.41) |
| 20–49 years | 917 (31.06) |
| 50–79 years | 1844 (62.47) |
| ≥80 years | 179 (6.06) |
| Sex | |
| Male | 1196 (40.51) |
| Female | 1756 (59.49) |
| Education level | |
| None or <1 year | 238 (8.06) |
| <8 years | 1172 (39.70) |
| 8–10 years | 264 (8.94) |
| 11–14 years | 640 (21.68) |
| >15 years | 155 (6.47) |
| Not reported | 447 (15.14) |
| Previous diagnosis/treatment | |
| With diagnosis/without treatment | 2577 (87.30) |
| With diagnosis/with treatment | 373 (12.64) |
| Not reported | 2 (0.06) |
| Color/ethnicity | |
| Yellow | 183 (6.20) |
| Brown | 1865 (63.18) |
| Black | 52 (1.76) |
| White | 85 (2.88) |
| Indigenous | 4 (0.14) |
| Not reported | 763 (25.85) |
The numbers and percentages of cancer cases confirmed and recorded by OLCH are shown in Table 2, compared with the number of cases in Pará state and Brazil, in 2020.
Table 2.
Detailed breakdown of the number of cancer cases by primary tumor location in Pará (OLCH) in 2020.
| Primary Tumor Location | OLCH | Pará | Brazil |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Breast | 489 (16.5) | 646 (15.56) | 51,025 (11.96) |
| Cervix | 396 (13.4) | 532 (12.82) | 25,683 (6.02) |
| Stomach | 265 (8.98) | 338 (8.14) | 16,680 (3.91) |
| Prostate | 225 (7.72) | 312 (7.52) | 32,681 (7.66) |
| Non-melanoma skin | 214 (7.25) | 373 (8.99) | 58,306 (13.67) |
| Colon and rectum | 191 (6.47) | 251 (6.05) | 36,426 (8.54) |
| Leukemia, bone marrow | 120 (4.07) | 178 (4.29) | 7236 (1.70) |
| Thyroid | 108 (3.66) | 134 (3.23) | 6526 (1.53) |
| Lungs | 92 (3.12) | 128 (3.08) | 12,755 (2.99) |
| Lymphoma, unspecified | 87 (2.95) | 122 (2.94) | 19,464 (4.56) |
| Lip and oral cavity | 81 (2.74) | 98 (2.36) | 12,975 (3.04) |
| Kidney | 75 (2.54) | 90 (2.17) | 5001 (1.17) |
| Bones and soft tissues | 67 (2.27) | 120 (2.89) | 15,493 (3.63) |
| CNS | 54 (1.83) | 94 (2.26) | 6570 (1.54) |
| Ovary | 51 (1.73) | 70 (1.69) | 6593 (1.55) |
| Liver and intrahepatic bile ducts | 50 (1.69) | 63 (1.52) | 3292 (0.77) |
| Pancreas | 47 (1.59) | 65 (1.57) | 4222 (0.99) |
| Body of uterus | 43 (1.46) | 53 (1.28) | 7244 (1.70) |
| Esophagus | 38 (1.29) | 67 (1.61) | 7794 (1.83) |
| Bladder | 37 (1.25) | 49 (1.18) | 7365 (1.73) |
| Larynx | 33 (1.12) | 44 (1.06) | 5383 (1.26) |
| Penis | 32 (1.08) | 44 (1.06) | 1162 (0.27) |
| Not specified | 30 (1.02) | 59 (1.42) | 48,056 (11.27) |
| Hematopoietic and reticulo endothelial systems, not bone marrow | 23 (0.78) | 49 (1.18) | 3383 (0.79) |
| Testicle | 17 (0.58) | 27 (0.65) | 1873 (0.44) |
| Gallbladder and extrahepatic bile ducts | 15 (0.51) | 19 (0.46) | 2356 (0.55) |
| Small intestine | 10 (0.34) | 11 (0.26) | 1863 (0.44) |
| Nasopharynx | 10 (0.34) | 12 (0.29) | 1097 (0.26) |
| Vulva | 8 (0.27) | 13 (0.31) | 2375 (0.56) |
| Digestive system, unspecified | 8 (0.27) | 8 (0.19) | 3629 (0.85) |
| Ocular | 7 (0.24) | 18 (0.43) | 1035 (0.24) |
| Pharynx | 6 (0.20) | 31 (0.75) | 7114 (1.67) |
| Mediastinum | 5 (0.17) | 7 (0.17) | 1276 (0.30) |
| Nasal cavity and middle ear | 5 (0.17) | 5 (0.12) | 629 (0.15) |
| Paranasal sinuses | 4 (0.14) | 6 (0.14) | 810 (0.19) |
| Vagina | 4 (0.14) | 8 (0.19) | 554 (0.15) |
| Placenta | 3 (0.10) | 3 (0.07) | 307 (0.07) |
| Respiratory system, unspecified | 1 (0.03) | 2 (0.05) | 131 (0.03) |
| Thymus | 1 (0.03) | 2 (0.05) | 145 (0.03) |
| Total | 2952 (100.00) | 4151 (100.00) | 426,509 (100.00) |
The numbers and percentages of cancer cases confirmed and recorded by OLCH are shown in Table 3, compared with the number of cases in Brazil, in 2020.
Table 3.
Distribution of cases per sex (by primary tumor location) treated at OLCH and Brazil, in 2020.
| Primary Tumor Location | OLCH n (%) |
Brazil n (%) |
||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Stomach | 179 (67.55) | 86 (32.45) | 8799 (52.75) | 7881 (47.25) |
| Non-melanoma skin | 116 (54.21) | 98 (45.79) | 27,827 (76.39) | 30,479 (23.61) |
| Colon and rectum | 89 (46.60) | 102 (53.40) | 17,965 (49.32) | 18,461 (50.68) |
| Leukemia, bone marrow | 69 (57.50) | 51 (42.50) | 4033 (55.74) | 3203 (44.26) |
| Thyroid | 15 (13.89) | 93 (86.11) | 1027 (15.74) | 5499 (84.26) |
| Lungs | 50 (54.35) | 42 (45.65) | 6950 (54.49) | 5805 (45.51) |
| Lymphoma, unspecified | 53 (60.92) | 34 (39.08) | 10,170 (52.25) | 9294 (47.75) |
| Lip and oral cavity | 50 (61.73) | 31 (38.27) | 8992 (69.30) | 3983 (30.70) |
| Kidney | 43 (57.33) | 32 (42.67) | 2856 (57.11) | 2145 (42.89) |
| Bones and soft tissues | 27 (40.30) | 40 (59.70) | 7747 (50.00) | 7746 (50.00) |
| CNS | 31 (57.41) | 23 (42.59) | 3433 (52.25) | 3137 (47.75) |
| Liver and intrahepatic bile ducts | 26 (52.00) | 24 (48.00) | 1710 (51.94) | 1582 (48.06) |
| Pancreas | 26 (55.32) | 21 (44.68) | 2098 (49.69) | 2124 (50.31) |
| Esophagus | 23 (60.53) | 15 (39.47) | 5457 (70.02) | 2337 (29.98) |
| Bladder | 30 (81.08) | 7 (18.92) | 5116 (69.63) | 2231 (30.37) |
| Larynx | 25 (75.76) | 8 (24.24) | 4610 (85.64) | 773 (14.36) |
| Hematopoietic and reticuloendothelial systems, not bone marrow | 11 (47.83) | 12 (52.17) | 1506 (44.52) | 1877 (55.48) |
| Gallbladder and extrahepatic bile ducts | 4 (26.67) | 11 (73.33) | 929 (39.43) | 1427 (60.57) |
| Small intestine | 4 (40.00) | 6 (60.00) | 910 (48.85) | 953 (51.15) |
| Nasopharynx | 4 (40.00) | 6 (60.00) | 768 (70.01) | 329 (29.99) |
| Digestive system, unspecified | 4 (50.00) | 4 (50.00) | 1662 (45.80) | 1967 (54.20) |
| Ocular | 6 (85.71) | 1 (14.29) | 569 (54.98) | 466 (45.02) |
| Pharynx | 4 (66.67) | 2 (33.33) | 5903 (82.98) | 1211 (17.02) |
| Mediastinum | 3 (60.00) | 2 (40.00) | 597 (46.79) | 679 (53.21) |
| Nasal cavity and middle ear | 2 (40.00) | 3 (60.00) | 398 (63.28) | 231 (36.72) |
| Paranasal sinuses | 2 (50.00) | 2 (50.00) | 456 (56.30) | 354 (43.70) |
| Respiratory system, unspecified | 1 (100.00) | - | 69 (52.67) | 62 (47.33) |
| Thymus | - | 1 (100.00) | 72 (49.66) | 73 (50.34) |
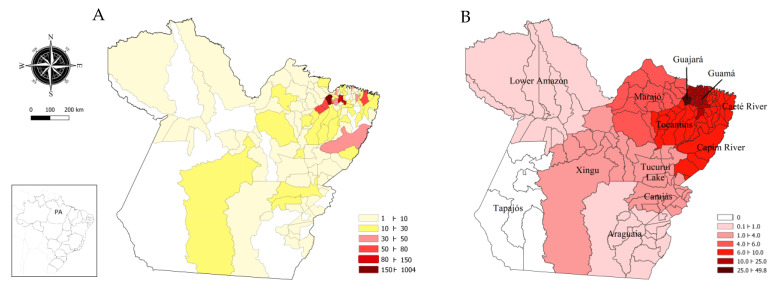
The spatial distribution of the total number of cases, by the location of the primary solid tumor, was heterogeneous with regard to the number of cases per municipality and per integration region (Figure 1). The municipality of Belém had the highest number of cases (34.01%), followed by the municipalities of Nonindium (11.62%), Castanhal (4.51%), Abaetetuba (2.47%), Bragança and Marituba (2.47%), Paragominas (1.69%), and Capanema (1.63%). Guajará accounted for 49.86% of the registered cases, with Marajó, Tocantins, Capim River, Caeté River, and Guamá accounting for 43.77% (1343 cases); Lower Amazon, Tucuruí Lake, Carajás, Araguaia and Xingu accounted for 5.72% (169 cases), with other states accounting for 0.64% (Table 4 and Table 5).
Figure 1.
Spatial distribution of cases treated at OLCH in 2020. (A) Absolute number of cases per municipality. (B) Percentage of cases by integration region of Pará.
Table 4.
Distribution of the incidence of principal solid tumors by location (ICD-11 and ICD-O) and sex in Guajará, Guamá, Caeté River, Capim River, Marajó, and Tocantins in 2020.
| Location (ICD-O) | Female (%) | Male (%) |
|---|---|---|
| Guajará | 864 (100) | 608 (100) |
| Stomach (C16) | 52 (6.02) | 86 (14.14) |
| Colon and rectum (C18–C21) | 48 (5.56) | 48 (7.9) |
| Bone marrow leukemia (C42.1) | 36 (4.17) | 41 (6.74) |
| Breast (C50) | 279 (32.29) | - |
| Cervix (C53) | 164 (18.98) | - |
| Prostate (C61) | - | 128 (21.05) |
| Thyroid (C73) | 39 (4.51) | 8 (1.32) |
| Guamá | 239 (100) | 143 (100) |
| Stomach (C16) | 13 (5.44) | 31 (21.68) |
| Colon and rectum (C18–C21) | 13 (5.44) | 10 (7.0) |
| Breast (C50) | 61 (25.52) | 2 (1.4) |
| Cervix (C53) | 59 (24.69) | - |
| Prostate (C61) | - | 25 (17.48) |
| Thyroid (C73) | 11 (4.6) | 2 (1.4) |
| Caeté River | 162 (100) | 103 (100) |
| Stomach (C16) | 7 (4.32) | 22 (21.36) |
| Colon and rectum (C18–C21) | 16 (9.87) | 8 (7.76) |
| Breast (C50) | 34 (20.99) | 1 (4.0) |
| Cervix (C53) | 48 (29.63) | - |
| Prostate (C61) | - | 17 (16.50) |
| Thyroid (C73) | 5 (3.09) | - |
| Lymphoma (C77) | 2 (1.23) | 5 (4.85) |
| Capim River | 148 (100) | 91 (100) |
| Stomach (C16) | 8 (5.41) | 17 (18.68) |
| Colon and rectum (C18–C21) | 8 (5.41) | 7 (7.7) |
| Breast (C50) | 29 (19.59) | 1 (1.10) |
| Cervix (C53) | 38 (25.68) | - |
| Prostate (C61) | - | 13 (14.29) |
| Thyroid (C73) | 10 (6.76) | - |
| Marajó | 82 (100) | 61 (100) |
| Stomach (C16) | - | 8 (13.11) |
| Breast (C50) | 16 (19.51) | 1 (4.0) |
| Cervix (C53) | 39 (47.56) | - |
| Prostate (C61) | - | 10 (16.39) |
| Thyroid (C73) | 6 (7.32) | - |
| Lymphoma (C77) | 2 (2.44) | 5 (8.20) |
| Tocantins | 148 (100) | 115 (100) |
| Stomach (C16) | 3 (2.03) | 12 (10.43) |
| Colon and rectum (C18–C21) | 13 (8.78) | 7 (6.09) |
| Breast (C50) | 34 (22.97) | - |
| Cervix (C53) | 31 (20.95) | - |
| Prostate (C61) | - | 26 (22.61) |
| Thyroid (C73) | 8 (5.41) | - |
Table 5.
Distribution of the incidence of principal solid tumors by location (ICD-11 and ICD-O) and sex in Tucuruí Lake, Carajás, Xingu, Araguaia, and Lower Amazon in 2020.
| Topography (ICD-O) | Female (%) | Male (%) |
|---|---|---|
| Tucuruí Lake | 26 (100) | 25 (100) |
| Stomach (C16) | 1 (3.85) | 1 (4.0) |
| Liver and intrahepatic bile ducts (C22) | 1 (3.85) | 3 (12.0) |
| Breast (C50) | 7 (26.92) | 1 (4.0) |
| Cervix (C53) | 4 (15.38) | - |
| Thyroid (C73) | 6 (23.08) | 1 (4.0) |
| Carajás | 36 (100) | 16 (100) |
| Stomach (C16) | 1 (2.78) | 1 (6.25) |
| Larynx (C32) | - | 2 (12.5) |
| Lung (C34) | 1 (2.78) | 1 (6.25) |
| Bone marrow leukemia (C42.1) | 3 (8.33) | 4 (25.0) |
| Breast (C50) | 12 (33.33) | - |
| Cervix (C53) | 3 (8.33) | - |
| Thyroid (C73) | 6 (16.67) | 2 (12.5) |
| Xingu | 19 (100) | 19 (100) |
| Bone marrow leukemia (C42.1) | - | 4 (21.05) |
| Breast (C50) | 5 (26.32) | - |
| Cervix (C53) | 4 (21.05) | - |
| Prostate (C61) | - | 2 (10.53) |
| Araguaia | 11 (100) | 6 (100) |
| Bone marrow leukemia (C42.1) | - | 1 (16.67) |
| Breast (C50) | 2 (18.18) | 1 (16.67) |
| Cervix (C53) | 3 (27.27) | - |
| Prostate (C61) | - | 1 (16.67) |
| Frontal lobe of the CNS (C71) | 2 (18.18) | - |
| Lower Amazon | 7 (100) | 4 (100) |
| Stomach (C16) | 1 (14.29) | - |
| Lung (C34) | 2 (28.57) | - |
| Breast (C50) | 2 (28.57) | - |
| Cervix (C53) | 1 (14.29) | - |
| Prostate (C61) | - | 1 (25.0) |
| Thyroid (C73) | - | 2 (50.0) |
The main solid tumors also had a heterogeneous incidence profile in the population of Pará (Table 4 and Table 5). Breast cancer was the most common cancer, which occurred in 489 patients (16.57%), followed by cervical cancer, which occurred in 396 patients (13.41%), stomach cancer, which occurred in 265 patients (8.98%), prostate cancer, which occurred in 225 patients (7.62%),cancer of the colon and rectum, which occurred in 191 patients (6.47%), leukemias, which occurred in 120 patients (4.07%), lung cancer, which occurred in 92 patients (3.12%) and lymphomas, without a specific location, which occurred in 87 patients (2.95%). Other neoplasms accounted for 36.81% of the total number of cases.
Cancer within the digestive tract was the most common, with 624 cases in 2020 [265 (42.47%) cases of stomach cancer, 191 (30.61%) cases of colon and rectum cancer, 65 (10.41%) cases of cancer affecting the liver and intrahepatic bile ducts as well as the gallbladder and extrahepatic bile ducts, and 47 (7.53%) cases affecting the pancreas]; 8.98% of cases were distributed among the esophagus and small intestine and in unspecified locations.
Cervical cancer accounted for 396 (78.42%) of 505 cases of gynecological cancers, followed by ovarian cancer (51 cases, 10.10%) and uterine body cancer (43 cases, 8.51%). In addition, when mastology was included within this group, breast cancer accounted for 49.20% of the total registered cases, bringing the total number to 994 cases.
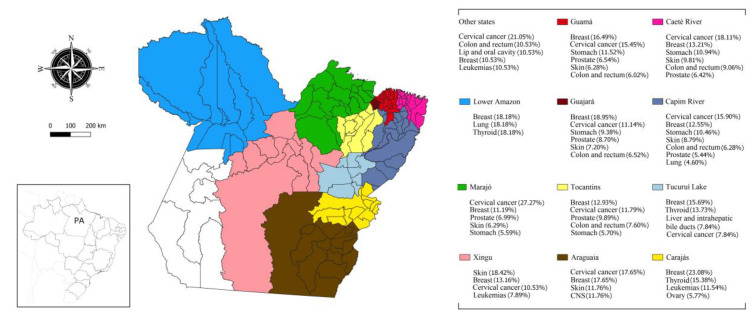
For solid tumors located in the head and neck, there were 254 cases [108 (42.52%) cases of thyroid cancer, 81 (31.89%) cases of lip and oral cavity cancer, and 33 (12.99%) cases oflarynx cancer]. The remaining cancers (nasal cavity and middle ear, pharynx, nasopharynx, paranasal sinuses, and ocular) accounted for 12.96% of the cases. Finally, there were 386 cases of urological cancer [225 (58.29%) cases of prostate cancer; 75 (19.43%) cases of kidney and renal cancer, 37 (9.59%) cases of bladder cancer, 32 (8.29%) cases of penile cancer, and 17 (4.40%) cases of testicular cancer] (Figure 2).
Figure 2.
Percentage of the most frequent cancers by integration region.
Within the relationship between females and males, except for cervical, breast, and prostate cancers and excluding non-melanoma skin cancer, there was a higher proportion of cases of stomach cancer among men in all IRs. However, the highest observed number ofthyroid cancer cases occurred among women (Table 3).
4. Discussion
The results of this study indicate the importance of OLCH as the reference center in Pará for the care and treatment of cancer patients. Based on the sociodemographic data analyzed, there was a high incidence of cases among patients between 20 and 49 years of age (31.06%), with the majority being female (59.49%). Additionally, approximately 87.30% of the people referred to OLCH had a previous diagnosis but had not started treatment, and only 12.64% had a previous diagnosis and had started the first therapeutic modality.
In terms of spatial representation, Pará encompasses 1,253,164.5 km2, making it the second largest state in Brazil in territorial extension, 35.54% larger than the Southeast Region (924,558,341 km2), and larger than countries such as France, Spain, and Ukraine. In this regard, the Legal Amazon accounts for approximately 24.9% of the total extension (5,016,478.27 km2) of Pará as well as 30.9% of its resident population (28,419,712 inhabitants), thus being the second most populous IR [15]. Thus, given its importance in area and population, the flow of patients to OLCH is noteworthy.
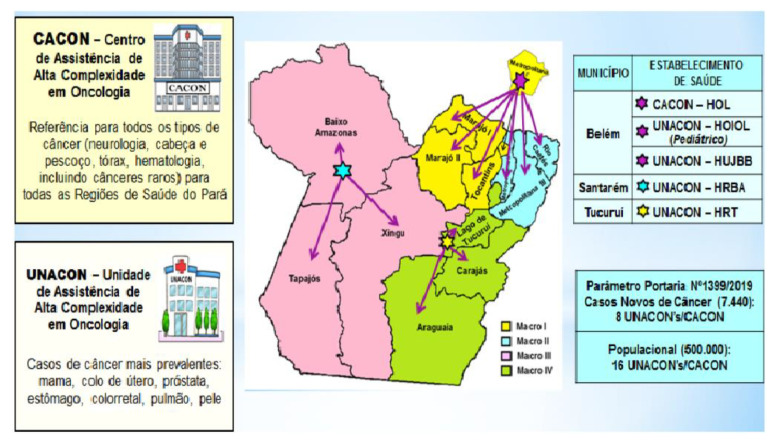
To identify the distribution of cancer cases, Figure 3 details the access protocol of the medium and high-complexity assistance network in oncology of the State of Pará (2021) [16], which directs case flow based on the macro-region and type of cancer. Case flow is determined by institution availability, confirmed diagnosis or clinical suspicion of cancer, and tumor profile, leading to referrals to the high-complexity care units and high-complexity center at OLCH [16].
Figure 3.
Flow of patients to high-complexity care centers and units. Source: adapted from the access protocol of the medium and high-complexity assistance network in oncology of the State of Pará, 2020.
However, due to the limited number of beds and thus the ability to treat a reduced number of patients with cancers at each UNACON, CACON/OLCH has become the reference institution for patients who need a hospital bed, a lower cost of care, and radiotherapy as well as for patients who have cancers that are not treated at certain UNACONs. Of the estimated number of cancer cases in Pará for 2020 [17], the percentage recorded in the INCA hospital cancer registries (HCR/INCA) for OLCH accounted for approximately 71.11% of all patients in the state, corroborating the high demand observed [16].
In addition to this analysis, the comparison of this percentage of patients who begin follow-up at OLCH with the total registered in Brazil in 2020, indicates a strong prevalence of preventable neoplasms. However, the flow of oncology patients to high-complexity centers in 2020, compared to 2019, wasdrastically reduced, following the manifestation of the first waves of the COVID-19 pandemic, by approximately 45% to 66% [18,19]. Furthermore, the primary tumors with the highest percentage of reduction in diagnosis were colorectal, prostate, and bladder [20].
The coherence found that justifies this reality is directly associated with the re-formulation of surveillance plans and technical support for health services, in accordance with the need for isolation and reduction of the risk of contagion [21]. Furthermore, the findings found in the 2020 records at OLCH suggest the need for an epidemiological description of the reality of a large oncology care center in the eastern Amazon region.
Figure 1 illustrates the spatial distribution of cases by municipality and the percentage by IR, representing the flow of referrals to OLCH from 119 municipalities in Pará and fivein other states. In this context, the highest flows originated in Guajará (49.86%) and Guamá (12.94%). Furthermore, as seen in Figure 2, the distribution of malignant neoplasms in the state followed the prevalence of cancer in each region of Brazil, with breast, cervical, stomach, and colorectal cancers being the most prominent.
In several regions of Brazil, breast cancer overlaps with cervical cancer or has a similar profile [22]. However, in patients treated at CACON/OLCH, for example, from some integration regions, the proportions of these cancers were different (Figure 2, Table 4 and Table 5). In the state of Pará, breast cancer is the most common and causes the highest mortality, which is different from the national data where it may occupy the second or third position (Table 2).
Stomach cancer was also prevalent among patients treated at CACON/OLCH (Figure 2, Table 4 and Table 5). An association between the regional culture of excessive consumption of foods with a hypersodium diet and the high incidence and mortality in Paráhas already been scientifically established [23,24,25] and is not observed in other regions and other states [26] (Table 2 and Table 3). This evidence explains the proportion of cases in the Salgado micro-region, which is composed of the municipalities of the Guamá, Caeté River, and Guajará IRs in northeastern Pará (Figure 2, Table 4 and Table 5). Thus, the epigenetic favoring of mutated genes present in gastric cancer caused by a high-sodium diet is closely associated with the prevalence of this neoplasm in Pará [24].
In Tucuruí Lake and Carajás, thyroid cancer was the second most frequent neoplasms, and in the Lower Amazon, it was the third most frequent among patients treated at OLCH (Figure 2, Table 4 and Table 5). In contrast, this neoplasm only ranks asnineteenth on the national scene. Previous studies have indicated the high mining activity in Carajás and the Lower Amazon with a strong relationship between methyl mercury and mercury contamination in the Tapajós River and the adjacent gold extraction territory and the high load of these materials in fish distributed regionally for human consumption [27,28,29]. In addition, exposure to mercury increases the risk of developing thyroid cancer [30].
Of the total solid tumors identified in the 2952 patients, the 10 most incident tumors (except non-melanoma skin cancer) were breast cancer, cervical cancer, stomach cancer, prostate cancer, colon and rectum cancer, leukemia (bone marrow), thyroid cancer, lung cancer, lymphoma (unspecified), and lip and oral cavity cancer.
Breast cancer accounted for the highest number of cases (Table 2), corroborating its high prevalence in the northern regions in an ecological study conducted by Camargo et al. (2021) [31]. In the data recorded by CACON, approximately 35.79% of cases were in patients aged between 20 and 49 years, 24.95% were in patients aged between 40 and 49 years, and 64.21% were in patients over 50 years of age. However, in recent years, there has been a trend in Brazil toward an increase in the proportion of cases in patients under 40 years of age [32].
The increase in this frequency and the predominance in areas with greater human and socioeconomic development have been associated with greater access to diagnostic and detection tools, as exemplified by the high prevalence of advanced-stage disease (III and IV) in women under 50 years of age [33]. However, the high number of cases is also associated with low preventive coverage, with a deficit in monitoring and offering screening tests in more precarious regions [32].
On a global level, cervical cancer is the fourth most prevalent cancer among all types and the second most prevalent among females, in 2020 reaching an estimated 604 thousand cases and 342 thousand deaths [34]. In Brazil, cervical cancer is the third most common disease in women, especially in the Centra-West Region [32]. However, in Pará, the incidence of this neoplasm ranked second among women treated at OLCH (Table 1 and Table 2), with 48.23% being between 20 and 49 years, 48.74% being older than 50 years and only 3.03% being younger than 20 years.
In reference to this reality, although screening and detection tests are available for this malignancy, in the last decade there was a significant decline in the coverage of preventive tests, such as cytopathological and histopathological tests, in regions of Brazil, especially in the North, Southeast and South, in addition to an increase in the time it takes to start treatment [35,36]. Additionally, the North Region has one of the highest rates of cervical cancer in Brazil but does not have the same prevalence of confirmed cases of HPV [37]. Thus, the decline in early detection strategies and the limited vaccination coverage in the last 3 years may be closely linked to the incidence of this neoplasia in Pará.
Gastric cancer is a public health problem affecting onein 54 men and onein 126 women worldwide [38]. In Brazil, stomach cancer ranks fourth among male malignancies, mainly affecting those aged between 60 years and 74 years [26]. In the general Brazilian population, in 2020, this primary tumor location ranked eighth (Table 2), and there were approximately 13,360 new cases among men and 7870 among women in the period from 2020 to 2022 [17].
Among the Brazilian regions, for males, gastric cancer is more common in the South (16.02/100,000), Southeast (13.99/100,000), and North (11.75/100,000) Regions [17]. At CACON/OLCH, the incidence of gastric cancer ranks third, with 265 cases, with a predominance of cases in males (67.55%) than in females (32.45%); 24.15% of patients with these neoplasms were younger than 50 years, with a higher incidence among those between 50 and 69 years of age (49.05%).
As the second most common malignancy among men worldwide, prostate cancer accounts for an estimated 65,000 cases in Brazil alone, being the second most lethal cancer in this population [39]. Of the 225 patients treated at OLCH, the majority were over 70 years of age (51.55%), and 46.67% were between 50 and 69 years of age; prevalence rates that are close to the national rate [39], which for 2020 was 33.08/100,000 inhabitants, approximately 3 times higher than that for lung cancer and trachea cancer, thus contradicting the global trend, which is the opposite [17].
Malignant neoplasms of the colon and rectum were the fourth most frequent and the third most frequent cancers among females at OLCH (Table 2). Worldwide, the incidence of these neoplasms ranks second, with the fifth-highest mortality rate among men [34]. In Brazil, the estimated rate of new diagnoses per year between 2020 and 2022 was 20,540 for males and 20,470 for females, associated with an increased influence of modifiable risk factors [40,41]; these values differ from the proportions at CACON/OLCH, with malignant neoplasms affecting more women (53.40%) than men (46.60%) in 2020 [42]. Therefore, in this study, of the total number of cases with a diagnosis or clinical suspicion of colorectal cancer, 39.27% were between 40 and 59 years of age, 49.21% were older than 60 years, and 11.52% were between 20 and 39 years of age.
Furthermore, some sociocultural factors suggest explanations directly related to the different incidences of this neoplasia in the IRs of Pará (Table 4 and Table 5). Recent studies indicate that the coverage of specialized diagnostic services and preventive follow-up for colorectal cancer are poorly distributed between the interior and the capital of the states in the North Region, in addition to the rapid growth, in the last decade, of sedentary lifestyles and obesity [43,44].
The global epidemiological profile of thyroid cancer indicates a higher incidence in females, with an estimated risk of 3 to 10 times greater than that for males (Table 3), depending on the region [45]. In Brazil, for this malignancy, the estimate for 2020 was 13,780 cases, with a higher prevalence in the South and Southeast Regions [17].
Based on the local data from OLCH, thyroid cancer, in general, was the seventh most frequent, excluding non-melanoma skin cancer (Table 4 and Table 5), with 86.11% of cases in females and only 13.89% in males. In addition, 39.82% of those with thyroid cancer were between 20 and 39 years of age, 44.44% were between 40 and 59 years of age, and 15.74% were older than 60 years. In recent years, there has been a significant increase in the incidence of these neoplasms in the population due to the carcinogenic profile itself and advances in diagnosis, which have allowed the early detection of subtypes of differentiated papillary, follicular, and Hürthle cell carcinomas [34,46].
5. Conclusions
Considering the admissions and treatments to CACON/OLCH for 2020, there were high incidences of breast, cervical, stomach, prostate, colon, and rectal cancersin individuals with an average age close to that expected based on the national average. However, the disparities in the incidence rates and the growth in the number of less expressive types of cancer show that the carcinogenic profile and the influence of environmental factors specific to each IR promote a specific epidemiological profile for the eastern Amazon.
In addition, Guajará stands out as the IR with the highest flow of referrals, and Marajó has the highest proportion of patients with cancer who underwent screening and the lowest coverage of preventive follow-up.
Acknowledgments
We acknowledge Ophir Loyola Cancer Hospital (OLCH); the University of Pará (UEPA); the Nucleus of Research in Oncology (NPO/UFPA); and the Federal University of Pará (UFPA).
Author Contributions
Conceptualization, J.R.C., D.D.F.Á.A., M.I.T.O., A.P.B.d.S., M.M.B.G.I. and R.M.R.B.; methodology, M.I.T.O., J.R.C., J.S.d.C.R., D.D.F.Á.A. and R.M.R.B.; software, M.I.T.O., J.R.C. and D.D.F.Á.A.; validation, J.R.C. and D.D.F.Á.A.; formal analysis, M.I.T.O., J.R.C., A.P.B.d.S. and D.D.F.Á.A.; investigation, M.I.T.O., J.R.C., D.D.F.Á.A., A.P.B.d.S. and R.M.R.B.; resources, J.R.C., D.D.F.Á.A. and R.M.R.B.; data curation, R.S.L. and M.I.T.O.; writing—original draft preparation, M.I.T.O., J.R.C., J.S.d.C.R. and D.D.F.Á.A.; writing—review and editing, M.I.T.O., J.R.C., D.D.F.Á.A. and R.M.R.B.; visualization, M.I.T.O., J.R.C. and D.D.F.Á.A.; supervision, J.R.C., D.D.F.Á.A. and R.M.R.B.; project administration, J.R.C.; and funding acquisition, J.R.C., D.D.F.Á.A., M.M.B.G.I. and R.M.R.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Federal University of Pará/Dean’s Office for Research and Graduate Studies (PROPESP)-Public Notice-Support Program for Qualified Publication (PAPQ) and University of the State of Pará/Dean’s Office for Research and Graduate Studies (PROPESP).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Lee R.J., Madan R.A., Kim J., Posadas E.M., Yu E.Y. Disparities in Cancer Care and the Asian American Population. Oncologist. 2021;26:453–460. doi: 10.1002/onco.13748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Moura R.R., Coelho A.V., Balbino V.Q., Crovella S., Brandão L.A. Meta-analysis of Brazilian genetic admixture and comparison with other Latin America countries. Am. J. Hum. Biol. 2015;27:674–680. doi: 10.1002/ajhb.22714. [DOI] [PubMed] [Google Scholar]
- 4.Allemani C., Matsuda T., Di Carla V., Harewood R., Matz M., Niksic M., Bonaventure A., Valkov M., Johnson C.J., Estève J., et al. Global surveillance of trendsin cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Oliveira N.P.D., Santos Siqueira C.A.D., Lima K.Y.N., de Camargo Cancela M., Souza D.L.B. Association of cervical and breast cancer mortality with socioeconomic indicators and availability of health services. Cancer Epidemiol. 2020;64:101660. doi: 10.1016/j.canep.2019.101660. [DOI] [PubMed] [Google Scholar]
- 6.Korkes F., Rodrigues A.F.S., Baccaglini W., Cunha F.T.S., Slongo J., Spiess P., Glina S. Penile cancer trends and economic burden in the Brazilian public health system. Einstein. 2020;18:eAO5577. doi: 10.31744/einstein_journal/2020AO5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lima C.A., da Silva B.E.B., Hora E.C., Lima M.S., Brito E.A.C., Santos M.O., da Silva A.M., Nunes M.A.P., Brito H.L.F., Lima M.M.M. Trends in prostate cancer incidence and mortality to monitor control policies in a northeastern Brazilian state. PLoS ONE. 2021;16:e0249009. doi: 10.1371/journal.pone.0249009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iser D.A., Cobalchini G.R., Oliveira M.M., Teixeira R., Malta D.C., Naghavi M., Iser B.P.M. Prostate cancer mortality in Brazil 1990–2019: Geographical distribution and trends. Rev. Soc. Bras. Med. Trop. 2022;55:e0277. doi: 10.1590/0037-8682-0277-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filho M.F.B., Santana M.E., Mendes C.P., Jesus Costa D., Santos C.A.A.S.D., Araújo M.F.M., Oliveira Serra M.A.A. Cultural, social, and healthcare access factors associated with delays in gastric cancer presentation, diagnosis, and treatment: A cross-sectional study. J. Cancer Policy. 2021;28:100277. doi: 10.1016/j.jcpo.2021.100277. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira M.M., Silva I.P.B., Teixeira R., Malta D.C., Iser B.P.M. Esophageal cancer mortality in brazil: A time-series analysis from the global burden of disease study. Arq. Gastroenterol. 2021;58:100–106. doi: 10.1590/s0004-2803.202100000-17. [DOI] [PubMed] [Google Scholar]
- 11.Zamudio R., Pereira L., Rocha C.D., Berg D.E., Muniz-Queiroz T., Anna H.P.S., Cabrera L., Combe J.M., Herrera P., Jahuira M.H., et al. Population, Epidemiological, and Functional Genetics of Gastric Cancer Candidate Genes in Peruvians with Predominant Amerindian Ancestry. Dig. Dis. Sci. 2016;61:107–116. doi: 10.1007/s10620-015-3859-6. [DOI] [PubMed] [Google Scholar]
- 12.Gibbon S. Translating Population Difference: The Use and Re-Use of Genetic Ancestry in Brazilian Cancer Genetics. Med. Anthropol. 2016;35:58–72. doi: 10.1080/01459740.2015.1091818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffecker J.F., Scott A.E., O’Rourke D.H., Scott G.R., Bigelow N.H. Beringiaand the global dispersal of modern humans. Evol. Anthropol. 2016;25:64–78. doi: 10.1002/evan.21478. [DOI] [PubMed] [Google Scholar]
- 14.Carroll C.B., Gomide M. Análise de redesnaregulação do tratamento do câncer do aparelhodigestivo. Cad. SaúdePública. 2020;36:e00041518. doi: 10.1590/0102-311X00041518. [DOI] [PubMed] [Google Scholar]
- 15.Instituto Brasileiro de Geografia e Estatística . Estimativas da Populaçãoresidente para Osmunicípios e para as Unidades da Federaçãobrasileiros com Datadereferênciaem 1º de Julho de 2019. IBGE; Rio de Janeiro, Brazil: 2019. [(accessed on 15 March 2023)]. Available online: https://biblioteca.ibge.gov.br/visualizacao/livros/liv101662.pdf. [Google Scholar]
- 16.Pará (Estado) Governo do Estado do Pará. Secretaria de Estado de SaúdePública. CoordenaçãoEstadual de AtençãoOncológica . Protocolo de Acesso da Rede de Assistência de Média e Altacomplexidadeemoncologia do Estado do Pará. CEAO/DDRA/SESPA; Belém, Pará, Brazil: 2021. [Google Scholar]
- 17.Instituto Nacional de Câncer José Alencar Gomes da Silva . Estimativa 2020: Incidência de Câncer no Brasil. INCA; Rio de Janeiro, Brazil: 2019. [Google Scholar]
- 18.De Vincentiis L., Carr R.A., Mariani M.P., Ferrara G. Cancer diagnostic rates during the 2020 ‘lockdown’, due to COVID-19 pandemic, compared with the 2018–2019: An audit study from cellular pathology. J. Clin. Pathol. 2020;74:187–189. doi: 10.1136/jclinpath-2020-206833. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman H.W., Chen Z., Niles J., Fesko Y. Changes in the number of US patients with newly identified cancer before and during the Coronavirus Disease 2019 (COVID-19) pandemic. JAMA Netw. Open. 2020;3:e2017267. doi: 10.1001/jamanetworkopen.2020.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Shamsi H.O., Alhazzani W., Alhuraiji A., Coomes E.A., Chemaly R.F., Almuhanna M., Wolff R.A., Ibrahim N.K., Chua M.L., Hotte S.J., et al. A practical approach to the management of cancer patients during the novel Coronavirus disease 2019 (COVID-19) Pandemic: An International Collaborative Group. Oncologist. 2020;25:e936–e945. doi: 10.1634/theoncologist.2020-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simões e Silva A.C., Oliveira E.A., Martelli Júnior H. Coronavirus disease pandemic is a real challenge for Brazil. Front. Public Health. 2020;8:268. doi: 10.3389/fpubh.2020.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nascimento J.H.F., Vieira A.T.S., Souza Filho B.M., Tomaz S.C., Delgado Bocanegra R.E., Melo Costa V.S., Johnson L.F.P., Gusmão-Cunha A., Silva Neto M.M., Andrade A.B. Breast cancer in Brazil: Screening program and surgical approach. Cancer Epidemiol. 2021;73:101970. doi: 10.1016/j.canep.2021.101970. [DOI] [PubMed] [Google Scholar]
- 23.Lee O.P., Cesario F.C. Relationship between food choices and the development of gastric cancer: A systematic review. Braz. J. Health Rev. 2019;2:2640–2656. doi: 10.34119/bjhrv2n4-036. [DOI] [Google Scholar]
- 24.das Neves I.S., Cruz M.S.Q.V., de Jesus D.L., Lima F.G.F., Nazeba K.V.J.F.O., Monteiro Júnior M.A.C. Epidemiologicalanalysis of deaths from stomach cancer in Northern Brazil. Res. Soc. Dev. 2021;10:e39410917503. doi: 10.33448/rsd-v10i9.17503. [DOI] [Google Scholar]
- 25.Braga L.L.B.C., Ferreira A.F., Pinheiro F.A.S., Benigno T.G.D.S., Heukelbach J., de Castro D.B., Queiroz D.M.M., Miyajima F., Ramos A.N., Jr. Temporal trends and spatial clusters of gastric cancer mortality in Brazil. Rev. Panam. Salud. Publica. 2022;46:e101. doi: 10.26633/RPSP.2022.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hora B.K.S., Pereira P.C., Brito M.S., Cedraz M.E.S., Britto Neto H.S., de Melo A.C.C., Gois Y.D.C., de Jesus C.V.F., Batista J.F.C., Lima S.O. Spatialand temporal analysis of mortality from gastric cancer in Brazil, 2001 to 2020. Res. Soc. Dev. 2022;11:e550111436909. doi: 10.33448/rsd-v11i14.36909. [DOI] [Google Scholar]
- 27.Meneses H.d.N.d.M., Oliveira-da-Costa M., Basta P.C., Morais C.G., Pereira R.J.B., de Souza S.M.S., Hacon S.d.S. Mercury Contamination: A Growing Threatto Riverineand Urban Communities in the Brazilian Amazon. Int. J. Environ. Res. Public Health. 2022;19:2816. doi: 10.3390/ijerph19052816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Custódio F.B., Andrade A.M.G.F., Guidi L.R., Leal C.A.G., Gloria M.B.A. Total mercury in commercialfishesandestimationofBraziliandietaryexposuretomethylmercury. J. Trace Elem. Med. Biol. 2020;62:126641. doi: 10.1016/j.jtemb.2020.126641. [DOI] [PubMed] [Google Scholar]
- 29.Hacon S., Barrocas P.R.G., de Vasconcellos A.C.S., Barcellos C., Wasserman J.C., Campos R.C., Ribeiro C., Azevedo-Carloni F.B. An over view of mercury contamination research in the Amazon basin with an emphasison Brazil. Cad. SaúdePública. 2008;24:1479–1492. doi: 10.1590/S0102-311X2008000700003. [DOI] [PubMed] [Google Scholar]
- 30.Kim S., Song S.H., Lee C.W., Kwon J.T., Park E.Y., Oh J.K., Kim H.J., Park E., Kim B. Low-Level Environmental Mercury Exposureand Thyroid Cancer Risk among Residents Living Near National Industrial Complexes in South Korea: A Population-Based Cohort Study. Thyroid. 2022;32:1118–1128. doi: 10.1089/thy.2022.0084. [DOI] [PubMed] [Google Scholar]
- 31.Camargo J.D.A.S., dos Santos J., Simões T.C., Carvalho J.B.L., Silva G.W.D.S., Dantas E.S.O., Rodrigues W.T.D.S., Freire F.H.M.A., Meira K.C. Mortality due to breast cancer in a region of high socioeconomic vulnerability in Brazil: Analysis of the effect of age-period and cohort. PLoS ONE. 2021;16:e0255935. doi: 10.1371/journal.pone.0255935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonadio R.C., Moreira O.A., Testa L. Breast cancer trends in women younger than 40 years in Brazil. Cancer Epidemiol. 2022;78:102139. doi: 10.1016/j.canep.2022.102139. [DOI] [PubMed] [Google Scholar]
- 33.Santos T.B.D., Borges A.K.D.M., Ferreira J.D., Meira K.C., Souza M.C., Guimarães R.M., Jomar R.T. Prevalence and factors associated with advanced stage breast cancer diagnosis. Cien. Saude Colet. 2022;27:471–482. doi: 10.1590/1413-81232022272.36462020. [DOI] [PubMed] [Google Scholar]
- 34.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 35.Silva G.A.E., Alcantara L.L.M., Tomazelli J.G., Ribeiro C.M., Girianelli V.R., Santos É.C., Claro I.B., Almeida P.F., Lima L.D. Evaluationof cervical cancer control actions within Braziland its regions based on data recorded in the Brazilian Unified National Health System. Cad. Saude Publica. 2022;38:e00041722. doi: 10.1590/0102-311xpt041722. [DOI] [PubMed] [Google Scholar]
- 36.Corrêa F.M., Migowski A., de Almeida L.M., Soares M.A. Cervical cancerscreening, treatment and prophylaxis in Brazil: Currentand future perspectives for cervical cancer elimination. Front. Med. 2022;9:945621. doi: 10.3389/fmed.2022.945621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colpani V., Falcetta F.S., Bidinotto A.B., Kops N.L., Falavigna M., Hammes L.S., Benzaken A.S., Maranhão A.G.K., Domingues C.M.A.S., Wendland E.M. Prevalence of human papillomavirus (HPV) in Brazil: A systematic review and meta-analysis. PLoS ONE. 2020;15:e0229154. doi: 10.1371/journal.pone.0229154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thrift A.P., El-Serag H.B. Burden of Gastric Cancer. Clin. Gastroenterol. Hepatol. 2020;18:534–542. doi: 10.1016/j.cgh.2019.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Oliveira R.A.R., Mourão T.C., Santana T.B.M., Favaretto R.L., Zequi S.C., Guimarães G.C. Cost-Effectiveness Analysis of Prostate Cancer Screening in Brazil. Value Health Reg. Issues. 2021;26:89–97. doi: 10.1016/j.vhri.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 40.GBD 2019 Colorectal Cancer Collaborators Global, regional, and national burden of colorectal cancer and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022;7:627–647. doi: 10.1016/S2468-1253(22)00044-9. Erratum in Lancet Gastroenterol. Hepatol. 2022, 7, 704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nascimento A.Q., Dantas D.B., Melo G.S., Gomes F.C., de Melo Neto J.S. Impact of sociodemographic factors and screening, diagnosis, and treatment strategies on colorectal cancer mortality in Brazil: A 20-year ecological study. PLoS ONE. 2022;17:e0274572. doi: 10.1371/journal.pone.0274572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dobiesz B.A., Oliveira R.R., Souza M.P., Pedroso R.B., Stevanato K.P., Pelloso F.C., Carvalho M.D.B., Pelloso S.M. Colorectal cancer mortality in women: Trendanalysis in Braziland its regions and states. Rev. Bras. Enferm. 2022;75:e20210751. doi: 10.1590/0034-7167-2021-0751. [DOI] [PubMed] [Google Scholar]
- 43.Kupper B.E.C., Ferreira F.O., Nakagawa W.T., Calsavara V.F., Chulam T.C., Lopes A., Aguiar-Junior S. Colorectal cancer: Association between sociodemographic variables and the adherence to cancer screening. Arq. Bras. Cir. Dig. 2023;36:e1729. doi: 10.1590/0102-672020230002e1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sampaio A.P.N., de Souza L.P., de Lima Moreira J.P., Luiz R.R., Fogaça H.S., de Souza H.S. Geographic Distribution and Time Trends of Colorectal Cancer in Brazil from 2005 to 2018. Dig. Dis. Sci. 2022;67:4708–4718. doi: 10.1007/s10620-021-07357-9. [DOI] [PubMed] [Google Scholar]
- 45.Borges A.K.d.M., Ferreira J.D., Koifman S., Koifman R.J. Câncer de Thyroid no Brasil: Estudo descritivo dos casosinformadospelos registros hospitalares de câncer, 2000–2016. Epidemiol. Serv. Saúde. 2020;29:e2019503. doi: 10.5123/S1679-49742020000400012. [DOI] [PubMed] [Google Scholar]
- 46.de Morais Fernandes F.C.G., de Souza D.L.B., Curado M.P., de Souza T.A., de Almeida Medeiros A., Barbosa I.R. Incidence and mortality from thyroid cancer in Latin America. Trop. Med. Int. Health. 2021;26:800–809. doi: 10.1111/tmi.13585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.