Abstract
This review explores the evolving landscape of antibody-based therapies in neuro-oncology, in particular, immune checkpoint inhibitors and immunomodulatory antibodies. We discuss their mechanisms of action, blood-brain barrier (BBB) penetration, and experience in neuro-oncological conditions. Evidence from recent trials indicates that while these therapies can modulate the tumor immune microenvironment, their clinical benefits remain uncertain, largely due to challenges with BBB penetration and tumor-derived immunosuppression. This review also examines emerging targets such as TIGIT and LAG3, the potential of antibodies in modulating the myeloid compartment, and tumor-specific targets for monoclonal antibody therapy. We further delve into advanced strategies such as antibody–drug conjugates and bispecific T cell engagers. Lastly, we explore innovative techniques being investigated to enhance antibody delivery, including CAR T cell therapy. Despite current limitations, these therapies hold significant therapeutic potential for neuro-oncology. Future research should focus on optimizing antibody delivery to the CNS, identifying novel biological targets, and discovering combination therapies to address the hostile tumor microenvironment.
Keywords: immunotherapy, brain tumor, antibodies, immunomodulation, glioblastoma, meningioma
1. Background
Neuro-oncology is a rapidly evolving field focusing on the management of primary and secondary brain and spinal cord tumors. The American Cancer Society predicts that 24,810 adults in the US (14,280 males and 10,530 females) will be diagnosed with primary malignant tumors of the brain and spinal cord in 2023 [1]. Around 18,990 fatalities (11,020 males and 7970 females) due to primary malignant brain and central nervous system (CNS) tumors, the tenth leading cause of death for both genders, are expected to occur in the US in 2023 [1]. Given the high mortality rate of patients with neuro-oncological conditions, new therapeutic approaches are needed to improve the standard of care.
Antibody-based immunotherapy holds great potential for patients with CNS malignancies, with a myriad ongoing preclinical and clinical efforts. With advances in producing humanized antibodies and “fully-human” proteins [2], antibodies have been shown to be potent therapeutic tools in the treatment of various cancers starting from the late 1980s [3,4]. However, the CNS presents unique challenges for antibody therapies, in large part due to the immunosuppressive microenvironment [5] and limited access hampered by the blood-brain barrier (BBB) [6].
This review aims to discuss the role of antibody-based therapies in neuro-oncology, focusing on immune checkpoint inhibitors and immunomodulatory antibodies. We will explore their mechanisms of action, their ability to penetrate the BBB, and their efficacy in treating neuro-oncological conditions.
2. The Challenge of Crossing the BBB
The BBB, a highly selective semipermeable structure, restricts the passage of substances from the bloodstream into the brain. This barrier poses a significant challenge for the delivery of antibody-based therapeutic agents [7]. Monoclonal antibodies are proteins that are traditionally considered to be too large to penetrate the blood-brain barrier (BBB), limiting their efficacy against tumors within the CNS [8]. Additionally, the expression of the neonatal Fc receptor (FcRn) in the capillary endothelium of the BBB has a putative role in preventing the delivery of antibodies to the brain parenchyma. Specifically, FcRn is theorized to cause reverse transcytosis of IgG antibodies from the brain to the blood [9,10]. Modifying the antibody Fc domain to prevent their interaction with FcRn has been shown preclinically to improve the distribution of antibodies to the brain [9,11].
However, the traditional limitations of the BBB may be less relevant to brain tumors that disrupt the integrity of the BBB. In the case of high-grade gliomas, the accumulation of contrast agents like gadolinium and a higher distribution of high molecular weight proteins (i.e., monoclonal antibodies) within tumor tissue compared to normal brain tissue suggests that the compromised barrier may permit the passage of molecules and/or biologics to the brain from systemic circulation [12,13,14]. Although a more permeable BBB is a hallmark of high-grade glioma and perhaps beneficial for drug delivery, it is thought that much of the infiltrative component may still be protected by intact regions of the BBB [12], representing a persistent challenge for antibody-based therapeutics. Both the timing of antibody administration and the extent of BBB permeability in neuro-oncologic disease are areas of ongoing research.
Evidence from other neurological antibody-based therapies and autoimmune neurological diseases suggest some BBB penetration potential for monoclonal antibodies. In mouse studies of Alzheimer’s disease, peripherally administered antibodies against amyloid-beta were able to enter the CNS, localize to plaques, and clear them [15]. This suggests some degree of BBB crossing, but theoretically, the antibodies may have accumulated outside the BBB and sequestered plaques there. In paraneoplastic neurologic disorders, several antibodies that are generated peripherally against a tumor can cross-react with neurological structures and cause morbidity. This in turn suggests that these paraneoplastic antibodies such as anti-Ma, anti-Ri, and anti-amphiphysin can cross the BBB and bind the brain parenchyma [16].
Finally, clinical studies using radiolabeled antibody–drug conjugates have shown selective tumor localization of these antibodies in patients with gliomas and other intracranial malignancies, providing further credence to the ability of monoclonal antibodies to accumulate in intracerebral sites [17,18]. However, rigorous comparison of intratumoral versus systemic delivery of therapeutic antibodies is ongoing, and radiolabeling of other frequently infused monoclonal antibodies like immune checkpoint inhibitors has not been studied comprehensively in patients with brain tumors. Recently, bispecific antibodies that bind to transferrin receptors (TfR) associated with the BBB have been shown to have enhanced distribution to the brain through co-opting the mechanism of TfR-mediated endocytosis [19]. Bispecific antibodies targeting other components of the BBB may prove effective in facilitating the delivery of therapeutic agents to the brain.
Even if these barriers posed by the BBB prevent monoclonal antibodies from entering the brain, there remains an open question on whether immunomodulatory antibodies, such as the immune checkpoint inhibitors that we will discuss in the next section, need to reach the brain to have efficacy. Antibodies could theoretically bind to and modulate T cells in the periphery, such that these cells are activated in some way prior to homing to the CNS for their cytotoxic activities.
3. Immune Checkpoint Inhibitors and Immunomodulatory Antibodies
3.1. Immune Checkpoint Inhibitors: Anti-PD-1 and Anti-CTLA-4
Immune checkpoint inhibitors (ICIs), such as anti-programmed cell death protein 1 (PD-1) and anti-cytotoxic T-lymphocyte-antigen 4 (CTLA-4), have revolutionized cancer treatment by augmenting the immune system’s ability to destroy cancer cells through the activation and prevention of T cell exhaustion. ICIs are not involved in tumor recognition but rather target the tumor–immune cell interface. Specifically, these inhibitors work by blocking the signals that cancer cells use to evade immune responses, thereby allowing immune cells to attack tumors.
PD-1 is a cell surface receptor expressed predominantly on activated T cells, B cells, and some monocytes. It plays a crucial role in downregulating the immune system and promoting self-tolerance by suppressing T cell inflammatory activity. The mechanism of action of PD-1 revolves around its interaction with its ligands, PD-L1 and PD-L2. When these ligands, often expressed on tumor cells or on cells in the tumor microenvironment, bind to PD-1, they transmit an inhibitory signal into the T cell [20]. This signal reduces the proliferation of T cells in lymph nodes and their activity in peripheral tissues, resulting in reduced autoimmunity and protection of tissues from immune-mediated damage. However, cancers exploit this pathway, using PD-L1 and PD-L2 expression to shut down the immune response against them, thereby allowing them to grow unchecked. One strategy for antibody-based immune checkpoint therapies is the use of anti-PD-1 antibodies (e.g., nivolumab and pembrolizumab) [21]. Therapeutic antibodies targeting the PD-1/PD-L1 pathway are designed to block this interaction, thereby releasing the “brakes” on the immune system and enabling it to target and destroy tumor cells. The function of these antibodies is to block the binding of PDL-1 to PD-1 on the surface of T cells. Use of PD-1 blockade on PD-1 expressing T cells has been associated with reversing T-cell exhaustion and improved cytokine production [22].
CTLA-4 is another critical immune checkpoint receptor, predominantly expressed on the surface of T cells. It shares structural similarities with the T cell co-stimulatory protein, CD28, and both bind to CD80 and CD86 on antigen-presenting cells (APCs). The binding of CD28 to CD80 and CD86, along with the binding of the T cell receptor (TCR) to MHC1, promotes T cell activation and proliferation [23]. However, unlike CD28, which promotes T cell activation when engaged, CTLA-4 acts as an “immune brake” by outcompeting CD28 for its ligands and delivering inhibitory signals to the T cell. In essence, CTLA-4′s engagement reduces T cell proliferation, IL-2 production, and cell cycle progression [23]. Tumor cells can exploit the CTLA-4 pathway, indirectly facilitating a suppressed immune environment favorable for tumor growth. Monoclonal antibodies, such as ipilimumab, bind to CTLA-4 to block its interaction with ligands, CD80 and CD86, thereby augmenting T cell activation and proliferation. Furthermore, CTLA-4 signaling in regulatory T cells (Tregs) promotes their suppressive function, contributing to peripheral tolerance. While some studies have previously shown that anti-CTLA-4 monoclonal antibodies selectively depleted intratumoral FOXP3+ regulatory T cells via an Fc-dependent mechanism, clinical evidence has not confirmed this mechanism of action [24]. In neuro-oncology, these ICIs have shown promise for a subset of patients with glioblastoma (GBM), the most common and aggressive primary brain tumor, suggesting that certain biomarkers may be good for stratifying therapies [25]. Investigators have delved into the potential biomarkers associated with ICI responsiveness in GBM patients [26]. These include relationships with the differentiation status of tumor-infiltrating lymphocytes and the role of cytotoxic CD8+ tumor-infiltrating lymphocytes (TILs) [27]. For example, EomeshiT-betlo CD8+ TILs were associated with lower T cell activation in the setting of ICIs [27]. Additionally, higher densities of proliferating CD8+ T cells and a higher ratio of CD8+ to CD4+ cells in tumor infiltrates were found to be associated with improved survival [28].
Anti-PD-1 therapies are also currently being actively investigated in meningiomas. Preclinical evidence suggests that there is great intra- and intertumor heterogeneity of PD-L1 expression in meningiomas [29]. PD-L1 expression was also shown as a tumor marker that can predict recurrence [29]. Thus, a number of clinical trials have been conducted to evaluate the efficacy of anti-PD-1 therapy in recurrent meningiomas, as we will discuss below [30].
Although ICIs offer a promising avenue for glioma and meningioma treatment, their efficacy may be influenced by a range of factors, including the unique immune environment of the CNS, the presence and differentiation status of TILs, and the potential for synergistic combination therapies. Additional research is essential to fully realize the potential of ICIs in brain tumor treatment.
3.2. Combination Treatment Strategies
ICIs may be best used alongside conventional treatments like radiotherapy and chemotherapy, as well as novel modalities like virotherapy [31]. Combining virotherapy with immunotherapy may offer a novel approach to GBM in particular. Saha et al., investigated the combination of oncolytic herpes simplex virus (oHSV) with ICIs [32]. Their findings suggest that a specific oHSV expressing IL-12, when combined with anti-PD-1 and anti-CTLA-4 checkpoint inhibitors, could be curative. This combination therapy was found to be particularly effective due to the involvement of CD4+ and CD8+ T cells, as well as macrophages, creating a complex but effective interplay in combating tumors.
Radiation, which is intrinsically immunogenic and can reprogram an immunosuppressive tumor microenvironment [33], may be an effective combination therapy to facilitate immune cell recruitment and subsequent priming. Radiation has been shown to cause dynamic alterations in tumor-associated macrophages and microglia in glioma, but the extent of implications for immunotherapy still are unclear [34]. One preclinical study showed that the combination of an anti-PD-1 blockade and localized radiotherapy improved long-term survival in mice with intracranial glioma [35].
3.3. Clinical Trials for Anti-PD-1 and Anti-CTLA-4 Therapies
Over the past decade, a variety of trials have been conducted for ICIs in GBM [36,37,38,39,40,41,42,43,44,45]. In some of these trials, there was evidence of tumor immune microenvironment changes such as enhanced expression of chemokine transcripts, increased immune cell infiltration, and expanded T cell clonal diversity, suggesting that that antibody-based therapy can mediate immunomodulatory effects for tumors in the brain [45]. The majority of these trials utilized intravenous delivery, which raises the question regarding whether adequate BBB penetration was achieved or whether CNS localization was necessary for biological activity. One phase I trial examined intracerebral administration of CTLA-4 and a PD-1 immune checkpoint blockade in recurrent GBM [38]. While there was encouraging improvement in overall survival compared to a historical cohort, no significant effect was observed for progression-free survival. Nivolumab failed to show efficacy in the large randomized, multi-centered phase 3 Checkmate 143 trial, with comparable overall survival to bevacizumab [37]. Interestingly, the neoadjuvant administration of anti-PD-1 in an early-phase randomized trial was more effective in the setting of recurrent GBM compared to the adjuvant group [39].
Most recently, a phase 2 study of pembrolizumab in patients with recurrent and residual high-grade meningiomas achieved a 6-month progression-free survival rate of 0.48 and had a median progression-free survival (PFS) of 7.6 months. [46]. However, one in five patients experienced at least one grade 3 or higher treatment-related adverse event. Another phase 2 trial of nivolumab in patients with recurrent meningioma found no clinical benefit overall, but the drug was well tolerated [47]. The baseline TIL density in these patients was low, but three patients had increased TIL density post-treatment, and two of the three patients had an elevated tumor mutational burden greater than 10/Mb. Tumor mutational burden has been associated with positive immunotherapy responses in other tumors [48].
A phase 1/2 trial examining oncolytic virotherapy plus pembrolizumab in recurrent GBM was also recently completed [49]. In this trial, patients with GBM received intratumoral delivery of virus DNX-2401, followed by intravenous pembrolizumab. The objective response rate was 10.4% and the overall survival rate at 12 months was 52.7%. Notably, 3 of the 49 patients in the trial had durable responses and remained alive at 60 months. Genomic and immunological analysis of these tumors suggest that immune cell infiltration and checkpoint expression may be predictors of response to treatment.
The endpoint results of key clinical trials of ICIs in GBM and meningioma are summarized in Table 1.
Table 1.
Key trials of immune checkpoint inhibitors for central nervous system tumors.
| Author | Year | Phase | Tumor Type | Immunotherapy Agent | Combination Treatment | Number of Patients | Progression-Free Survival | Overall Survival |
|---|---|---|---|---|---|---|---|---|
| Brastianos PK, et al. [46] | 2022 | Phase II | recurrent high-grade meningioma | Pembrolizumab | None | 25 | 7.6 months (90% CI, 3.4–12.9) | 20.2 months (90% CI, 14.8–25.8) |
| Nassiri F, et al. [49] | 2023 | Phase I/II | recurrent glioblastoma | Pembrolizumab | Oncolytic Virotherapy | 49 | Not reported | 12.5 months (10.7–13.5) |
| Reardon DA, et al. [36] | 2021 | Phase I | recurrent glioblastoma | Pembrolizumab | None | 111 | 2.8 months (95% Cl, 1.8–8.1) | 13.1 months (95% CI 8.0–26.6) |
| Reardon DA, et al. [37] | 2020 | Phase III | recurrent glioblastoma | Nivolumab | Ipilimumab | 369 | 1.5 months (95% CI, 1.5–1.6) | 9.8 months (95% CI, 8.2–11.8) |
| Duerinck J, et al. [38] | 2021 | Phase I | recurrent glioblastoma | Ipilimumab | Nivolumab | 27 | 2.9 months (95% CI, 2.5–3) | 9.5 months (95% CI: 6.8–12.3) |
| Cloughesy TF, et al. [39] | 2019 | Phase II | recurrent glioblastoma | Pembrolizumab | None | 35 | 3.3 months | 13.7 months |
| Nayak L, et al. [40] | 2021 | Phase II | recurrent glioblastoma | Pembrolizumab | Bevacizumab | 80 | 4.1 months (95% CI, 2.8–8.6) | 8.8 months (95% CI, 7.7–14.2) |
| Sahebjam S, et al. [41] | 2020 | Phase I | recurrent high-grade glioma | Pembrolizumab | Hypofractionated Stereotactic Irradiation | 32 | 7.9 months (95% CI, 6.3–12.5) | 13.34 months (95% CI, 9.5–18.5) |
| Bi WL, et al. [47] | 2022 | Phase II | recurrent high-grade meningioma | Nivolumab | None | 25 | 5.6 months (95% CI, 3.2–7.4) | 30.9 months (95% CI, 17.6, NA) |
| Omuro A, et al. [42] | 2023 | Phase III | newly diagnosed glioblastoma | Nivolumab | Radiation Therapy | 560 | 6.0 months (95% CI, 5.7–6.2) | 13.4 (95% CI, 1.1–1.6) |
| Lim M, et al. [43] | 2022 | Phase III | newly diagnosed glioblastoma | Nivolumab | Temozolomide Plus Radiation Therapy | 716 | 10.6 months (95% CI, 8.9–11.8) | 28.9 months (95% CI, 24.4–31.6) |
| Omuro A, et al. [44] | 2018 | Phase I | recurrent glioblastoma | Nivolumab | Ipilimumab | 40 | 1.5 months (95% CI, 0.5–2.8) | 9.2 months (95% CI, 3.9–12.7) |
| Schalper KA, et al. [45] | 2019 | Phase II | recurrent glioblastoma | Nivolumab | None | 30 | 4.1 months (95% CI, 2.8–5.5) | 7.3 months (95% CI, 5.4–7.9) |
3.4. Additional Immune Checkpoints: TIGIT and LAG3
T cell immunoreceptor with Ig and ITIM domains (TIGIT) and Lymphocyte-activation gene 3 (LAG3) are other immune checkpoints that have been targeted for cancer treatment [50,51]. These checkpoints are expressed on various immune cells, including T cells and natural killer (NK) cells, and their blockade can enhance immune responses against cancer cells. While research on these targets in neuro-oncology is still in its early stages, preliminary studies suggest potential therapeutic benefits. For instance, the co-blockade of PD-1 and TIGIT has been shown to enhance T cell responses in preclinical models of GBM, leading to reduced tumor growth and improved survival [52]. Additionally, TIGIT expression on TILs in patient-derived GBM samples has been found to be elevated. LAG-3 blockade via monoclonal antibodies alone or in combination with anti-PD-1 has been shown to eradicate GBM tumors in mice [53]. Clinical trials testing antibodies against TIGIT (NCT04656535) and LAG3 (NCT02658981) are currently ongoing.
3.5. Modulating the Myeloid Compartment with Antibodies
Most antibody-directed targets in gliomas are focused on T cell modulation, despite a growing understating of the crucial role of innate immunity and the need to redirect predominant immunosuppressive myeloid cell populations in malignant gliomas [54]. Myeloid-derived suppressor cells (MDSCs), along with immunosuppressive M2 tumor-associated macrophages, can significantly blunt the efficacy of natural and engineered anti-tumor immunity.
Distinct from immune checkpoint molecules, phosphatidylserine (PS) is an immunosuppressive target widely expressed on the surface of cancer cells, including GBM [55]. Blocking PS signaling with anti-PS monoclonal antibodies (e.g., bavituximab) has demonstrated durable anti-tumor responses, especially in combination with RT in various preclinical tumor models [56], including GBM [57]. Anti-PS therapy has been shown to repolarize the immunosuppressive myeloid compartment towards a pro-inflammatory state [56,58]. A recently published phase 2 single-arm trial evaluated the efficacy of bavituximab in combination with temozolomide and radiation in 33 newly diagnosed patients with GBM and showed a marked reduction in infiltrating myeloid-derived suppressor cells (MDSCs) after treatment [59]. Although this study demonstrates a potential on-target effect, the mechanisms by which PS targeting antibodies interfere with the inhibitory signaling and function of the myeloid compartment are not well understood and require further investigation.
The CD47 protein in lay terms is a “don’t eat me signal” that inhibits macrophage phagocytosis of tumor cells which overexpress this protein. Researchers have also investigated using anti-CD47 monoclonal antibodies to reprogram the tumor-associated macrophages in the microenvironment. Testing this therapy on human glioblastoma cell lines and xenografts, they showed that the addition of anti-CD47 led to increased tumor-cell phagocytosis in both M1 and M2 macrophages, but this increase was more prominent in M1 macrophages [60]. Anti-CD47 therapy has also led to durable responses in various preclinical pediatric brain tumor models [61]. However, the trials of anti-CD47, which were initially tested in hematological malignancies were discontinued due adverse effects of significant anemia, which may suggest off-target toxicity because CD47 is expressed on non-malignant blood cells. Further study and optimization of the anti-CD47 may allow the optimization of the therapy.
3.6. Neurotoxicity Profile of Immune Checkpoint and Modulatory Antibodies
The use of antibody therapies has the potential to mediate off-tumor, on-target effects [62,63,64]. These immune-related adverse effects (irAEs) can include colitis, thyroiditis, pneumonitis, rashes, hepatitis, and less commonly, neurologic syndromes. Proposed mechanisms include overactivation of the peripheral immune system, activation from viral or co-administered drug antigens, and failure of self-tolerance [65,66]. Multiple case reports have described neurologic irAEs (irAEs-N), and previous research has found that up to 4.2% of cancer patients treated with anti-PD-1 therapy exhibited some level of neurologic dysfunction, with most occurring within 3 months of starting therapy [67]. Other studies estimate 1–2% will have irAEs-N [68]. Combination therapies can increase this likelihood up to a 12% risk [69]. The heterogeneity of irAEs-N within this population is notable for the involvement of both the central and peripheral nervous system, with syndromes including headaches, seizures, peripheral neuropathy, demyelinating diseases, neuromuscular receptor dysfunction, diffuse encephalopathy, CNS vasculitis, and aseptic meningitis [70,71,72].
For patients with pre-existing immune-related neurologic diseases (e.g., myasthenia gravis), exacerbation of the host immune response can be fatal [70]. The distribution of severity influence grading on the Common Terminology Criteria for Adverse Events (CTCAE) ranges from grade 1 (representing asymptomatic and mild disease) to grade 3 (requiring hospitalization) and grade 5 (mortality) [73]. For grade 3 and higher, ASCO (2021) and NCNN (2022) guidelines recommend hospitalization and treatment with intravenous glucocorticoids and additional appropriate immunomodulating/immunosuppressive therapies including intravenous immunoglobulin (IVIG), CD20 inhibition, and plasmapheresis [74,75,76]. After discontinuation of immunotherapy and treatment with immunosuppression, the majority of patients have robust clinical improvement, although residual sequalae may persist [77]. Balancing efficacy with toxicity remains a critical component of immunotherapy, and further research into the prediction and effective management of irAEs-N could improve tumor response to therapy. The pattern of adverse events in ICI neuro-oncology trials conducted to date has not been significantly different from that of other types of cancer. However, most neuro-oncology trials have utilized intravenous delivery. One trial for GBM which used intracerebral ICI delivery reported that immune-related adverse events were mild and infrequent [38]. It will be important to further characterize the neurotoxicity risk associated with intracerebral delivery.
If immune checkpoint blockade therapy is concurrently used with chimeric antigen receptor (CAR) T cell delivery, additional neurotoxic effects including cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), and tumor inflammation-associated neurotoxicity (TIAN) should be considered [78,79,80]. CRS is defined by a fever and nonspecific symptoms, including fatigue, tachycardia, and a rash, which may be self-limiting or require additional critical care interventions [81,82]. Patients with ICANS develop neurologic dysfunction with characteristic aphasia, apraxia, and dysgraphia which can progress to encephalopathy [83,84]. The Immune Effector Cell Encephalopathy Score (ICE) characterizes the severity, informing appropriate interventions for ICANS [85].
4. Tumor-Specific Targets for Monoclonal Antibody-Based Therapy
Reaching optimal treatment outcomes in brain tumors largely depends on the appropriate selection of targets. Some frequently explored surface targets in the field of neuro-oncology are discussed below.
4.1. EGFRvIII
This is a variant of the Epidermal Growth Factor Receptor (EGFR), resulting from a deletion of exons 2 to 7 [86,87]. In GBM, nearly 40% of newly identified patients show EGFR gene amplification, with roughly half of those expressing EGFRvIII [88]. The modified extracellular domain structure brought about by this mutation provides an ideal tumor-specific target due to its unique epitope, which may be recognized by specific monoclonal antibodies [89], reducing the chance of unwanted toxicity. EGFRvIII-targeted vaccination therapy using a peptide vaccine has been successful in redirecting cellular and humoral immunity against this target in cancer cells, preclinically and clinically [90,91,92].
4.2. IL13Rα2
IL-13′s function is to manage inflammation and immune responses upon binding to IL13Rα1. Furthermore, IL-13 also attaches to IL13Rα2 with high affinity. IL13Rα2 is found in more than 75% of GBMs and is associated with tumor aggressiveness and poor prognosis [93,94,95]. However, it is not significantly present in normal brain tissue or most other normal tissues, except for the testes [96]. Given the relatively specific presence of IL13Rα2 in GBM, it may serve as a promising target.
4.3. HER2
Human Epidermal Growth Factor Receptor 2 (HER2), a type of receptor tyrosine kinase, is overexpressed in around 40% of medulloblastomas and 80% of GBMs [81,97], with no expression in normal brain tissues. Thus, it appears to be an ideal target antigen for antibody-based immunotherapy in neuro-oncology. However, the use of HER2-targeted immunotherapy carries a risk of harmful off-target effects, as HER2 is also found in several essential normal tissues [98]. Targeting HER2 with chimeric antigen receptor (CAR) T cells has led to severe immune toxicity and subsequent multi-organ system failure in clinical studies [99].
4.4. Disialoganglioside (GD2)
GD2 exhibits high expression in several cancers including neuroblastoma and GBMs, making it an attractive target for GBM therapy as it is present on GBM cell lines and patient samples [100]. GD2 has been effectively and safely targeted by CAR T cells in diffuse midline gliomas and neuroblastoma in recently published clinical trials [101,102].
4.5. CD147
CD147 is found in GBM and is associated with a poorer prognosis for patients [103]. It plays a role in the degradation of the extracellular matrix (ECM) and promotes tumor progression, invasion, and metastasis.
4.6. SMARCAL1 Mutations
A certain group of primary GBMs which carry mutations in the SWI/SNF-related matrix-associated actin-dependent regulator of the chromatin subfamily A-like protein 1 (SMARCAL1) are seen as a potential target [104].
4.7. Delta-Like Canonical Notch Ligand 3 (DLL3)
DLL3, part of the Delta/Serrate/Lag2 (DSL) Notch receptor ligand family, plays a role in Notch signaling, impacting various cell processes such as differentiation, proliferation, survival, and apoptosis. Cell surface DLL3 may represent an effective target for certain subsets of gliomas and is expressed at very low or absent levels in normal tissues [105,106]. Rovalpituzumab tesirine (Rova-T) is a DLL3-targeting antibody–drug conjugate, and Tarlatamab [107], a first-of-its-kind DLL3-targeted bispecific T Cell engager, was recently studied in a phase I trial for recurrent small-cell lung cancer patients [108].
5. Advanced Antibody-Based Strategies
5.1. Antibody–Drug Conjugates (ADCs)
Antibody–drug conjugates consist of a monoclonal antibody fused with a potent cytotoxic agent. These cytotoxic components fall into two categories: immunotoxins and radiolabeled antibodies. Immunotoxins represent antibodies that are linked to naturally derived bacterial toxins, such as exotoxin A from Pseudomonas aeruginosa or diphtheria toxin. Some examples of these are ABT-414 [109], AMG-595 [110], Cintredexin Besudotoxin [111], NBI-3001 [112], TP-38 (IVAX) [113], and Tf-CRM107 [114].
Radiolabeled antibodies, also known as radioimmunoconjugates, are monoclonal antibodies connected to a radionuclide. These radiolabeled antibodies employ isotopes like iodine-124 or iodine-131 as their toxic payloads. Compared to traditional radiation therapy, radioimmunotherapy (RIT) reduces toxicity and boosts the effectiveness of monoclonal antibodies. Several radionuclides, including actinium-225 (225 Ac), astatine-211 (211 At), bismuth-213 (213 Bi), indium-111 (111 In), iodine-123 (123 I), iodine-124 (124 I), iodine-131 (131 I), lead-212 (212 Pb), lutetium-177 (177 Lu), technetium-99 m (99 mTc), copper-64 (64 Cu), gallium-68 (68 Ga), yttrium-86 (86 Y), yttrium-90 (90 Y), and zirconium-89 (89 Zr), can be used for labeling in therapeutic applications [115].
In the late 1980s, Bigner and colleagues began investigating iodine-131-labeled antibodies against tenascin, a glioma-associated extracellular matrix antigen, in intracranial human xenografts [116]. The preclinical studies showed significant survival prolongation in mice bearing these tumors. Later, Bigner and Pastan led studies on using Pseudomonas exotoxin conjugated to antibodies targeting IL-4, EGFR, and EGFRvIII which were translated to phase 1 trials using intratumoral administration [117]. Despite the impressive potency of these antibody–drug conjugates, early clinical trials showed limited success and some notable toxicity [118], especially with EGFR-targeting ADCs [119]. More recently, ABT-806 (Depatuxizumab), an ADC against EGFRvIII, failed to show any survival benefit in a phase III clinical trial [120]. Thus, the therapeutic potential of ADCs in clinical settings remains to be clinically realized, but the optimization of ADC targets and payloads, along with combinatorial strategies, continues to be explored.
5.2. Bispecific T Cell Engagers (BiTEs)
BiTE antibodies typically consist of one scFv that specifically identifies a surface antigen on a target cell, and another scFv that recognizes CD3, a portion of the activating complex found on T cells. BiTEs have demonstrated the ability to redirect T cells in the treatment of hematologic malignancies [121]. In preclinical studies, a BiTE that targets EGFRvIII has been observed to stimulate T cells and facilitate the antigen-specific destruction of EGFRvIII-expressing gliomas [122]. BiTEs have also been shown to be potent in modifying the tumor microenvironment by converting and redirecting the regulatory T cells into anti-tumor effector cells [123]. One theorized mechanism of how BiTEs reach the brain is that they piggyback on circulating T cells that then traffic to the brain parenchyma [124]. In support of this hypothesis, data show that the adoptive transfer of activated T cells increases BiTE CNS tumor penetrance [125]. This may represent a novel mechanism for enhancing antibody delivery to the brain through the hijacking of T cell migration.
5.3. Using Adoptive Cell Therapy to Enhance Antibody Delivery to the CNS
As aforementioned, antibody-based therapies for brain tumors are often systemically administered, and their therapeutic success may often hinge on their ability to cross the BBB or act on target cells that can ultimately traffic past the endothelial wall of the BBB [8]. Designing a strategy to optimize antibody-based delivery in gliomas is quite challenging; however, several novel approaches are currently being investigated, such as receptor mediated-transcytosis, focused ultrasound, nanoparticles, chemical modifications, and enzyme-mediated BBB disruption [126,127,128].
Another method to enhance antibody delivery in a controlled manner includes utilizing adoptive cell transfer (ACT) [129]. CAR T cells can be modified to possess the ability to secrete proteins (i.e., antibodies, cytokines, chemokines, or enzymes) and act as living “micro-pharmacies” [130]. A key benefit of this strategy is exploiting the T cell’s ability to successfully traverse the BBB, traffic to tumor sites, and deliver a payload. Moreover, the localized delivery of antibodies in the TME has been shown to lower the likelihood of toxicities associated with systemic infusion [131]. For example, localized delivery of a bispecific T cell engager (BiTE) specific for wild-type EGFR by an EGFRvIII-directed CAR T cell in GBM prevented on-target toxicity in normal tissues [132]. Engineering T cells to secrete other immunomodulating antibody-based molecules locally in the glioma TME is an attractive approach that offers the potential for versatility along with safety.
6. Conclusions
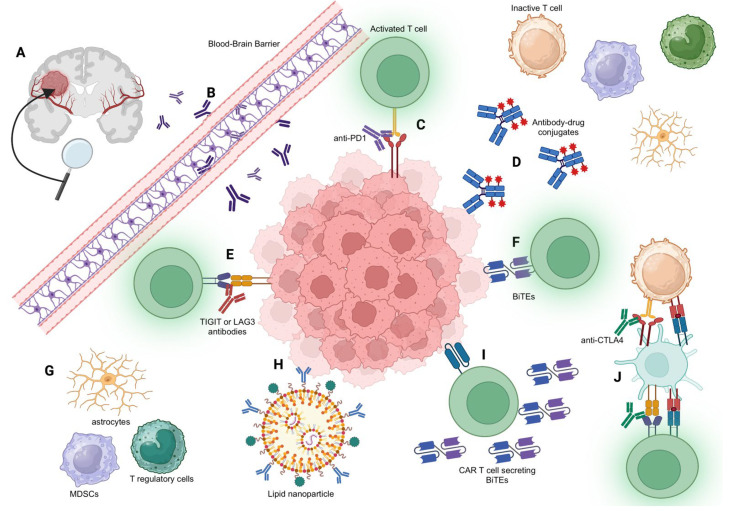
In conclusion, antibody-based therapies represent a promising frontier in neuro-oncology (Figure 1). ICIs, despite their current limitations, show potential in modulating immune microenvironments in the CNS. Ultimately, the success of antibody-based therapies will depend on overcoming both the challenges posed by the BBB and the immunosuppressive TME. Various strategies to enhance delivery into the CNS, such as receptor-mediated transcytosis, focused ultrasound, and nanoparticles, are under active investigation and should be explored in combination with antibody-based therapy for brain tumors. There also remain fundamental questions regarding the immune dynamics of the brain, which may influence antibody-delivery strategies. For example, it is unclear whether ICIs need to penetrate the BBB to have efficacy or if they can peripherally activate T cells that then traffic to the brain. The location of T cell priming and activation with relation to brain tumors, which has not been fully elucidated, may also influence the choice between delivering immunomodulatory antibodies locally (e.g., intratumoral or intrathecal injection) versus peripherally. Combination therapy of immune checkpoint inhibitors with radiotherapy and virotherapy, as well as combinatorial antibody strategies to target both T cells and the TME in tandem, are promising. Questions related to the optimal sequencing and specific combinations of such therapies merit further study. Continued research and innovation are essential to unlock the full potential of these promising therapeutic avenues in neuro-oncology.
Figure 1.
Illustration of Antibody Mechanisms in Neuro-oncology. (A) Overview of a centrally located brain tumor, with its vascular supply highlighted. (B) Detailed view of antibodies attempting to cross the blood-brain barrier, emphasizing the inherent challenges of this process. (C) PD-1 antibodies in action, illustrating their role in enhancing T cell functionality against tumor cells. (D) Depiction of antibody–drug conjugates (ADCs) that are attached to a cytotoxic drug, targeting and destroying tumor cells. (E) TIGIT or LAG-3 antibodies activating T cells, emphasizing their function in boosting immune response against tumor cells. (F) BiTE (Bispecific T cell Engager) mechanism, showcasing how it links T cells to tumor cells, facilitating the destruction of the tumor cells by T cells. (G) Illustration of other cells within the tumor microenvironment (TME) such as astrocytes, monocytes, and myeloid-derived suppressor cells (MDSCs). These cells can exert immunosuppressive effects, hindering effective anti-tumor immune responses. (H) Representation of lipid nanoparticles carrying antibody receptors, illustrating the potential for targeted drug delivery. (I) CAR T cell mechanism depicted as it releases BiTEs upon activation, further enhancing T cell engagement with tumor cells. (J) Detailed depiction of the anti-CTLA4 antibody mechanism: The anti-CTLA4 antibody blocks the interaction between CTLA-4 on both T regulatory and T effector cells and B7 on dendritic cells. In effector cells, CTLA-4 acts as an inhibitory receptor, reducing T cell activation. By blocking this interaction, the anti-CTLA4 antibody promotes prolonged T cell activation and increased T effector cell activity against tumor cells.
Author Contributions
Conceptualization, R.R. and B.D.C.; methodology, R.R.; investigation, R.R., J.S., A.C., L.G.R., T.R. and M.A.G.; data curation, R.R., J.S. and A.C.; writing—original draft preparation, R.R., J.S., A.C., L.G.R., T.R. and M.A.G.; writing—review and editing, P.C.G., E.R.G., W.T.C. and B.D.C.; visualization, R.R. and A.C.; supervision, W.T.C. and B.D.C.; All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Cancer Facts & Figures 2023. [(accessed on 24 July 2023)]. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2023-cancer-facts-figures.html.
- 2.Smith K., Garman L., Wrammert J., Zheng N.-Y., Capra N.D., Ahmed R., Wilson P.C. Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nat. Protoc. 2009;4:372–384. doi: 10.1038/nprot.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin S.U., Morrison S.L. Production and properties of chimeric antibody molecules. Methods Enzymol. 1989;178:459–476. doi: 10.1016/0076-6879(89)78034-4. [DOI] [PubMed] [Google Scholar]
- 4.Riechmann L., Clark M., Waldmann H., Winter G. Reshaping human antibodies for therapy. Nature. 1988;332:323–327. doi: 10.1038/332323a0. [DOI] [PubMed] [Google Scholar]
- 5.Himes B.T., Geiger P.A., Ayasoufi K., Bhargav A.G., Brown D.A., Parney I.F. Immunosuppression in Glioblastoma: Current Understanding and Therapeutic Implications. Front. Oncol. 2021;11:770561. doi: 10.3389/fonc.2021.770561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu D., Chen Q., Chen X., Han F., Chen Z., Wang Y. The blood–brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023;8:217. doi: 10.1038/s41392-023-01481-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arvanitis C.D., Ferraro G.B., Jain R.K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer. 2020;20:26–41. doi: 10.1038/s41568-019-0205-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pardridge W.M. Blood-Brain Barrier and Delivery of Protein and Gene Therapeutics to Brain. Front. Aging Neurosci. 2019;11:373. doi: 10.3389/fnagi.2019.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruiz-López E., Schuhmacher A.J. Transportation of Single-Domain Antibodies through the Blood–Brain Barrier. Biomolecules. 2021;11:1131. doi: 10.3390/biom11081131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roopenian D.C., Akilesh S. FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 11.Tien J., Leonoudakis D., Petrova R., Trinh V., Taura T., Sengupta D., Jo L., Sho A., Yun Y., Doan E., et al. Modifying antibody-FcRn interactions to increase the transport of antibodies through the blood-brain barrier. mAbs. 2023;15:2229098. doi: 10.1080/19420862.2023.2229098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarkaria J.N., Hu L.S., Parney I.F., Pafundi D.H., Brinkmann D.H., Laack N.N., Giannini C., Burns T.C., Kizilbash S.H., Laramy J.K., et al. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro-Oncology. 2018;20:184–191. doi: 10.1093/neuonc/nox175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solar P., Hendrych M., Barak M., Valekova H., Hermanova M., Jancalek R. Blood-Brain Barrier Alterations and Edema Formation in Different Brain Mass Lesions. Front. Cell Neurosci. 2022;16:922181. doi: 10.3389/fncel.2022.922181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolburg H., Noell S., Fallier-Becker P., Mack A.F., Wolburg-Buchholz K. The disturbed blood-brain barrier in human glioblastoma. Mol. Asp. Med. 2012;33:579–589. doi: 10.1016/j.mam.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Bard F., Cannon C., Barbour R., Burke R.-L., Games D., Grajeda H., Guido T., Hu K., Huang J., Johnson-Wood K., et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 16.Dalmau J., Rosenfeld M.R. Paraneoplastic syndromes of the CNS. Lancet Neurol. 2008;7:327–340. doi: 10.1016/S1474-4422(08)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Day E.D., Lassiter S., Woodhall B., Mahaley J.L., Mahaley M.S. The localization of radioantibodies in human brain tumors: I. Preliminary exploration. Cancer Res. 1965;25:773–778. [PubMed] [Google Scholar]
- 18.Zalutsky M.R., Moseley R.P., Coakham H.B., Coleman R.E., Bigner D.D. Pharmacokinetics and tumor localization of 131I-labeled anti-tenascin monoclonal antibody 81C6 in patients with gliomas and other intracranial malignancies. Cancer Res. 1989;49:2807–2813. [PubMed] [Google Scholar]
- 19.Faresjö R., Bonvicini G., Fang X.T., Aguilar X., Sehlin D., Syvänen S. Brain pharmacokinetics of two BBB penetrating bispecific antibodies of different size. Fluids Barriers CNS. 2021;18:26. doi: 10.1186/s12987-021-00257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun C., Mezzadra R., Schumacher T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity. 2018;48:434–452. doi: 10.1016/j.immuni.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waldman A.D., Fritz J.M., Lenardo M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020;20:651–668. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honda T., Egen J.G., Lämmermann T., Kastenmüller W., Torabi-Parizi P., Germain R.N. Tuning of Antigen Sensitivity by T Cell Receptor-Dependent Negative Feedback Controls T Cell Effector Function in Inflamed Tissues. Immunity. 2014;40:235–247. doi: 10.1016/j.immuni.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma A., Subudhi S.K., Blando J., Scutti J., Vence L., Wargo J., Allison J.P., Ribas A., Sharma P. Anti-CTLA-4 Immunotherapy Does Not Deplete FOXP3+ Regulatory T Cells (Tregs) in Human Cancers. Clin. Cancer Res. 2019;25:1233–1238. doi: 10.1158/1078-0432.CCR-18-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arrieta V.A., Dmello C., McGrail D.J., Brat D.J., Lee-Chang C., Heimberger A.B., Chand D., Stupp R., Sonabend A.M. Immune checkpoint blockade in glioblastoma: From tumor heterogeneity to personalized treatment. J. Clin. Investig. 2023;133 doi: 10.1172/JCI163447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garg A.D., Vandenberk L., Van Woensel M., Belmans J., Schaaf M., Boon L., De Vleeschouwer S., Agostinis P. Preclinical efficacy of immune-checkpoint monotherapy does not recapitulate corresponding biomarkers-based clinical predictions in glioblastoma. OncoImmunology. 2017;6:e1295903. doi: 10.1080/2162402X.2017.1295903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park J., Kwon M., Kim K.H., Kim T.-S., Hong S.-H., Kim C.G., Kang S.-G., Moon J.H., Kim E.H., Park S.-H., et al. Immune Checkpoint Inhibitor-induced Reinvigoration of Tumor-infiltrating CD8+ T Cells is Determined by Their Differentiation Status in Glioblastoma. Clin. Cancer Res. 2019;25:2549–2559. doi: 10.1158/1078-0432.CCR-18-2564. [DOI] [PubMed] [Google Scholar]
- 28.Mauldin I.S., Jo J., Wages N.A., Yogendran L.V., Mahmutovic A., Young S.J., Lopes M.B., Slingluff C.L., Erickson L.D., Fadul C.E. Proliferating CD8+ T Cell Infiltrates Are Associated with Improved Survival in Glioblastoma. Cells. 2021;10:3378. doi: 10.3390/cells10123378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karimi S., Mansouri S., Mamatjan Y., Liu J., Nassiri F., Suppiah S., Singh O., Aldape K., Zadeh G. Programmed death ligand-1 (PD-L1) expression in meningioma; prognostic significance and its association with hypoxia and NFKB2 expression. Sci. Rep. 2020;10:14115. doi: 10.1038/s41598-020-70514-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ijad N., Dahal A., Kim A.E., Wakimoto H., Juratli T.A., Brastianos P.K. Novel Systemic Approaches for the Management of Meningiomas: Immunotherapy and Targeted Therapies. Neurosurg. Clin. 2023;34:447–454. doi: 10.1016/j.nec.2023.02.012. [DOI] [PubMed] [Google Scholar]
- 31.Huang J., Liu F., Liu Z., Tang H., Wu H., Gong Q., Chen J. Immune Checkpoint in Glioblastoma: Promising and Challenging. Front. Pharmacol. 2017;8:242. doi: 10.3389/fphar.2017.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saha D., Martuza R.L., Rabkin S.D. Oncolytic herpes simplex virus immunovirotherapy in combination with immune checkpoint blockade to treat glioblastoma. Immunotherapy. 2018;10:779–786. doi: 10.2217/imt-2018-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menon H., Ramapriyan R., Cushman T.R., Verma V., Kim H.H., Schoenhals J.E., Atalar C., Selek U., Chun S.G., Chang J.Y., et al. Role of Radiation Therapy in Modulation of the Tumor Stroma and Microenvironment. Front. Immunol. 2019;10:193. doi: 10.3389/fimmu.2019.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akkari L., Bowman R.L., Tessier J., Klemm F., Handgraaf S.M., de Groot M., Quail D.F., Tillard L., Gadiot J., Huse J.T., et al. Dynamic changes in glioma macrophage populations after radiotherapy reveal CSF-1R inhibition as a strategy to overcome resistance. Sci. Transl. Med. 2020;12:eaaw7843. doi: 10.1126/scitranslmed.aaw7843. [DOI] [PubMed] [Google Scholar]
- 35.Zeng J., See A.P., Phallen J., Jackson C.M., Belcaid Z., Ruzevick J., Durham N., Meyer C., Harris T.J., Albesiano E., et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2013;86:343–349. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reardon D.A., Kim T.M., Frenel J., Simonelli M., Lopez J., Subramaniam D.S., Siu L.L., Wang H., Krishnan S., Stein K., et al. Treatment with pembrolizumab in programmed death ligand 1–positive recurrent glioblastoma: Results from the multicohort phase 1 KEYNOTE-028 trial. Cancer. 2021;127:1620–1629. doi: 10.1002/cncr.33378. [DOI] [PubMed] [Google Scholar]
- 37.Reardon D.A., Brandes A.A., Omuro A., Mulholland P., Lim M., Wick A., Baehring J., Ahluwalia M.S., Roth P., Bähr O., et al. Effect of Nivolumab vs. Bevacizumab in Patients with Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6:1003. doi: 10.1001/jamaoncol.2020.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duerinck J., Schwarze J.K., Awada G., Tijtgat J., Vaeyens F., Bertels C., Geens W., Klein S., Seynaeve L., Cras L., et al. Intracerebral administration of CTLA-4 and PD-1 immune checkpoint blocking monoclonal antibodies in patients with recurrent glioblastoma: A phase I clinical trial. J. Immunother. Cancer. 2021;9:e002296. doi: 10.1136/jitc-2020-002296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cloughesy T.F., Mochizuki A.Y., Orpilla J.R., Hugo W., Lee A.H., Davidson T.B., Wang A.C., Ellingson B.M., Rytlewski J.A., Sanders C.M., et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat. Med. 2019;25:477–486. doi: 10.1038/s41591-018-0337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nayak L., Molinaro A.M., Peters K., Clarke J.L., Jordan J.T., de Groot J., Nghiemphu L., Kaley T., Colman H., McCluskey C., et al. Randomized Phase II and Biomarker Study of Pembrolizumab plus Bevacizumab versus Pembrolizumab Alone for Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2021;27:1048–1057. doi: 10.1158/1078-0432.CCR-20-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sahebjam S., A Forsyth P., Tran N.D., A Arrington J., Macaulay R., Etame A.B., Walko C.M., Boyle T., Peguero E.N., Jaglal M., et al. Hypofractionated stereotactic re-irradiation with pembrolizumab and bevacizumab in patients with recurrent high-grade gliomas: Results from a phase I study. Neuro-Oncology. 2021;23:677–686. doi: 10.1093/neuonc/noaa260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Omuro A., A Brandes A., Carpentier A.F., Idbaih A., A Reardon D., Cloughesy T., Sumrall A., Baehring J., Bent M.v.D., Bähr O., et al. Radiotherapy combined with nivolumab or temozolomide for newly diagnosed glioblastoma with unmethylated MGMT promoter: An international randomized phase III trial. Neuro-Oncology. 2023;25:123–134. doi: 10.1093/neuonc/noac099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim M., Weller M., Idbaih A., Steinbach J., Finocchiaro G., Raval R.R., Ansstas G., Baehring J., Taylor J.W., Honnorat J., et al. Phase III trial of chemoradiotherapy with temozolomide plus nivolumab or placebo for newly diagnosed glioblastoma with methylated MGMT promoter. Neuro-Oncology. 2022;24:1935–1949. doi: 10.1093/neuonc/noac116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Omuro A., Vlahovic G., Lim M., Sahebjam S., Baehring J., Cloughesy T., Voloschin A., Ramkissoon S.H., Ligon K.L., Latek R., et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: Results from exploratory phase I cohorts of CheckMate 143. Neuro-Oncology. 2018;20:674–686. doi: 10.1093/neuonc/nox208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schalper K.A., Rodriguez-Ruiz M.E., Diez-Valle R., López-Janeiro A., Porciuncula A., Idoate M.A., Inogés S., De Andrea C., López-Diaz De Cerio A., Tejada S., et al. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat. Med. 2019;25:470–476. doi: 10.1038/s41591-018-0339-5. [DOI] [PubMed] [Google Scholar]
- 46.Brastianos P.K., Kim A.E., Giobbie-Hurder A., Lee E.Q., Wang N., Eichler A.F., Chukwueke U., Forst D.A., Arrillaga-Romany I.C., Dietrich J., et al. Phase 2 study of pembrolizumab in patients with recurrent and residual high-grade meningiomas. Nat. Commun. 2022;13:1325. doi: 10.1038/s41467-022-29052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bi W.L., Nayak L., Meredith D.M., Driver J., Du Z., Hoffman S., Li Y., Lee E.Q., Beroukim R., Rinne M., et al. Activity of PD-1 blockade with nivolumab among patients with recurrent atypical/anaplastic meningioma: Phase II trial results. Neuro-Oncology. 2022;24:101–113. doi: 10.1093/neuonc/noab118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strickler J.H., Hanks B.A., Khasraw M. Tumor Mutational Burden as a Predictor of Immunotherapy Response: Is More Always Better? Clin. Cancer Res. 2021;27:1236–1241. doi: 10.1158/1078-0432.CCR-20-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nassiri F., Patil V., Yefet L.S., Singh O., Liu J., Dang R.M.A., Yamaguchi T.N., Daras M., Cloughesy T.F., Colman H., et al. Oncolytic DNX-2401 virotherapy plus pembrolizumab in recurrent glioblastoma: A phase 1/2 trial. Nat. Med. 2023;29:1370–1378. doi: 10.1038/s41591-023-02347-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ge Z., Peppelenbosch M.P., Sprengers D., Kwekkeboom J. TIGIT, the Next Step Towards Successful Combination Immune Checkpoint Therapy in Cancer. Front. Immunol. 2021;12:699895. doi: 10.3389/fimmu.2021.699895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andrews L.P., Marciscano A.E., Drake C.G., Vignali D.A.A. LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev. 2017;276:80–96. doi: 10.1111/imr.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hung A.L., Maxwell R., Theodros D., Belcaid Z., Mathios D., Luksik A.S., Kim E., Wu A., Xia Y., Garzon-Muvdi T., et al. TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity and survival in GBM. OncoImmunology. 2018;7:e1466769. doi: 10.1080/2162402X.2018.1466769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harris-Bookman S., Mathios D., Martin A.M., Xia Y., Kim E., Xu H., Belcaid Z., Polanczyk M., Barberi T., Theodros D., et al. Expression of LAG-3 and efficacy of combination treatment with anti-LAG-3 and anti-PD-1 monoclonal antibodies in glioblastoma. Int. J. Cancer. 2018;143:3201–3208. doi: 10.1002/ijc.31661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ott M., Prins R.M., Heimberger A.B. The immune landscape of common CNS malignancies: Implications for immunotherapy. Nat. Rev. Clin. Oncol. 2021;18:729–744. doi: 10.1038/s41571-021-00518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Birge R.B., Boeltz S., Kumar S., Carlson J., Wanderley J., Calianese D., Barcinski M., A Brekken R., Huang X., Hutchins J.T., et al. Phosphatidylserine is a global immunosuppressive signal in efferocytosis, infectious disease, and cancer. Cell Death Differ. 2016;23:962–978. doi: 10.1038/cdd.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Budhu S., Giese R., Gupta A., Fitzgerald K., Zappasodi R., Schad S., Hirschhorn D., Campesato L.F., De Henau O., Gigoux M., et al. Targeting Phosphatidylserine Enhances the Anti-tumor Response to Tumor-Directed Radiation Therapy in a Preclinical Model of Melanoma. Cell Rep. 2021;34:108620. doi: 10.1016/j.celrep.2020.108620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He J., Yin Y., Luster T.A., Watkins L., Thorpe P.E. Antiphosphatidylserine antibody combined with irradiation damages tumor blood vessels and induces tumor immunity in a rat model of glioblastoma. Clin. Cancer Res. 2009;15:6871–6880. doi: 10.1158/1078-0432.CCR-09-1499. [DOI] [PubMed] [Google Scholar]
- 58.Yin Y., Huang X., Lynn K.D., Thorpe P.E. Phosphatidylserine-targeting antibody induces M1 macrophage polarization and promotes myeloid-derived suppressor cell differentiation. Cancer Immunol. Res. 2013;1:256–268. doi: 10.1158/2326-6066.CIR-13-0073. [DOI] [PubMed] [Google Scholar]
- 59.Ly K.I., Richardson L.G., Liu M., Muzikansky A., Cardona J., Lou K., Beers A.L., Chang K., Brown J.M., Ma X., et al. Bavituximab decreases immunosuppressive myeloid-derived suppressor cells in newly diagnosed glioblastoma patients. Clin Cancer Res. 2023;29:3017–3025. doi: 10.1158/1078-0432.CCR-23-0203. [DOI] [PubMed] [Google Scholar]
- 60.Zhang M., Hutter G., Kahn S.A., Azad T.D., Gholamin S., Xu C.Y., Liu J., Achrol A.S., Richard C., Sommerkamp P., et al. Anti-CD47 Treatment Stimulates Phagocytosis of Glioblastoma by M1 and M2 Polarized Macrophages and Promotes M1 Polarized Macrophages In Vivo. PLoS ONE. 2016;11:e0153550. doi: 10.1371/journal.pone.0153550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gholamin S., Mitra S.S., Feroze A.H., Liu J., Kahn S.A., Zhang M., Esparza R., Richard C., Ramaswamy V., Remke M., et al. Disrupting the CD47-SIRPα anti-phagocytic axis by a humanized anti-CD47 antibody is an efficacious treatment for malignant pediatric brain tumors. Sci. Transl. Med. 2017;9:eaaf2968. doi: 10.1126/scitranslmed.aaf2968. [DOI] [PubMed] [Google Scholar]
- 62.Wang X., Guo G., Guan H., Yu Y., Lu J., Yu J. Challenges and potential of PD-1/PD-L1 checkpoint blockade immunotherapy for glioblastoma. J. Exp. Clin. Cancer Res. 2019;38:87. doi: 10.1186/s13046-019-1085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang W., Huang Q., Xiao W., Zhao Y., Pi J., Xu H., Zhao H., Xu J., Evans C.E., Jin H. Advances in Anti-Tumor Treatments Targeting the CD47/SIRPα Axis. Front. Immunol. 2020;11:18. doi: 10.3389/fimmu.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oldenborg P.A. Role of CD47 in erythroid cells and in autoimmunity. Leuk. Lymphoma. 2004;45:1319–1327. doi: 10.1080/1042819042000201989. [DOI] [PubMed] [Google Scholar]
- 65.Mirabile A., Brioschi E., Ducceschi M., Piva S., Lazzari C., Bulotta A., Viganò M.G., Petrella G., Gianni L., Gregorc V. PD-1 Inhibitors-Related Neurological Toxicities in Patients with Non-Small-Cell Lung Cancer: A Literature Review. Cancers. 2019;11:296. doi: 10.3390/cancers11030296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mangan B.L., McAlister R.K., Balko J.M., Johnson D.B., Moslehi J.J., Gibson A., Phillips E.J. Evolving insights into the mechanisms of toxicity associated with immune checkpoint inhibitor therapy. Br. J. Clin. Pharmacol. 2020;86:1778–1789. doi: 10.1111/bcp.14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnson D.B., Manouchehri A., Haugh A.M., Quach H.T., Balko J.M., Lebrun-Vignes B., Mammen A., Moslehi J.J., Salem J.-E. Neurologic toxicity associated with immune checkpoint inhibitors: A pharmacovigilance study. J. Immunother. Cancer. 2019;7:134. doi: 10.1186/s40425-019-0617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang D.Y., Johnson D.B., Davis E.J. Toxicities associated with PD-1/PD-L1 blockade. Cancer J. 2018;24:36–40. doi: 10.1097/PPO.0000000000000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cuzzubbo S., Javeri F., Tissier M., Roumi A., Barlog C., Doridam J., Lebbe C., Belin C., Ursu R., Carpentier A. Neurological adverse events associated with immune checkpoint inhibitors: Review of the literature. Eur. J. Cancer. 2017;73:1–8. doi: 10.1016/j.ejca.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 70.Kolb N.A., Trevino C.R., Waheed W., Sobhani F., Landry K.K., Thomas A.A., Hehir M. Neuromuscular complications of immune checkpoint inhibitor therapy. Muscle Nerve. 2018;58:10–22. doi: 10.1002/mus.26070. [DOI] [PubMed] [Google Scholar]
- 71.Touat M., Talmasov D., Ricard D., Psimaras D. Neurological toxicities associated with immune-checkpoint inhibitors. Curr. Opin. Neurol. 2017;30:659–668. doi: 10.1097/WCO.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 72.Hottinger A.F. Neurologic complications of immune checkpoint inhibitors. Curr. Opin. Neurol. 2016;29:806–812. doi: 10.1097/WCO.0000000000000391. [DOI] [PubMed] [Google Scholar]
- 73.U.S. Department of Health and Human Services Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0. Published Online November 27, 2017. [(accessed on 6 November 2023)]; Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5×7.pdf.
- 74.Brahmer J.R., Lacchetti C., Schneider B.J., Atkins M.B., Brassil K.J., Caterino J.M., Chau I., Ernstoff M.S., Gardner J.M., Ginex P., et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schneider B.J., Naidoo J., Santomasso B.D., Lacchetti C., Adkins S., Anadkat M., Atkins M.B., Brassil K.J., Caterino J.M., Chau I., et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021;39:4073–4126. doi: 10.1200/JCO.21.01440. [DOI] [PubMed] [Google Scholar]
- 76.Thompson J.A., Schneider B.J., Brahmer J., Achufusi A., Armand P., Berkenstock M.K., Bhatia S., Budde L.E., Chokshi S., Davies M., et al. Management of Immunotherapy-Related Toxicities, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022;20:387–405. doi: 10.6004/jnccn.2022.0020. [DOI] [PubMed] [Google Scholar]
- 77.Feng S., Coward J., McCaffrey E., Coucher J., Kalokerinos P., O’Byrne K. Pembrolizumab-Induced Encephalopathy: A Review of Neurological Toxicities with Immune Checkpoint Inhibitors. J. Thorac. Oncol. 2017;12:1626–1635. doi: 10.1016/j.jtho.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 78.Mahdi J., Dietrich J., Straathof K., Roddie C., Scott B.J., Davidson T.B., Prolo L.M., Batchelor T.T., Campen C.J., Davis K.L., et al. Tumor inflammation-associated neurotoxicity. Nat. Med. 2023;29:803–810. doi: 10.1038/s41591-023-02276-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sheth V., Gauthier J. Taming the Beast: CRS and ICANS after CAR T-cell therapy for ALL. Bone Marrow Transplant. 2021;56:552–566. doi: 10.1038/s41409-020-01134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Santomasso B., Bachier C., Westin J., Rezvani K., Shpall E.J. The Other Side of CAR T-Cell Therapy: Cytokine Release Syndrome, Neurologic Toxicity, and Financial Burden. Am. Soc. Clin. Oncol. Educ. Book. 2019;39:433–444. doi: 10.1200/EDBK_238691. [DOI] [PubMed] [Google Scholar]
- 81.Orentas R.J., Lee D.W., Mackall C. Immunotherapy targets in pediatric cancer. Front. Oncol. 2012;2:3. doi: 10.3389/fonc.2012.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Locke F.L., Ghobadi A., Jacobson C.A., Miklos D.B., Lekakis L.J., Oluwole O.O., Lin Y., Braunschweig I., Hill B.T., Timmerman J.M., et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20:31–42. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee D.W., Santomasso B.D., Locke F.L., Ghobadi A., Turtle C.J., Brudno J.N., Maus M.V., Park J.H., Mead E., Pavletic S., et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019;25:625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PubMed] [Google Scholar]
- 84.Brown B.D., Tambaro F.P., Kohorst M., Chi L., Mahadeo K.M., Tewari P., Petropoulos D., Slopis J.M., Sadighi Z., Khazal S. Immune Effector Cell Associated Neurotoxicity (ICANS) in Pediatric and Young Adult Patients Following Chimeric Antigen Receptor (CAR) T-Cell Therapy: Can We Optimize Early Diagnosis? Front. Oncol. 2021;11:634445. doi: 10.3389/fonc.2021.634445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rees J.H. Table 27.1, [Immune Effector Cell Encephalopathy (ICE) Score] [(accessed on 26 July 2023)];2022 Available online: https://www.ncbi.nlm.nih.gov/books/NBK584157/table/ch27.Tab1/
- 86.Sugawa N., Ekstrand A.J., James C.D., Collins V.P. Identical splicing of aberrant epidermal growth factor receptor transcripts from amplified rearranged genes in human glioblastomas. Proc. Natl. Acad. Sci. USA. 1990;87:8602–8606. doi: 10.1073/pnas.87.21.8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ekstrand A.J., Sugawa N., James C.D., Collins V.P. Amplified and rearranged epidermal growth factor receptor genes in human glioblastomas reveal deletions of sequences encoding portions of the N- and/or C-terminal tails. Proc. Natl. Acad. Sci. USA. 1992;89:4309–4313. doi: 10.1073/pnas.89.10.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hatanpaa K.J., Burma S., Zhao D., Habib A.A. Epidermal Growth Factor Receptor in Glioma: Signal Transduction, Neuropathology, Imaging, and Radioresistance. Neoplasia. 2010;12:675–684. doi: 10.1593/neo.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rutkowska A., Stoczyńska-Fidelus E., Janik K., Włodarczyk A., Rieske P. EGFRvIII: An Oncogene with Ambiguous Role. J. Oncol. 2019;2019:1092587. doi: 10.1155/2019/1092587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Choi B.D., Archer G.E., Mitchell D.A., Heimberger A.B., McLendon R.E., Bigner D.D., Sampson J.H. EGFRvIII-Targeted Vaccination Therapy of Malignant Glioma. Brain Pathol. 2009;19:713–723. doi: 10.1111/j.1750-3639.2009.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brown C.E., Alizadeh D., Starr R., Weng L., Wagner J.R., Naranjo A., Ostberg J.R., Blanchard M.S., Kilpatrick J., Simpson J., et al. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N. Engl. J. Med. 2016;375:2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li G., Wong A.J. EGF receptor variant III as a target antigen for tumor immunotherapy. Expert. Rev. Vaccines. 2008;7:977–985. doi: 10.1586/14760584.7.7.977. [DOI] [PubMed] [Google Scholar]
- 93.Kim G.B., Aragon-Sanabria V., Randolph L., Jiang H., Reynolds J.A., Webb B.S., Madhankumar A., Lian X., Connor J.R., Yang J., et al. High-affinity mutant Interleukin-13 targeted CAR T cells enhance delivery of clickable biodegradable fluorescent nanoparticles to glioblastoma. Bioact. Mater. 2020;5:624–635. doi: 10.1016/j.bioactmat.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zeng J., Zhang J., Yang Y.-Z., Wang F., Jiang H., Chen H.-D., Wu H.-Y., Sai K., Hu W.-M. IL13RA2 is overexpressed in malignant gliomas and related to clinical outcome of patients. Am. J. Transl. Res. 2020;12:4702–4714. [PMC free article] [PubMed] [Google Scholar]
- 95.Brown C.E., Aguilar B., Starr R., Yang X., Chang W.-C., Weng L., Chang B., Sarkissian A., Brito A., Sanchez J.F., et al. Optimization of IL13Rα2-Targeted Chimeric Antigen Receptor T Cells for Improved Anti-tumor Efficacy against Glioblastoma. Mol. Ther. 2018;26:31–44. doi: 10.1016/j.ymthe.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.IL13RA2 Protein Expression Summary—The Human Protein Atlas. [(accessed on 26 July 2023)]. Available online: https://www.proteinatlas.org/ENSG00000123496-IL13RA2.
- 97.Mineo J.-F., Bordron A., Baroncini M., Maurage C.-A., Ramirez C., Siminski R.-M., Berthou C., Hieu P.D. Low HER2-expressing glioblastomas are more often secondary to anaplastic transformation of low-grade glioma. J. Neurooncol. 2007;85:281–287. doi: 10.1007/s11060-007-9424-1. [DOI] [PubMed] [Google Scholar]
- 98.Gutierrez C., Schiff R. HER 2: Biology, Detection, and Clinical Implications. Arch. Pathol. Lab. Med. 2011;135:55–62. doi: 10.5858/2010-0454-RAR.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Morgan R.A., Yang J.C., Kitano M., Dudley M.E., Laurencot C.M., Rosenberg S.A. Case Report of a Serious Adverse Event Following the Administration of T Cells Transduced with a Chimeric Antigen Receptor Recognizing ERBB2. Mol. Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nazha B., Inal C., Owonikoko T.K. Disialoganglioside GD2 Expression in Solid Tumors and Role as a Target for Cancer Therapy. Front. Oncol. 2020;10:1000. doi: 10.3389/fonc.2020.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Del Bufalo F., De Angelis B., Caruana I., Del Baldo G., De Ioris M.A., Serra A., Mastronuzzi A., Cefalo M.G., Pagliara D., Amicucci M., et al. GD2-CART01 for Relapsed or Refractory High-Risk Neuroblastoma. N. Engl. J. Med. 2023;388:1284–1295. doi: 10.1056/NEJMoa2210859. [DOI] [PubMed] [Google Scholar]
- 102.Majzner R.G., Ramakrishna S., Yeom K.W., Patel S., Chinnasamy H., Schultz L.M., Richards R.M., Jiang L., Barsan V., Mancusi R., et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature. 2022;603:934–941. doi: 10.1038/s41586-022-04489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang M., Yuan Y., Zhang H., Yan M., Wang S., Feng F., Ji P., Li Y., Li B., Gao G., et al. Prognostic significance of CD147 in patients with glioblastoma. J. Neurooncol. 2013;115:19–26. doi: 10.1007/s11060-013-1207-2. [DOI] [PubMed] [Google Scholar]
- 104.Brosnan-Cashman J.A., Davis C.M., Diplas B.H., Meeker A.K., Rodriguez F.J., Heaphy C.M. SMARCAL1 loss and alternative lengthening of telomeres (ALT) are enriched in giant cell glioblastoma. Mod. Pathol. 2021;34:1810–1819. doi: 10.1038/s41379-021-00841-7. [DOI] [PubMed] [Google Scholar]
- 105.Spino M., Kurz S.C., Chiriboga L., Serrano J., Zeck B., Sen N., Patel S., Shen G., Vasudevaraja V., Tsirigos A., et al. Cell Surface Notch Ligand DLL3 is a Therapeutic Target in Isocitrate Dehydrogenase-mutant Glioma. Clin. Cancer Res. 2019;25:1261–1271. doi: 10.1158/1078-0432.CCR-18-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Noor H., Whittaker S., McDonald K.L. DLL3 expression and methylation are associated with lower-grade glioma immune microenvironment and prognosis. Genomics. 2022;114:110289. doi: 10.1016/j.ygeno.2022.110289. [DOI] [PubMed] [Google Scholar]
- 107.Mansfield A.S., Hong D.S., Hann C.L., Farago A.F., Beltran H., Waqar S.N., Hendifar A.E., Anthony L.B., Taylor M.H., Bryce A.H., et al. A phase I/II study of rovalpituzumab tesirine in delta-like 3—Expressing advanced solid tumors. NPJ Precis. Oncol. 2021;5:74. doi: 10.1038/s41698-021-00214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Paz-Ares L., Champiat S., Lai W.V., Izumi H., Govindan R., Boyer M., Hummel H.-D., Borghaei H., Johnson M.L., Steeghs N., et al. Tarlatamab, a First-in-Class DLL3-Targeted Bispecific T-Cell Engager, in Recurrent Small-Cell Lung Cancer: An Open-Label, Phase I Study. J. Clin. Oncol. 2023;41:2893–2903. doi: 10.1200/JCO.22.02823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Von Achenbach C., Silginer M., Blot V., Weiss W.A., Weller M. Depatuxizumab Mafodotin (ABT-414)-induced Glioblastoma Cell Death Requires EGFR Overexpression, but not EGFRY1068 Phosphorylation. Mol. Cancer Ther. 2020;19:1328–1339. doi: 10.1158/1535-7163.MCT-19-0609. [DOI] [PubMed] [Google Scholar]
- 110.Rosenthal M., Curry R., Reardon D.A., Rasmussen E., Upreti V.V., Damore M.A., Henary H.A., Hill J.S., Cloughesy T. Safety, tolerability, and pharmacokinetics of anti-EGFRvIII antibody-drug conjugate AMG 595 in patients with recurrent malignant glioma expressing EGFRvIII. Cancer Chemother. Pharmacol. 2019;84:327–336. doi: 10.1007/s00280-019-03879-2. [DOI] [PubMed] [Google Scholar]
- 111.Mut M., Sherman J.H., Shaffrey M.E., Schiff D. Cintredekin besudotox in treatment of malignant glioma. Expert. Opin. Biol. Ther. 2008;8:805–812. doi: 10.1517/14712598.8.6.805. [DOI] [PubMed] [Google Scholar]
- 112.Weber F.W., Floeth F., Asher A., Bucholz R., Berge M., Pradoss M., Chang S., Bruces J., Hall W., Raino N.G., et al. Therapies for Glioma Present Status and Future Developments. Volume 88. Springer; Vienna, Austria: 2003. Local convection enhanced delivery of IL4-Pseudomonas exotoxin (NBI-3001) for treatment of patients with recurrent malignant glioma; pp. 93–103. [DOI] [PubMed] [Google Scholar]
- 113.Sampson J.H., Reardon D.A., Friedman A.H., Friedman H.S., Coleman R.E., McLendon R.E., Pastan I., Bigner D.D. Sustained radiographic and clinical response in patient with bifrontal recurrent glioblastoma multiforme with intracerebral infusion of the recombinant targeted toxin TP-38: Case study. Neuro-Oncology. 2005;7:90–96. doi: 10.1215/S1152851703000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Weaver M., Laske D.W. Transferrin receptor ligand-targeted toxin conjugate (Tf-CRM107) for therapy of malignant gliomas. J. Neurooncol. 2003;65:3–13. doi: 10.1023/A:1026246500788. [DOI] [PubMed] [Google Scholar]
- 115.Goldenberg D.M., Juweid M., Dunn R.M., Sharkey R.M. Cancer imaging with radiolabeled antibodies: New advances with technetium-99m-labeled monoclonal antibody Fab’ fragments, especially CEA-Scan and prospects for therapy. J. Nucl. Med. Technol. 1997;25:18–23. [PubMed] [Google Scholar]
- 116.Lee Y., Bullard D.E., Humphrey P.A., Colapinto E.V., Friedman H.S., Zalutsky M.R., Coleman R.E., Bigner D.D. Treatment of Intracranial Human Glioma Xenografts with 131I-labeled Anti-Tenascin Monoclonal Antibody 81C61. Cancer Res. 1988;48:2904–2910. [PubMed] [Google Scholar]
- 117.Leshem Y., Pastan I. Pseudomonas Exotoxin Immunotoxins and Anti-Tumor Immunity: From Observations at the Patient’s Bedside to Evaluation in Preclinical Models. Toxins. 2019;11:20. doi: 10.3390/toxins11010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mair M.J., Bartsch R., Le Rhun E., Berghoff A.S., Brastianos P.K., Cortes J., Gan H.K., Lin N.U., Lassman A.B., Wen P.Y., et al. Understanding the activity of antibody–drug conjugates in primary and secondary brain tumours. Nat. Rev. Clin. Oncol. 2023;20:372–389. doi: 10.1038/s41571-023-00756-z. [DOI] [PubMed] [Google Scholar]
- 119.Parakh S., Nicolazzo J., Scott A.M., Gan H.K. Antibody Drug Conjugates in Glioblastoma—Is There a Future for Them? Front. Oncol. 2021;11:718590. doi: 10.3389/fonc.2021.718590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lassman A.B., Pugh S.L., Wang T.J.C., Aldape K., Gan H.K., Preusser M., A Vogelbaum M., Sulman E.P., Won M., Zhang P., et al. Depatuxizumab mafodotin in EGFR-amplified newly diagnosed glioblastoma: A phase III randomized clinical trial. Neuro-Oncol. 2022;25:339–350. doi: 10.1093/neuonc/noac173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bargou R., Leo E., Zugmaier G., Klinger M., Goebeler M., Knop S., Noppeney R., Viardot A., Hess G., Schuler M., et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 122.Choi B.D., Kuan C.-T., Cai M., Archer G.E., Mitchell D.A., Gedeon P.C., Sanchez-Perez L., Pastan I., Bigner D.D., Sampson J.H. Systemic administration of a bispecific antibody targeting EGFRvIII successfully treats intracerebral glioma. Proc. Natl. Acad. Sci. USA. 2013;110:270–275. doi: 10.1073/pnas.1219817110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Choi B.D., Gedeon P.C., Ii J.E.H., Archer G.E., Reap E.A., Sanchez-Perez L., Mitchell D.A., Bigner D.D., Sampson J.H. Human regulatory T cells kill tumor cells through granzyme-dependent cytotoxicity upon retargeting with a bispecific antibody. Cancer Immunol. Res. 2013;1:163. doi: 10.1158/2326-6066.CIR-13-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Choi B.D., Pastan I., Bigner D.D., Sampson J.H. A novel bispecific antibody recruits T cells to eradicate tumors in the “immunologically privileged” central nervous system. OncoImmunology. 2013;2:e23639. doi: 10.4161/onci.23639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gedeon P.C., Suryadevara C.M., Choi B.D., Sampson J.H. The effect of adoptive transfer of ex vivo activated T cells on the efficacy and tumor penetrance of intravenously-administered CD3-engaging bispecific antibody. J. Clin. Oncol. 2019;37:30. doi: 10.1200/JCO.2019.37.8_suppl.30. [DOI] [Google Scholar]
- 126.Chacko A.M., Li C., Pryma D.A., Brem S., Coukos G., Muzykantov V. Targeted delivery of antibody-based therapeutic and imaging agents to CNS tumors: Crossing the blood-brain barrier divide. Expert. Opin. Drug Deliv. 2013;10:907–926. doi: 10.1517/17425247.2013.808184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhao P., Zhang N., An Z. Engineering antibody and protein therapeutics to cross the blood-brain barrier. Antib. Ther. 2022;5:311–331. doi: 10.1093/abt/tbac028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Karmur B.S., Philteos J., Abbasian A., Zacharia B.E., Lipsman N., Levin V., Grossman S., Mansouri A. Blood-Brain Barrier Disruption in Neuro-Oncology: Strategies, Failures, and Challenges to Overcome. Front. Oncol. 2020;10:563840. doi: 10.3389/fonc.2020.563840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rafiq S., Hackett C.S., Brentjens R.J. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat. Rev. Clin. Oncol. 2020;17:147–167. doi: 10.1038/s41571-019-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gardner T.J., Bourne C.M., Dacek M.M., Kurtz K., Malviya M., Peraro L., Silberman P.C., Vogt K.C., Unti M.J., Brentjens R., et al. Targeted Cellular Micropharmacies: Cells Engineered for Localized Drug Delivery. Cancers. 2020;12:2175. doi: 10.3390/cancers12082175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Evans A.N., Lin H.K., Hossian A.K.M.N., Rafiq S. Using Adoptive Cellular Therapy for Localized Protein Secretion. Cancer J. 2021;27:159–167. doi: 10.1097/PPO.0000000000000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Choi B.D., Yu X., Castano A.P., Bouffard A.A., Schmidts A., Larson R.C., Bailey S.R., Boroughs A.C., Frigault M.J., Leick M.B., et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat. Biotechnol. 2019;37:1049–1058. doi: 10.1038/s41587-019-0192-1. [DOI] [PubMed] [Google Scholar]