Abstract
Objective
To evaluate genetic evaluation practices in newborns with the most common birth defect, congenital heart defects (CHD), we determined the prevalence and the yield of genetic evaluation across time and across patient subtypes, before and after implementation of institutional genetic testing guidelines.
Study design
This was a retrospective, cross-sectional study of 664 hospitalized newborns with CHD using multivariate analyses of genetic evaluation practices across time and patient subtypes.
Results
Genetic testing guidelines for hospitalized newborns with CHD were implemented in 2014, and subsequently genetic testing increased (40% in 2013 and 75% in 2018, OR 5.02, 95% CI 2.84–8.88, P < .001) as did medical geneticists’ involvement (24% in 2013 and 64% in 2018, P < .001). In 2018, there was an increased use of chromosomal microarray (P < .001), gene panels (P = .016), and exome sequencing (P = .001). The testing yield was high (42%) and consistent across years and patient subtypes analyzed. Increased testing prevalence (P < .001) concomitant with consistent testing yield (P = .139) added an estimated 10 additional genetic diagnoses per year, reflecting a 29% increase.
Conclusions
In patients with CHD, yield of genetic testing was high. After implementing guidelines, genetic testing increased significantly and shifted to newer sequence-based methods. Increased use of genetic testing identified more patients with clinically important results with potential to impact patient care.
Congenital heart disease (CHD) remains the most prevalent birth defect and a leading cause of morbidity and mortality in newborns.1–7 CHD often has a genetic etiology, and recent studies indicate that genetic testing is abnormal in 25%–50% of patients with CHD.8–10 In recent years, technologic advancement has led to decreased cost, increased test options, and increased accessibility of genetic testing in clinical settings.11 As a result, the recommendations for genetic testing are evolving. In 2010 the International Standard Cytogenomic Array Consortium (ISCA) released a consensus statement recommending chromosome microarray analysis (CMA) as first-tier cytogenetic testing for patients with developmental delay/intellectual disability, autism, or multiple congenital anomalies.12 In 2021, the American College of Medical Genetics and Genomics released updated guidelines, reinforcing recommendations for genetic testing in pediatric patients with congenital anomalies and incorporating exome sequencing (ES) and genome sequencing (GS).13 For patients with CHD in particular, the American Heart Association (AHA) published a scientific statement in 2008 outlining the value of genetic testing14 and updated these guidelines in 2018 to reflect shifting technologies.15
The AHA guidelines highlight the need for genetic testing in CHD to assess risk for patients and their family members, identify risk for extracardiac pathologies, identify risk for developmental delays that may benefit from early intervention, and inform prognosis and outcomes. Genetic diagnoses in CHD recently have been linked to postoperative respiratory16 and surgical outcomes,17–21 further highlighting the benefit of genetic testing for this population. Although access to genetic testing was previously limited due to cost and other factors, genetic testing protocols have been shown to reduce cost and increase genetic diagnoses in CHD.9 In addition, many protocols also outline recommendations for consultation with medical geneticists, which is critical for optimizing testing strategies and the interpretation of results, especially in the setting of increased use, advancing technologies, and rapidly evolving recommendations.9 Importantly, geneticist involvement and the implementation of testing protocols has been shown in a single-site study to improve care in CHD.9
In 2014, our center implemented genetic testing guidelines for newborns in the intensive care unit with CHD. The guidelines expanded upon the ISCA statement and recommended medical genetics evaluation for all hospitalized infants with CHD, chromosome analysis for select patients with high likelihood of aneuploidy, and CMA for all other hospitalized infants with CHD. This is a single-center analysis of genetic evaluation practices in infants with CHD across multiple patient subtypes, during a period after the ISCA and AHA recommendations and spanning implementation at our center, of genetic evaluation guidelines.
Methods
We performed a retrospective analysis of genetic testing practices and results at Riley Hospital for Children at Indiana University Health. Cohort ascertainment used data submitted from our center to the Society of Thoracic Surgeons (STS) National Database to comprehensively identify patients with critical CHD. We included 664 children who underwent surgical repair for CHD at ≤14 months of age, with 474 between January 1, 2013, and December 31, 2015 (with data collection range November 1, 2011, to December 31, 2016), and 190 between January 1, 2018, and December 31, 2018 (with data-collection range November 1, 2016, to December 31, 2019). We implemented the algorithm for genetic testing in the fourth quarter of 2015. Thus, we chose a time frame before the algorithm (genetic testing in 2013–2015), and 2018, after the algorithm was firmly established. The study was approved by the Indiana University Institutional Review Board, including a waiver of consent for this minimal risk study.
Patient medical records were comprehensively reviewed, and study data were entered into a Research Electronic Data Capture (REDCap) database hosted at Indiana University.22,23 Data also were obtained from STS. All available genetic testing was collected from the medical record, including noninvasive prenatal screening (NIPS), chromosome analysis, CMA, fluorescent in situ hybridization analysis (FISH), single-gene sequencing, gene panel sequencing, ES, and GS. Result interpretation used the clinical testing laboratory report. Testing results from the clinical laboratory report were evaluated as “normal,” “abnormal,” or “not done/unknown.” Data on extracardiac congenital anomalies were extracted from the medical record. Participants with at least one extracardiac congenital anomaly were defined as having multiple congenital anomalies (MCAs) vs those with isolated CHD. The data were entered into the REDCap deidentified, except for date of birth, which was used for data integrity. Only summary results are presented within the research article and all data presented within the article are deidentified.
We identified the fundamental diagnosis listed in the STS database for each patient. Based on this diagnosis, we assigned an overall cardiac diagnosis type in the classification system developed by the National Birth Defects Prevention Study.24 This classification was done in a hierarchical fashion based on the approach used by previous epidemiologic studies.25 Cardiac classifications included anomalous pulmonary venous return, atrioventricular septal defect (AVSD), arteriopathy (included with “other” for estimates of testing yield, given low numbers), conotruncal defect (CTD), heterotaxy, left ventricle outflow tract obstruction, right ventricle outflow tract obstruction, and septal defect. All other lesions were classified as “other.”
The univariate comparisons of patients’ characteristics, testing rate, and testing yield across years were conducted using the Pearson χ2 test (all expected cell counts in a contingency table ≥5) or the Fisher exact test (any expected cell counts <5) for categorical variables and one-way ANOVA for continuous variables. The effect (ie, OR) of covariates on testing rate and testing yield was estimated via multivariate logistic regression models, including year, sex, race, family history of CHD, presence of other major congenital malformation, history of intrauterine growth restriction (IUGR) or born small for gestational age (SGA), CHD group, infant of mother with diabetes, maternal teratogens (alcohol, illegal drugs, tobacco, and prescription opioids), maternal infection, multiple gestation, gestational age, birth weight, number of cardiac surgeries, deceased status, and extracorporeal membrane oxygenation (ECMO). All analyses were conducted in R, version 4.0.2 (The R Foundation for Statistical Computing).
Results
There were 664 patients included in the study, including 534 with apparently isolated CHD and 130 with CHD + MCA (Table I). Within the cohort, 371 (55.9%) were male and 550 (82.2%) were White. In total, 511 of 664 (77.0%) were born full term, and 76 (11.4%) were IUGR or SGA with a mean birth weight of 2.93 kg in the overall cohort. A total of 84 of 664 (12.7%) were infants of a mother with diabetes, 82 (12.3%) were exposed to maternal teratogens (alcohol, tobacco, or illicit drugs), and 72 (10.8%) were exposed to maternal infection. The average number of cardiac surgeries was 1.6, with 90 of 664 (13.6%) requiring ECMO; 69 (10.4%) patients were deceased at the time of data collection (Table I).
Table I.
Patient characteristics
| Characteristics | Total (n = 664) | 2013 (n = 158) | 2014 (n = 170) | 2015 (n = 146) | 2018 (n = 190) | P value |
|---|---|---|---|---|---|---|
| Sex | .131 | |||||
| Male | 371 (55.9%) | 86 (54.4%) | 86 (50.6%) | 93 (63.7%) | 106 (55.8%) | |
| Female | 292 (44.0%) | 71 (44.9%) | 84 (49.4%) | 53 (36.3%) | 84 (44.2%) | |
| Unknown | 1 (0.2%) | 1 (0.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Race | .674 | |||||
| White | 550 (82.8%) | 134 (84.8%) | 139 (81.8%) | 123 (84.2%) | 154 (81.1%) | |
| Black/African American | 82 (12.3%) | 19 (12.0%) | 19 (11.2%) | 18 (12.3%) | 26 (13.7%) | |
| Other | 32 (4.8%) | 5 (3.2%) | 12 (7.1%) | 5 (3.5%) | 10 (5.3%) | |
| Family history of CHD: genetics provider only | .238 | |||||
| Yes | 38 (5.7%) | 1 (0.6%) | 5 (2.9%) | 8 (5.5%) | 24 (12.6%) | |
| No | 170 (25.6%) | 24 (15.2%) | 21 (12.4%) | 31 (21.2%) | 94 (49.5%) | |
| Unknown | 456 (68.7%) | 133 (84.2%) | 144 (84.7%) | 107 (73.3%) | 72 (37.9%) | |
| Family history of CHD: other specialty (nongenetics provider(s)) | .007 | |||||
| Yes | 8 (1.2%) | 0 (0.0%) | 1 (0.6%) | 0 (0.0%) | 7 (3.7%) | |
| No | 325 (48.9%) | 84 (53.2%) | 73 (42.9%) | 70 (47.9%) | 98 (51.6%) | |
| Unknown | 331 (49.8%) | 74 (46.8%) | 96 (56.5%) | 76 (52.1%) | 85 (44.7%) | |
| Family history of CHD | .004 | |||||
| Yes | 44 (6.6%) | 1 (0.6%) | 6 (3.5%) | 8 (5.5%) | 29 (15.3%) | |
| No | 374 (56.3%) | 83 (52.5%) | 69 (40.6%) | 65 (44.5%) | 157 (82.6%) | |
| Unknown | 246 (37.0%) | 74 (46.8%) | 95 (55.9%) | 73 (50.0%) | 4 (2.1%) | |
| Congenital malformations | ||||||
| Brain | .026 | |||||
| Yes | 28 (4.2%) | 7 (4.4%) | 6 (3.5%) | 12 (8.2%) | 3 (1.6%) | |
| No | 636 (95.8%) | 151 (95.6%) | 164 (96.5%) | 134 (91.8%) | 187 (98.4%) | |
| ENT | .305 | |||||
| Yes | 22 (3.3%) | 4 (2.5%) | 3 (1.8%) | 5 (3.4%) | 10 (5.3%) | |
| No | 642 (96.7%) | 154 (97.5%) | 167 (98.2%) | 141 (96.6%) | 180 (94.7%) | |
| Eye | >.999 | |||||
| Yes | 7 (1.1%) | 2 (1.3%) | 2 (1.2%) | 1 (0.7%) | 2 (1.1%) | |
| No | 657 (98.9%) | 156 (98.7%) | 168 (98.8%) | 145 (99.3%) | 188 (98.9%) | |
| Chest | .067 | |||||
| Yes | 5 (0.8%) | 0 (0.0%) | 0 (0.0%) | 1 (0.7%) | 4 (2.1%) | |
| No | 659 (99.2%) | 158 (100.0%) | 170 (100.0%) | 145 (99.3%) | 186 (97.9%) | |
| Lung | >.999 | |||||
| Yes | 3 (0.5%) | 1 (0.6%) | 1 (0.6%) | 0 (0.0%) | 1 (0.5%) | |
| No | 661 (99.5%) | 157 (99.4%) | 169 (99.4%) | 146 (100.0%) | 189 (99.5%) | |
| Diaphragm | >.999 | |||||
| Yes | 1 (0.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.5%) | |
| No | 663 (99.8%) | 158 (100.0%) | 170 (100.0%) | 146 (100.0%) | 189 (99.5%) | |
| Ribs/vertebrae | .285 | |||||
| Yes | 20 (3.0%) | 5 (3.2%) | 2 (1.2%) | 7 (4.8%) | 6 (3.2%) | |
| No | 644 (97.0%) | 153 (96.8%) | 168 (98.8%) | 139 (95.2%) | 184 (96.8%) | |
| Limbs | .414 | |||||
| Yes | 12 (1.8%) | 4 (2.5%) | 1 (0.6%) | 4 (2.7%) | 3 (1.6%) | |
| No | 652 (98.2%) | 154 (97.5%) | 169 (99.4%) | 142 (97.3%) | 187 (98.4%) | |
| Lymphatic: lymphatic dysplasia | .356 | |||||
| Yes | 2 (0.3%) | 1 (0.6%) | 0 (0.0%) | 1 (0.7%) | 0 (0.0%) | |
| No | 662 (99.7%) | 157 (99.4%) | 170 (100.0%) | 145 (99.3%) | 190 (100.0%) | |
| Skin | .356 | |||||
| Yes | 2 (0.3%) | 1 (0.6%) | 0 (0.0%) | 1 (0.7%) | 0 (0.0%) | |
| No | 662 (99.7%) | 157 (99.4%) | 170 (100.0%) | 145 (99.3%) | 190 (100.0%) | |
| AVM | .458 | |||||
| Yes | 1 (0.2%) | 1 (0.6%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| No | 663 (99.8%) | 157 (99.4%) | 170 (100.0%) | 146 (100.0%) | 190 (100.0%) | |
| Umbilical | .755 | |||||
| Yes | 11 (1.7%) | 2 (1.3%) | 2 (1.2%) | 2 (1.4%) | 5 (2.6%) | |
| No | 653 (98.3%) | 156 (98.7%) | 168 (98.8%) | 144 (98.6%) | 185 (97.4%) | |
| Others | .346 | |||||
| Yes | 74 (11.1%) | 16 (10.1%) | 24 (14.1%) | 18 (12.3%) | 16 (8.4%) | |
| No | 590 (88.9%) | 142 (89.9%) | 146 (85.9%) | 128 (87.7%) | 174 (91.6%) | |
| Any congenital malformation | .771 | |||||
| Yes | 130 (19.6%) | 28 (17.7%) | 35 (20.6%) | 32 (21.9%) | 35 (18.4%) | |
| No | 534 (80.4%) | 130 (82.3%) | 135 (79.4%) | 114 (78.1%) | 155 (81.6%) | |
| Infant of a mother with diabetes | .278 | |||||
| Yes | 84 (12.7%) | 15 (9.5%) | 20 (11.8%) | 24 (16.4%) | 25 (13.2%) | |
| No | 395 (59.5%) | 89 (56.3%) | 103 (60.6%) | 76 (52.1%) | 127 (66.8%) | |
| Unknown | 185 (27.9%) | 54 (34.2%) | 47 (27.6%) | 46 (31.5%) | 38 (20.0%) | |
| Maternal teratogens | .062 | |||||
| Yes | 82 (12.3%) | 23 (14.6%) | 25 (14.7%) | 18 (12.3%) | 16 (8.4%) | |
| No | 403 (60.7%) | 84 (53.2%) | 98 (57.6%) | 83 (56.8%) | 138 (72.6%) | |
| Unknown | 179 (27.0%) | 51 (32.3%) | 47 (27.6%) | 45 (30.8%) | 36 (18.9%) | |
| Maternal infection | <.001 | |||||
| Yes | 72 (10.8%) | 7 (4.4%) | 10 (5.9%) | 15 (10.3%) | 40 (21.1%) | |
| No | 399 (60.1%) | 96 (60.8%) | 106 (62.4%) | 84 (57.5%) | 113 (59.5%) | |
| Unknown | 193 (29.1%) | 55 (34.8%) | 54 (31.8%) | 47 (32.2%) | 37 (19.5%) | |
| Multiple gestation | .373 | |||||
| Yes | 40 (6.0%) | 5 (3.2%) | 12 (7.1%) | 13 (8.9%) | 10 (5.3%) | |
| No | 585 (88.1%) | 115 (72.8%) | 158 (92.9%) | 132 (90.4%) | 180 (94.7%) | |
| Unknown | 39 (5.9%) | 38 (24.1%) | 0 (0.0%) | 1 (0.7%) | 0 (0.0%) | |
| Average weight at birth, kg | .106 | |||||
| Mean (SD) | 2.93 (0.74) | 2.99 (0.73) | 2.92 (0.64) | 3.02 (0.64) | 2.83 (0.86) | |
| Gestational age | .019 | |||||
| Term (≥37 wk) | 511 (77.0%) | 123 (77.8%) | 128 (75.3%) | 114 (78.1%) | 146 (76.8%) | |
| Late preterm (32–37 wk) | 110 (16.6%) | 23 (14.6%) | 30 (17.6%) | 29 (19.9%) | 28 (14.7%) | |
| Preterm (<32 wk) | 27 (4.1%) | 8 (5.1%) | 3 (1.8%) | 1 (0.7%) | 15 (7.9%) | |
| Unknown | 16 (2.4%) | 4 (2.5%) | 9 (5.3%) | 2 (1.4%) | 1 (0.5%) | |
| History of IUGR or SGA | .733 | |||||
| Yes | 76 (11.4%) | 17 (10.8%) | 15 (8.8%) | 16 (11.0%) | 28 (14.7%) | |
| No | 413 (62.2%) | 90 (57.0%) | 105 (61.8%) | 85 (58.2%) | 133 (70.0%) | |
| Unknown | 175 (26.4%) | 51 (32.3%) | 50 (29.4%) | 45 (30.8%) | 29 (15.3%) | |
| Average number of cardiac surgeries | 0.038 | |||||
| Mean (SD) | 1.6 (0.99) | 1.55 (0.89) | 1.54 (0.91) | 1.51 (0.94) | 1.78 (1.14) | |
| ECMO required | 0.293 | |||||
| Yes | 90 (13.6%) | 15 (9.5%) | 26 (15.3%) | 24 (16.4%) | 25 (13.2%) | |
| No | 568 (85.5%) | 141 (89.2%) | 140 (82.4%) | 122 (83.6%) | 165 (86.8%) | |
| Unknown | 6 (0.9%) | 2 (1.3%) | 4 (2.4%) | 0 (0.0%) | 0 (0.0%) | |
| Deceased | 0.912 | |||||
| Yes | 69 (10.4%) | 16 (10.1%) | 20 (11.8%) | 15 (10.3%) | 18 (9.5%) | |
| Unknown | 595 (89.6%) | 142 (89.9%) | 150 (88.2%) | 131 (89.7%) | 172 (90.5%) |
AVM, arteriovenous malformation; CHD, congenital heart disease; ECMO, extracorporeal membrane oxygenation; ENT, ear, nose, throat; IUGR, intrauterine growth restriction; SGA, small for gestational age.
In the entire cohort, genetic testing occurred in 360 of 664 patients (54.2%) (Figure 1 and Table II). In patients with isolated CHD, genetic testing occurred in 271 of 534 (50.7%) (Figure 1, Table II), whereas testing occurred more frequently in the patients with CHD + MCA (89 of 130; 68.5%) (Figure 1, Table II). Overall, the most frequent genetic test was CMA, performed in 252 of 664 patients (38.0%), followed by chromosome analysis in 91 (13.7%), FISH in 61 (9.2%), NIPS in 13 (2.0%), gene panel in 11 (1.7%), and ES in 6 (0.9%) (Table III). Single gene testing and GS were least frequent, occurring in only 1 patient each (0.2%) (Table III).
Figure 1.
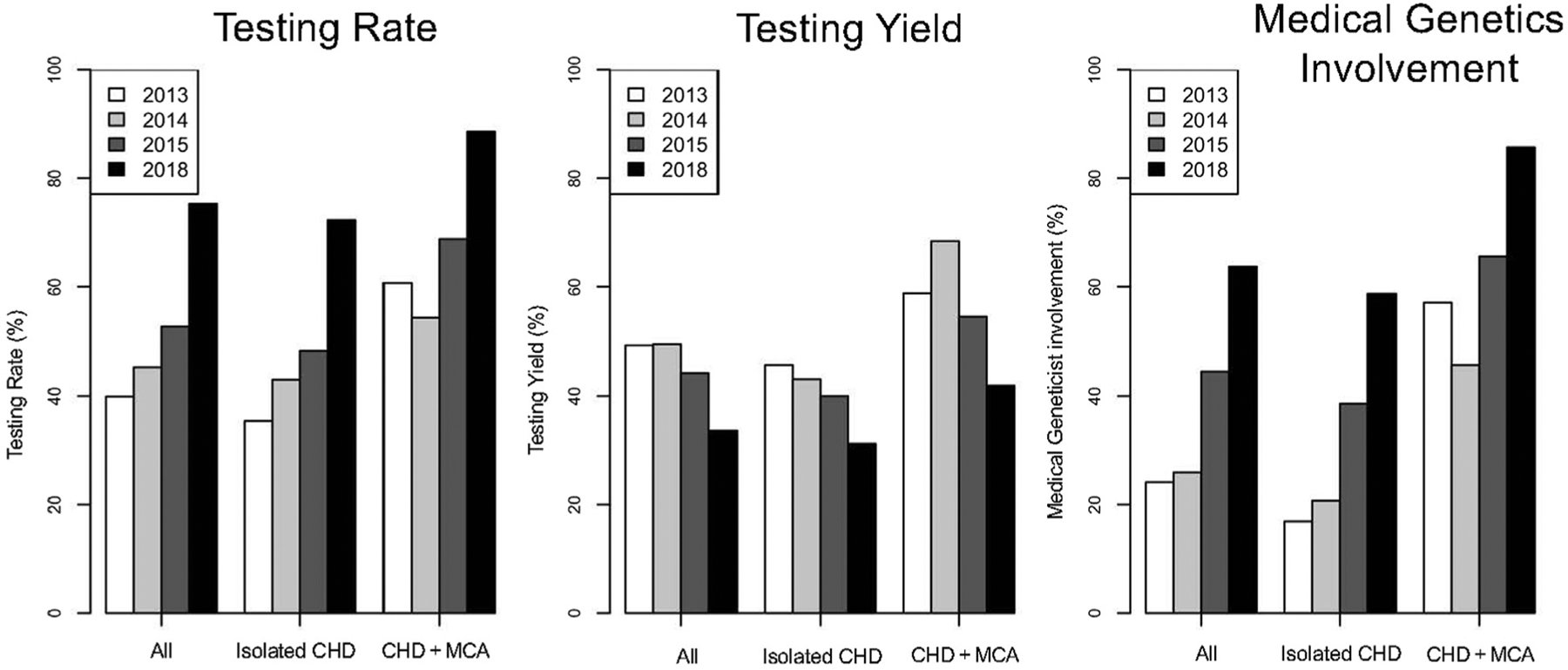
Comparison of testing rate, testing yield, and medical genetics involvement by year. Participants included children ≤14 months of age who underwent surgical repair for CHD. The proportion of patients who had genetic testing (testing rate), the proportion of patients whose genetic testing results were reported as abnormal (testing yield), and proportion of patients who were evaluated by a medical geneticist were analyzed. We separately analyzed patients with isolated CHD and patients with CHD + MCA.
Table II.
Testing rate, testing yield and medical genetics involvement by year
| Characteristics | Total | 2013 | 2014 | 2015 | 2018 | P value |
|---|---|---|---|---|---|---|
| All patients | ||||||
| Testing | ||||||
| No. | 664 | 158 | 170 | 146 | 190 | <.001 |
| Yes | 360 (54.2%) | 63 (39.9%) | 77 (45.3%) | 77 (52.7%) | 143 (75.3%) | |
| No | 304 (45.8%) | 95 (60.1%) | 93 (54.7%) | 69 (47.3%) | 47 (24.7%) | |
| Testing abnormal | ||||||
| No. | 360 | 63 | 77 | 77 | 143 | .061 |
| Yes | 151 (41.9%) | 31 (49.2%) | 38 (49.4%) | 34 (44.2%) | 48 (33.6%) | |
| No | 209 (58.1%) | 32 (50.8%) | 39 (50.6%) | 43 (55.8%) | 95 (66.4%) | |
| Medical genetics involvement | ||||||
| No. | 664 | 158 | 170 | 146 | 190 | <.001 |
| Yes | 268 (40.4%) | 38 (24.1%) | 44 (25.9%) | 65 (44.5%) | 121 (63.7%) | |
| No | 396 (59.6%) | 120 (75.9%) | 126 (74.1%) | 81 (55.5%) | 69 (36.3%) | |
| Isolated CHD | ||||||
| Testing | ||||||
| No. | 534 | 130 | 135 | 114 | 155 | <.001 |
| Yes | 271 (50.7%) | 46 (35.4%) | 58 (43.0%) | 55 (48.2%) | 112 (72.3%) | |
| No | 263 (49.3%) | 84 (64.6%) | 77 (57.0%) | 59 (51.8%) | 43 (27.7%) | |
| Testing abnormal | ||||||
| No. | 271 | 46 | 58 | 55 | 112 | .257 |
| Yes | 103 (38.0%) | 21 (45.7%) | 25 (43.1%) | 22 (40.0%) | 35 (31.2%) | |
| No | 168 (62.0%) | 25 (54.3%) | 33 (56.9%) | 33 (60.0%) | 77 (68.8%) | |
| Medical genetics involvement | ||||||
| No. | 534 | 130 | 135 | 114 | 155 | <.001 |
| Yes | 185 (34.6%) | 22 (16.9%) | 28 (20.7%) | 44 (38.6%) | 91 (58.7%) | |
| No | 349 (65.4%) | 108 (83.1%) | 107 (79.3%) | 70 (61.4%) | 64 (41.3%) | |
| CHD + MCA | ||||||
| Testing | ||||||
| No. | 130 | 28 | 35 | 32 | 35 | .014 |
| Yes | 89 (68.5%) | 17 (60.7%) | 19 (54.3%) | 22 (68.8%) | 31 (88.6%) | |
| No | 41 (31.5%) | 11 (39.3%) | 16 (45.7%) | 10 (31.2%) | 4 (11.4%) | |
| Testing abnormal | ||||||
| No. | 89 | 17 | 19 | 22 | 31 | .312 |
| Yes | 48 (53.9%) | 10 (58.8%) | 13 (68.4%) | 12 (54.5%) | 13 (41.9%) | |
| No | 41 (46.1%) | 7 (41.2%) | 6 (31.6%) | 10 (45.5%) | 18 (58.1%) | |
| Medical genetics involvement | ||||||
| No. | 130 | 28 | 35 | 32 | 35 | .005 |
| Yes | 83 (63.8%) | 16 (57.1%) | 16 (45.7%) | 21 (65.6%) | 30 (85.7%) | |
| No | 47 (36.2%) | 12 (42.9%) | 19 (54.3%) | 11 (34.4%) | 5 (14.3%) |
Table III.
Comparison of testing rate by genetic test type in 2013–2015 and 2018
| Testing modality | Total (n = 664) | 2013–2015 (n = 474) | 2018 (n = 190) | P value |
|---|---|---|---|---|
| NIPS | .535 | |||
| Yes | 13 (2.0%) | 8 (1.7%) | 5 (2.6%) | |
| No | 651 (98.0%) | 466 (98.3%) | 185 (97.4%) | |
| Chromosome analysis | .526 | |||
| Yes | 91 (13.7%) | 68 (14.3%) | 23 (12.1%) | |
| No | 573 (86.3%) | 406 (85.7%) | 167 (87.9%) | |
| FISH | <.001 | |||
| Yes | 61 (9.2%) | 56 (11.8%) | 5 (2.6%) | |
| No | 603 (90.8%) | 418 (88.2%) | 185 (97.4%) | |
| Microarray | <.001 | |||
| Yes | 252 (38.0%) | 126 (26.6%) | 126 (66.3%) | |
| No | 412 (62.0%) | 348 (73.4%) | 64 (33.7%) | |
| Single gene testing | .286 | |||
| Yes | 1 (0.2%) | 0 (0.0%) | 1 (0.5%) | |
| No | 663 (99.8%) | 474 (100.0%) | 189 (99.5%) | |
| Gene panel | .016 | |||
| Yes | 11 (1.7%) | 4 (0.8%) | 7 (3.7%) | |
| No | 653 (98.3%) | 470 (99.2%) | 183 (96.3%) | |
| Exome sequencing | .001 | |||
| Yes | 6 (0.9%) | 0 (0.0%) | 6 (3.2%) | |
| No | 658 (99.1%) | 474 (100.0%) | 184 (96.8%) | |
| Genome sequencing | .286 | |||
| Yes | 1 (0.2%) | 0 (0.0%) | 1 (0.5%) | |
| No | 663 (99.8%) | 474 (100.0%) | 189 (99.5%) | |
| Other | .328 | |||
| Yes | 23 (3.5%) | 19 (4.0%) | 4 (2.1%) | |
| No | 641 (96.5%) | 455 (96.0%) | 186 (97.9%) |
The number of patients who underwent genetic testing increased significantly across years; from 63 of 158 patients (39.9%) in 2013 to 77 of 170 (45.3%) in 2014, 77 of 146 (52.7%) in 2015, and 143 of 190 (75.3%) in 2018, (P < .001) (Figure 1, Table II). Testing showed a similar increase across subtypes, including an increase in patients with isolated CHD, from 46 of 130 (35.4%) in 2013 to 112 of 155 (72.3%) in 2018 (P < .001) (Figure 1, Table II) and in patients with CHD + MCA from 17 of 28 (60.7%) in 2013 to 31 of 35 (88.6%) in 2018, (P = .014) (Figure 1, Table II).
Our institution implemented genetic testing guidelines for newborns with CHD in 2014, which recommended chromosome analysis for patients with likely aneuploidy and CMA for all other patients with CHD. Therefore, we analyzed genetic testing, including a breakdown of testing modalities, in the cohort of patients from 2013 to 2015 compared with a cohort from 2018 (Table III). The increased genetic testing prevalence was accompanied by a shift in genetic testing modalities between the 2013–2015 and the 2018 cohort (Table III). Compared with 2013–2015, there was a significant increase in the use of 3 testing modalities: CMA, from 26.6% to 66.3% (P < .001); gene panels from 0.8% to 3.7% (P = .016); and ES from 0% to 3.2% (P = .001) in the 2018 cohort (Table III). Although there was no statistically significant difference across years, testing prevalence also increased for NIPS, from 1.7% to 2.6% (P = .535), single gene testing, from 0% to 0.5% (P = .286), and GS, from 0% to 0.5% (P = .286) (Table III). Only one testing modality had significantly decreased use, with the rate of FISH analysis falling from 11.8% to 2.6% (P < .001); and although not statistically significant, testing prevalence also decreased for chromosome analysis, from 14.3% to 12.1% (P = .525) (Table III).
Medical geneticists were involved in the care of 268 of 664 (40.4 %) patients, including 185 of the 534 (34.6%) patients with isolated CHD and 83 of the 130 (63.8%) patients with CHD + MCA (Figure 1, Table II).
The genetic testing guidelines implemented in 2014 recommended medical genetics evaluation for all patients with CHD. Medical geneticist involvement significantly increased across years (P < .001) from 38 of 158 patients (24.1%) in 2013, to 44 of 170 (25.9%) patients in 2014, 65 of 146 (44.5%) patients in 2015, and 121 of 190 (63.7%) patients in 2018. With respect to patient subtype, significant increases occurred for patients with isolated CHD, from 22 of 130 (16.9%) patients in 2013, to 91 of 155 (58.7%) patients in 2018, (across years, P < .001) as well as in patients with CHD + MCA from 16 of 28 (57.1%) patients in 2013; to 30 of 35 (85.7%) patients in 2018 (across years, P = .005) (Figure 1, Table II). The reported family history of CHD also increased from 1 of 158 (0.6%) in 2013 to 29 of 190 (15.3%) in 2018 (P = .004), possibly reflecting increased rigor in history taking and more formal documentation in the medical record due to the increased medical genetics involvement.
We analyzed factors affecting the prevalence of genetic testing by estimating the effects (OR; odds ratio) of multiple factors on genetic testing (Table IV, Figure 2). After adjusting for multiple factors, there was still a significant difference in genetic testing prevalence across years (P < .001), reflecting increased testing after implementing the algorithm in 2014 (Table IV, Figure 2). Compared with 2013, the genetic testing prevalence increased significantly in 2015 (OR 1.79, 95% CI 1.04–3.08, P = .036) and 2018 (OR 5.02, 95% CI 2.84–8.98, P < .001).
Table IV.
Effects of patient characteristics on genetic testing rate and yield
| Testing rate | Testing yield | |||
|---|---|---|---|---|
| Contributing factors | OR (95% CI) | P value | OR (95% CI) | P value |
| Year of surgery | ||||
| 2013 | Ref | <.001* | Ref | .139* |
| 2014 | 1.37 (0.81–2.32) | .242 | 1.21 (0.56–2.62) | .636 |
| 2015 | 1.79 (1.04–3.08) | .036 | 1.3 (0.59–2.85) | .520 |
| 2018 | 5.02 (2.84–8.88) | <.001 | 0.75 (0.36–1.56) | .438 |
| Sex | ||||
| Male | Ref | Ref | ||
| Female | 1.56 (1.05–2.32) | .026 | 1.91 (1.17–3.13) | .010 |
| Race | ||||
| White | Ref | .307* | Ref | .607* |
| African American | 0.68 (0.39–1.21) | .191 | 1.75 (0.85–3.60) | .128 |
| Other | 0.68 (0.28–1.64) | .394 | 0.96 (0.29–3.17) | .949 |
| Family history of CHD | ||||
| Yes | Ref | Ref | ||
| No | 0.15 (0.04–0.51) | .003 | 1.78 (0.75–4.24) | .194 |
| Infant of a diabetic mother | ||||
| No | Ref | Ref | ||
| Yes | 1.57 (0.89–2.79) | .121 | 0.57 (0.28–1.16) | .122 |
| Maternal teratogens | ||||
| No | Ref | Ref | ||
| Yes | 1.17 (0.66–2.08) | .590 | 1.24 (0.59–2.60) | .563 |
| Maternal infection | ||||
| No | Ref | Ref | ||
| Yes | 1.54 (0.79–2.98) | .201 | 0.91 (0.44–1.86) | .792 |
| Multiple gestation | ||||
| No | Ref | Ref | ||
| Yes | 1.51 (0.62–3.69) | .365 | 0.42 (0.14–1.26) | .122 |
| Weight at birth, kg | ||||
| 1-kg increase | 0.93 (0.63–1.39) | .733 | 0.61 (0.36–1.05) | .073 |
| Gestational age | ||||
| Term (≥37 wk) | Ref | <.001* | Ref | .937* |
| Late preterm (32–37 wk) | 1.12 (0.61–2.06) | .720 | 1.06 (0.50–2.24) | .876 |
| Preterm (<32 wk) | 0.15 (0.04–0.58) | .006 | 0.67 (0.10–4.56) | .684 |
| History of IUGR or SGA | ||||
| No | Ref | Ref | ||
| Yes | 3.77 (1.79–7.97) | <.001 | 0.97 (0.46–2.04) | .934 |
| Congenital malformation | ||||
| No | Ref | Ref | ||
| Yes | 2.13 (1.28–3.53) | .003 | 1.72 (0.97–3.05) | .065 |
| ECMO required | ||||
| No | Ref | Ref | ||
| Yes | 0.81 (0.44–1.47) | .481 | 1.08 (0.50–2.34) | .849 |
| Number of cardiac surgeries | ||||
| +1 cardiac surgery | 1.18 (0.95–1.48) | .141 | 0.8 (0.61–1.04) | .095 |
| Deceased | ||||
| Other | Ref | Ref | ||
| Yes | 1.33 (0.69–2.57) | .400 | 0.92 (0.40–2.07) | .832 |
| CHD groups | ||||
| CTD | Ref | .003* | Ref | .006* |
| APVR | 0.26 (0.11–0.62) | .002 | 0.54 (0.14–2.00) | .352 |
| Arteriopathy | 0.2 (0.04–0.92) | .039 | ||
| AVSD | 0.68 (0.34–1.37) | .284 | 3.8 (1.58–9.15) | .003 |
| HTX | 0.85 (0.25–2.89) | .793 | 0.54 (0.10–2.82) | .464 |
| LVOTO | 0.49 (0.29–0.83) | .008 | 0.92 (0.48–1.75) | .794 |
| RVOTO | 0.50 (0.21–1.21) | .125 | 0.87 (0.29–2.67) | .813 |
| Septal defect | 0.39 (0.20–0.77) | .007 | 2.97 (1.24–7.11) | .015 |
| Other | 0.25 (0.11–0.57) | .001 | 1.9 (0.69–5.23) | .212 |
APVR, anomalous pulmonary venous return; LVOTO, left ventricle outflow tract obstruction; RVOTO, right ventricle outflow tract obstruction.
P value for multilevel factors. Null hypothesis: the testing rate or testing yield is the same for all levels.
Figure 2.
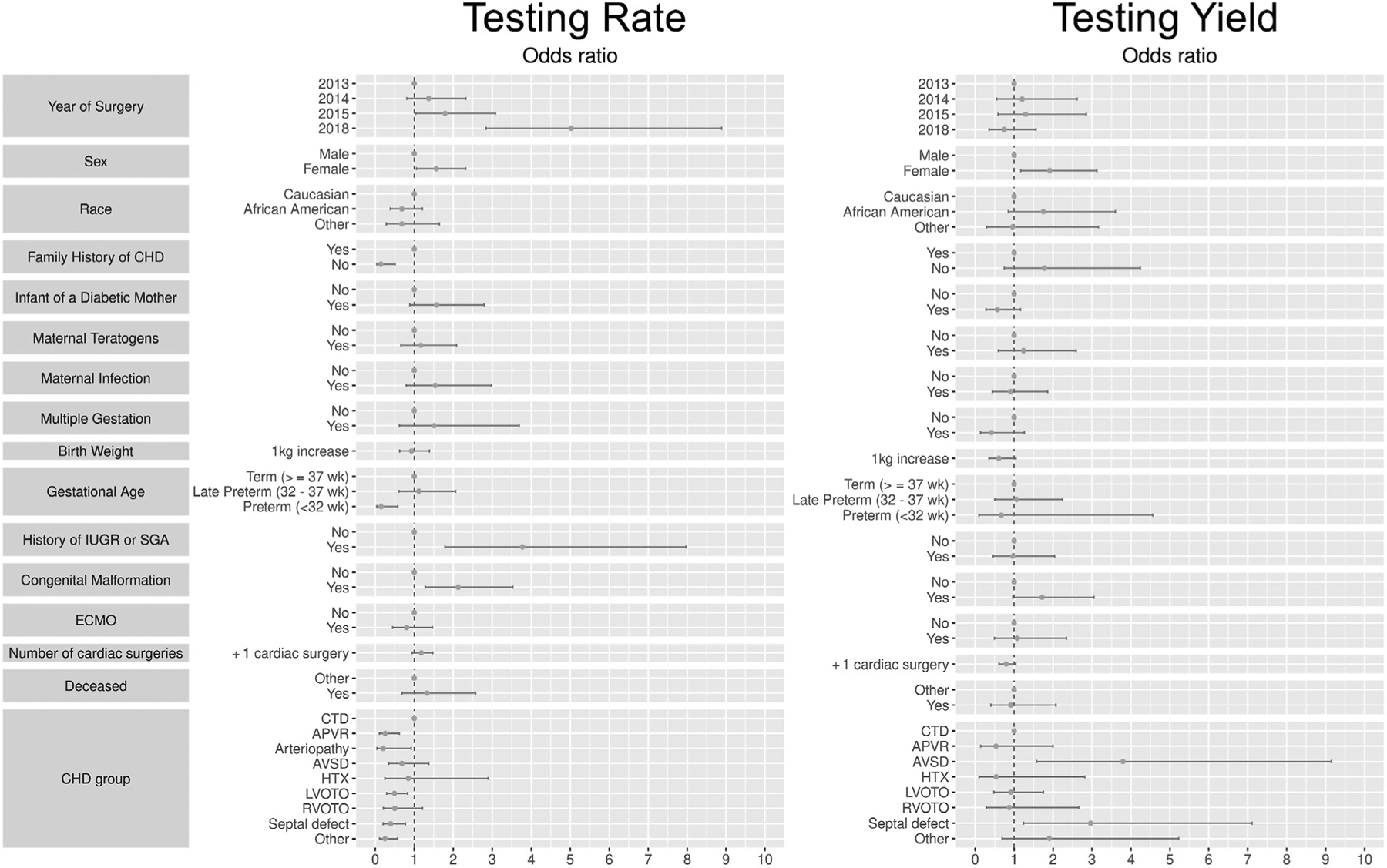
Effects of patient characteristics on genetic testing rate and yield. We estimated the effects (OR) of multiple factors on genetic testing rate and genetic testing yield, including year, sex, race, family history of CHD, maternal diabetes, maternal teratogen exposure, maternal infection, multiple gestation pregnancy, weight at birth, gestational age, IUGR or SGA, congenital malformation, requirement for ECMO, number of cardiac surgeries, mortality, and cardiac lesion. APVR, anomalous pulmonary venous return; AVSD, atrioventricular septal defect; CHD, congenital heart disease; CTD, conotruncal defect; ECMO, extracorporeal membrane oxygenation; HTX, heterotaxy; IUGR, intrauterine growth restriction; LVOTO, left ventricle outflow tract obstruction; RVOTO, right ventricle outflow tract obstruction; SGA, small for gestational age.
Our guidelines recommended genetic testing for all cardiac lesions outside of isolated septal defects. Compared with CTD (used as the reference, given longstanding recommendations for genetic testing in this group),14 multiple patient groups were tested less often, including anomalous pulmonary venous return (OR 0.26, 95% CI [0.11–0.62, P = .002), arteriopathy (OR 0.2, 95% CI 0.04–0.92, P = .039), left ventricle outflow tract obstruction (OR 0.49, 95% CI 0.29–0.83, P = .008), septal defects (OR 0.39, 95% CI 0.20–0.77, P = .007) and all other heart defects (OR 0.25, 95% CI 0.11–0.57, P = .001) (Table IV, Figure 2).
Additional factors drove genetic testing in patients with CHD. Infants who were IUGR or SGA were tested more frequently (OR 3.77, 95% CI 1.79–7.97, P < .001). Patients with CHD + MCA were also tested more frequently (OR 2.13, 95% CI 1.28–3.53, P = .003) (Table IV, Figure 2). Female patients were tested more frequently than male patients (OR 1.56, 95% CI 1.05–2.32, P = .026). Patients without a family history of CHD were tested less frequently (OR 0.15, 95% CI 0.04–0.51, P = .003). Compared with patients who were born at term, infants born preterm were tested less frequently (OR 0.15, 95% CI [0.04–0.58], P = .006) (Table IV, Figure 2). There was no significant difference in testing prevalence based on race, maternal diabetes, maternal teratogen exposure, maternal infection, multiple gestation pregnancy, weight at birth, requirement for ECMO, number of cardiac surgeries, or mortality.
Overall, the greatest effect on genetic testing prevalence occurred in 2018, the time point after implementation of genetic testing guidelines. We analyzed the testing yield or the proportion of patients for whom testing yielded abnormal results. Overall, 151 of 360 (41.9%) tested patients had abnormal results (Table II, Figure 1). Abnormal results were detected in 103 of 271 (38.0%) patients with isolated CHD and 48 of 89 (53.9%) patients with CHD + MCA (Table II, Figure 1).
As the genetic testing prevalence increased, the genetic testing yield decreased, but not significantly (yield of 49.2% in 2013, 49.4% in 2014, 44.2% in 2015, and 33.6% in 2018, across years P = .061). This held true for patients with isolated CHD (yield 45.7% in 2013, 43.1% in 2014, 40% in 2015, and 31.2% in 2018, across years, P = .257) as well as for patients with CHD + MCA (yield 58.8% in 2013, 68.4% in 2014, 54.5% in 2015 and 41.9% in 2018, across years, P = .312) (Table II, Figure 1).
We analyzed factors that affected the yield of genetic testing by estimating the effects of multiple factors (Table IV, Figure 2). Interestingly, after adjusting for multiple factors, despite the significantly increased testing prevalence across years, there was still not a significant decrease in testing yield (P = .139). Only cardiac lesion type and sex influenced yield. Female patients had a significantly greater yield of genetic testing (OR 1.91, 95% CI 1.17–3.13, P = .010). Our guidelines recommended genetic testing for hospitalized infants with all types of CHD except septal defects, which were left to the provider’s decision. Compared with CTD, septal defects actually had increased yield (OR 2.97, 95% CI 1.24–7.11, P = .015). AVSD also had significantly increased yield (OR 3.81, 95% CI 1.58–9.15, P = .003). There was no significant difference in testing yield for any other cardiac lesions. There was also no difference in testing yield based on family history of CHD, IUGR or SGA, congenital malformations, gestational age, race, maternal diabetes, maternal teratogen exposure, maternal infection, multiple gestation pregnancy, weight at birth, requirement for ECMO, number of cardiac surgeries, or mortality.
Our institution implemented genetic testing guidelines in 2014, recommending universal testing for patients with CHD. Our results indicate testing was still not universal in 2018, although there was a significant increase in testing prevalence from 39.9% in 2013 to 75.3% in 2018. The testing yield decreased from 49.2% in 2013 to 33.6% in 2018, although not significantly. With increased testing, the proportion of patients with a genetic diagnosis in our cohort increased, from 19% (31 of 158) in 2013 to 25% (48 of 190) in 2018. Through this time, our institution managed an average of 174 newborns undergoing surgery for CHD per year, and extrapolating testing rate and yield from 2013 compared with 2018 added an estimated 10 additional genetic diagnoses per year, reflecting a 29% increase, or genetic testing rate ratio of 1.29, after implementation of testing guidelines.
Discussion
Genetic testing demonstrated high yields across almost every patient subtype, including both isolated CHD (38.0%) and patients with MCA (53.9%). The overall diagnostic yield of 41.9% is similar to other studies in infants with CHD, with yields from 25% to 50%,8–10 despite distinct ascertainment protocols or comprehensiveness of genetic evaluation. Significantly increased testing rate (P < .001), with consistent testing yield (P = .139) increased the proportion of patients with CHD with a genetic diagnosis (Table IV, Figure 2). Although multiple factors influenced genetic testing rate, including a family history of CHD, IUGR/SGA at birth, and the presence of other congenital anomalies, none of these factors significantly impacted testing yield. In a recent study, Shikany et al identified specific congenital anomalies that increased the yield of abnormal genetic testing,10 although their study focused on patients younger than 1 month of age and included only patients evaluated by a genetics team (possibly with improved phenotyping). Another recent study reflected our results, where patient subtypes, including congenital anomalies, did not increase the odds of abnormal genetic testing.8 Together, these results seem to support universal testing in hospitalized infants with CHD requiring surgery.
Previous single-center studies indicate genetic testing is likely underused at many sites, with rates ranging from 25% to 87%.8–10,26–30 Our results indicate that genetic testing use was significantly improved with the implementation of genetic testing guidelines, with testing rates climbing from 39.9% in 2013 to 75.3% in 2018. We identified numerous factors that drove selective genetic testing, although none of these factors actually impacted testing yield. Universal genetic testing may allow us to better discern patient subpopulations who may not require testing. Despite the increased testing rate in 2018 to 75.3%, approaching universal testing, the diagnostic rate remained high at 33.6% and resulted in an increased proportion of patients with CHD with a genetic diagnosis.
Increased genetic testing and diagnosis has been shown to impact patient care and outcomes. Recommendations from the AHA, endorsed by the American Academy of Pediatrics, outline the importance of obtaining a genetic diagnosis in order to (1) Identify pathology in other organ systems, (2) to inform prognosis, (3) to identify recurrence risk, and (4) to inform family members at risk, and inform their screening.14 Multiple recent studies in patients with CHD demonstrate abnormal genetic testing correlates to poorer surgical outcomes.17–21 A study of critically ill newborns demonstrated a high prevalence of genetic disorders in this population and highlighted that increased genetic testing has potential to shorten the time to a diagnosis and targeted therapeutic interventions.31 Another more recent study in the same population demonstrated increased use of broad genetic testing improved the detection of genetic disease, which bolstered understanding of the patient’s condition and prognosis.32 Studies using rapid whole-genome sequencing in critically ill newborns have demonstrated diagnostic genetic testing led to avoided morbidity in up to 26% of patients, and had potential to reduce the likelihood of mortality, aid transition to palliative care, and led to changes in management that reduced inpatient cost.33 A follow-up study revealed genetic testing led to clinical change in one-third of affected patients and reduced net healthcare expenditures.34 Identifying a genetic diagnosis can inform the need for early intervention services, impacting long-term outcomes, and therefore should occur early, and genetic diagnoses should not be missed. These studies, along with our findings, highlight the need for broad genetic testing in CHD patients.
Our genetic testing guidelines expand upon the 2010 ISCA recommendation,12 for chromosomal analysis in select patients, and universal CMA in all others. This study shows the emerging use of molecular panels and ES technologies in 2018, suggesting an important role of these newer technologies in the identification of genetic etiologies that may have otherwise not been identified. To address the evolving landscape of genetic testing, the AHA updated guidelines in 2018 to reflect the availability of newer, more advanced, molecular based technologies, similar to those used in our study. Our data indicate that ES and GS were just beginning to be adopted in 2018 in select cases and the changing use of test modalities during the years of this study reflects the moving target nature of genetic testing. Our study was before the dramatic rise in clinical use of next-generation sequencing-based tests in recent years. In 2019, our algorithm was updated to include molecular panel testing of more than 500 genes. In 2021, American College of Medical Genetics and Genomics updated clinical guidelines and recommended ES and GS as a first- or second-tier test in pediatric patients with congenital anomalies or intellectual disability, based on a higher diagnostic yield and cost-effectiveness when ordered early in the diagnostic evaluation.13 This creates a new challenge and opportunity to compare tiered testing vs first-line GS. Notably, the use of rapid GS has been shown to improve care and reduce costs in the NICU in selected populations35,36 and was recently shown to impact care and improve cost specifically in infants with CHD, conferring a diagnostic yield up to 46%, albeit in a small, selected cohort.37 Rapid GS is now available at our NICU and other select institutions38,39 and has been incorporated into an updated guideline in our NICU. Our study has limited use of GS, and future analyses will be needed to monitor the impact of this new technology. Increased geneticist involvement will likely be necessary to guide testing and interpret increasingly complex results from newer modalities.
Our genetic testing guidelines recommend genetic testing in all cardiac lesions except septal defects; however, compared with CTD, multiple cardiac lesions were tested less frequently (Table IV, Figure 2). Furthermore, compared with CTD, patients with septal defects had increased diagnostic yield. It is possible that patients with septal defects selected to undergo genetic testing had obvious dysmorphisms or syndromic features that led to testing. In our results, AVSD had the greatest diagnostic yield, a finding that has been demonstrated in multiple studies and in part may reflect the common finding of these defects in patients with Down syndrome.8,26
For this study, a hierarchical cardiac classification system was used, grouping CHDs into umbrella categories (a single Botto Level 3 diagnosis) established by the National Birth Defects Prevention Study.24 At this broad level, all CHD subtypes appear to benefit from genetic testing for identifying etiology. Whether the more detailed Level I subtypes within each Level 3 category derive benefit remains a question for future investigation. Difficulties in resolving this question stem from incomplete genetic testing, small numbers of specific CHD subtypes at single institutions, and incomplete phenotyping in published literature. Future studies may need to employ a more complex nonhierarchical system of cardiac phenotyping to identify specific subtypes of CHD, or combinations thereof, that are highly associated with abnormal genetic testing results.10 Beyond CHD, sick neonates may require more detailed phenotyping, in order to implement broad use of genetic evaluation, including more detailed and rapid genetic testing.
One limitation of our study is that we used genetic testing results available within the electronic medical record and did not include testing that may have been performed at outside institutions, or outside the data collection window. Other limitations of our study include that our results reflect patients undergoing surgery and exclude less critically ill infants. The study also may also miss a small number of the most critically ill infants, those who died prior to surgery, a population that may be enriched for genetic anomalies. Future studies should explore larger sample sizes, across multiple institutions, with increased medical genetics involvement. Investigation of genotype–phenotype correlations using systems more focused on cardiac physiology or surgical repair type will help assess impact and the use of nonhierarchical cardiac classification systems may identify differences in genetic testing yield based on patient subtypes to better guide genetic testing.
Given the rapid expansion in genetic testing availability in recent years, the findings from this study have implications beyond CHD, to numerous pediatric patient populations affected by congenital anomalies and critical illnesses, where genetics evaluation and adherence to guidelines are likely beneficial.
Declaration of Competing Interest
This work was supported by the American Heart Association Transformational Award (grant number 19TPA34850054 [to [S.W.]), the Indiana University Health-Indiana University School of Medicine (Strategic Research Initiative and Physician Scientist Initiative [to [S.W.]), and National Institutes of Health (grant number 2P01HL134599 [to S.W. and M.D.] and grant number K08HL148508 [to M.D.]). The authors declare no conflicts of interest.
The authors are grateful to the patients and their families for their contribution.
Glossary
- AHA
American Heart Association
- AVSD
Atrioventricular septal defect
- CHD
Congenital heart disease
- CMA
Chromosomal microarray analysis
- CTD
Conotruncal defect
- ECMO
Extracorporeal membrane oxygenation
- ES
Exome sequencing
- FISH
Fluorescence in situ hybridization analysis
- GS
Genome sequencing
- ISCA
International Standard Cytogenomic Array Consortium
- IUGR
Intrauterine growth restriction
- MCA
Multiple congenital anomaly
- NIPS
Noninvasive prenatal screening
- SGA
Small for gestational age
- STS
Society of Thoracic Surgeons
Data Statement
Data sharing statement available at www.jpeds.com.
References
- 1.Thienpont B, Mertens L, de Ravel T, Eyskens B, Boshoff D, Maas N, et al. Submicroscopic chromosomal imbalances detected by array-CGH are a frequent cause of congenital heart defects in selected patients. Eur Heart J 2007;28:2778–84. [DOI] [PubMed] [Google Scholar]
- 2.Richards AA, Santos LJ, Nichols HA, Crider BP, Elder FF, Hauser NS, et al. Cryptic chromosomal abnormalities identified in children with congenital heart disease. Pediatr Res 2008;64:358–63. [DOI] [PubMed] [Google Scholar]
- 3.Breckpot J, Thienpont B, Peeters H, de Ravel T, Singer A, Rayyan M, et al. Array comparative genomic hybridization as a diagnostic tool for syndromic heart defects. J Pediatr 2010;156:810–7.e4. [DOI] [PubMed] [Google Scholar]
- 4.Goldmuntz E, Paluru P, Glessner J, Hakonarson H, Biegel JA, White PS, et al. Microdeletions and microduplications in patients with congenital heart disease and multiple congenital anomalies. Congenit Heart Dis 2011;6:592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lalani SR, Shaw C, Wang X, Patel A, Patterson LW, Kolodziejska K, et al. Rare DNA copy number variants in cardiovascular malformations with extracardiac abnormalities. Eur J Hum Genet 2013;21:173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Syrmou A, Tzetis M, Fryssira H, Kosma K, Oikonomakis V, Giannikou K, et al. Array comparative genomic hybridization as a clinical diagnostic tool in syndromic and nonsyndromic congenital heart disease. Pediatr Res 2013;73:772–6. [DOI] [PubMed] [Google Scholar]
- 7.Van Der Bom T, Zomer AC, Zwinderman AH, Meijboom FJ, Bouma BJ, Mulder BJ. The changing epidemiology of congenital heart disease. Nat Rev Cardiol 2011;8:50. [DOI] [PubMed] [Google Scholar]
- 8.Ahrens-Nicklas RC, Khan S, Garbarini J, Woyciechowski S, D’Alessandro L, Zackai EH, et al. Utility of genetic evaluation in infants with congenital heart defects admitted to the cardiac intensive care unit. Am J Med Genet 2016;170:3090–7. [DOI] [PubMed] [Google Scholar]
- 9.Geddes GC, Basel D, Frommelt P, Kinney A, Earing M. Genetic testing protocol reduces costs and increases rate of genetic diagnosis in infants with congenital heart disease. Pediatr Cardiol 2017;38:1465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shikany AR, Landis BJ, Parrott A, Miller EM, Coyan A, Walters L, et al. A comprehensive clinical genetics approach to critical congenital heart disease in infancy. J Pediatr 2020;227:231–8.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park ST, Kim J. Trends in next-generation sequencing and a new era for whole genome sequencing. Int Neurourol J 2016;20:S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller DT, Adam MP, Aradhya S, Biesecker LG, Brothman AR, Carter NP, et al. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet 2010;86: 749–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manickam K, McClain MR, Demmer LA, Biswas S, Kearney HM, Malinowski J, et al. Exome and genome sequencing for pediatric patients with congenital anomalies or intellectual disability: an evidence-based clinical guideline of the American College of Medical Genetics and Genomics (ACMG). Genet Med 2021;23:2029–37. [DOI] [PubMed] [Google Scholar]
- 14.Pierpont ME, Basson CT, Benson DW Jr, Gelb BD, Giglia TM, Goldmuntz E, et al. Genetic basis for congenital heart defects: current knowledge: a scientific statement from the American Heart Association Congenital Cardiac Defects Committee, Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation 2007;115:3015–38. [DOI] [PubMed] [Google Scholar]
- 15.Pierpont ME, Brueckner M, Chung WK, Garg V, Lacro RV, McGuire AL, et al. Genetic basis for congenital heart disease: revisited: a scientific statement from the American Heart Association. Circulation 2018;138: e653–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harden B, Tian X, Giese R, Nakhleh N, Kureshi S, Francis R, et al. Increased postoperative respiratory complications in heterotaxy congenital heart disease patients with respiratory ciliary dysfunction. J Thorac Cardiovasc Surg 2014;147:1291–8.e2. [DOI] [PubMed] [Google Scholar]
- 17.Carey AS, Liang L, Edwards J, Brandt T, Mei H, Sharp AJ, et al. The impact of CNVs on outcomes for infants with single ventricle heart defects. Circ Cardiovasc Genet 2013;6:444–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim DS, Kim JH, Burt AA, Crosslin DR, Burnham N, McDonald-McGinn DM, et al. Patient genotypes impact survival after surgery for isolated congenital heart disease. Ann Thorac Surg 2014;98:104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim DS, Kim JH, Burt AA, Crosslin DR, Burnham N, Kim CE, et al. Burden of potentially pathologic copy number variants is higher in children with isolated congenital heart disease and significantly impairs covariate-adjusted transplant-free survival. J Thorac Cardiovasc Surg 2016;151:1147–51.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landis BJ, Cooper DS, Hinton RB. CHD associated with syndromic diagnoses: peri-operative risk factors and early outcomes. Cardiol Young 2016;26:30–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landis BJ, Helm BM, Herrmann JL, Hoover MC, Durbin MD, Elmore LR, et al. Learning to crawl: determining the role of genetic abnormalities on postoperative outcomes in congenital heart disease. J Am Heart Assoc 2022;11:e026369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inf 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Botto LD, Lin AE, Riehle-Colarusso T, Malik S, Correa A. Seeking causes: classifying and evaluating congenital heart defects in etiologic studies. Birth Defects Res Part A Clin Mol Teratol 2007;79:714–27. [DOI] [PubMed] [Google Scholar]
- 25.Øyen N, Poulsen G, Boyd HA, Wohlfahrt J, Jensen PK, Melbye M. National time trends in congenital heart defects, Denmark, 1977–2005. Am Heart J 2009;157:467–73.e1. [DOI] [PubMed] [Google Scholar]
- 26.Buckley JR, Kavarana MN, Chowdhury SM, Scheurer MA. Current practice and utility of chromosome microarray analysis in infants undergoing cardiac surgery. Congenit Heart Dis 2015;10:E131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connor JA, Hinton RB, Miller EM, Sund KL, Ruschman JG, Ware SM. Genetic testing practices in infants with congenital heart disease. Congenit Heart Dis 2014;9:158–67. [DOI] [PubMed] [Google Scholar]
- 28.Baker K, Sanchez-de-Toledo J, Munoz R, Orr R, Kiray S, Shiderly D, et al. Critical congenital heart disease—utility of routine screening for chromosomal and other extracardiac malformations. Congenit Heart Dis 2012;7:145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cowan JR, Ware SM. Genetics and genetic testing in congenital heart disease. Clin Perinatol 2015;42:373–93. [DOI] [PubMed] [Google Scholar]
- 30.Helm BM, Landis BJ, Ware SM. Genetic evaluation of inpatient neonatal and infantile congenital heart defects: new findings and review of the literature. Genes 2021;12:1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swaggart KA, Swarr DT, Tolusso LK, He H, Dawson DB, Suhrie KR. Making a genetic diagnosis in a level IV neonatal intensive care unit population: who, when, how, and at what cost? J Pediatr 2019;213: 211–7.e4. [DOI] [PubMed] [Google Scholar]
- 32.Hagen L, Khattar D, Whitehead K, He H, Swarr DT, Suhrie K. Detection and impact of genetic disease in a level IV neonatal intensive care unit. J Perinatol 2022;42:580–8. [DOI] [PubMed] [Google Scholar]
- 33.Farnaes L, Hildreth A, Sweeney NM, Clark MM, Chowdhury S, Nahas S, et al. Rapid whole-genome sequencing decreases infant morbidity and cost of hospitalization. NPJ Genom Med 2018;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dimmock D, Caylor S, Waldman B, Benson W, Ashburner C, Carmichael JL, et al. Project Baby Bear: rapid precision care incorporating rWGS in 5 California children’s hospitals demonstrates improved clinical outcomes and reduced costs of care. Am J Hum Genet 2021;108: 1231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farnaes L, Hildreth A, Sweeney NM, Clark MM, Chowdhury S, Nahas S, et al. Rapid whole-genome sequencing decreases infant morbidity and cost of hospitalization. NPJ Genom Med 2018;3:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Willig LK, Petrikin JE, Smith LD, Saunders CJ, Thiffault I, Miller NA, et al. Whole-genome sequencing for identification of Mendelian disorders in critically ill infants: a retrospective analysis of diagnostic and clinical findings. Lancet Respir Med 2015;3:377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sweeney NM, Nahas SA, Chowdhury S, Batalov S, Clark M, Caylor S, et al. Rapid whole genome sequencing impacts care and resource utilization in infants with congenital heart disease. NPJ Genom Med 2021;6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saunders CJ, Miller NA, Soden SE, Dinwiddie DL, Noll A, Alnadi NA, et al. Rapid whole-genome sequencing for genetic disease diagnosis in neonatal intensive care units. Sci Transl Med 2012;4:154ra35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shickh S, Mighton C, Uleryk E, Pechlivanoglou P, Bombard Y. The clinical utility of exome and genome sequencing across clinical indications: a systematic review. Hum Genet 2021;140:1403–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing statement available at www.jpeds.com.


