Summary
The calcium binding protein parvalbumin (PV) in the mammalian neocortex is expressed in a subpopulation of cortical GABAergic inhibitory interneurons. PV – producing interneurons represent the largest subpopulation of neocortical inhibitory cells, exhibit mutual chemical and electrical synaptic contacts and are well known to generate gamma oscillation. This review summarizes basic data of the distribution, afferent and efferent connections and physiological properties of parvalbumin expressing neurons in the neocortex. Basic data about participation of PV-positive neurons in cortical microcircuits are presented. Autaptic connections, metabolism and perineuronal nets (PNN) of PV-positive neurons are also discussed.
Keywords: Interneurons, Parvalbumin, Inhibition, Neocortex, Inhibitory circuits
Introduction
The cerebral cortex of mammals is a complex structure located in the surface of hemisphere and parcellated on three- layered allocortex which comprise hippocampal formation (archicortex) and olfactory cortex (paleocortex), and six-layered neocortex [1,2]. The neocortex is the largest structure of the cortical mantle playing a role in motor, sensory and cognitive processes. There are transitional cortical fields (periarchicortex, peripaleocortex) between the neocortex and allocortical formations.
Neocortical neurons are organized into small units formed by cylinders or columns of tissue extending perpendicular to the surface of the cortex. Neurons within a column share similar functional properties that differ from those within adjacent column [3]. There is also tendency for cells with similar properties to be located together, more or less separated from cells with other properties. This is called modular organization. Modular organization was described in somatosensory and visual cortex and in primary motor cortex [4,5]. A general schema of cortical module containing excitatory and inhibitory neurons was proposed by Szentagothai [6].
All three basic cortical formations consist of two major classes of neurons: glutamatergic excitatory pyramidal neurons (principal cells) which constitute approximately 70 – 80 % of total neuronal population and inhibitory, GABAergic short-axonal interneurons (IN) which constitute only minor fraction of the total number of neocortical neurons (15 – 25 %). Although in an earlier period of neuroscience, S. Ramon y Cajal [7] considered cortical interneurons as an important neuronal elements modulating cortical functions and influencing higher mental processes (“butterflies of our souls”; Ramon y Cajal) [8] not until the last intensive research collecting many new data and concepts related to their structure, functions, connectivity, development, and chemical phenotypes [9–13]. Progress in measuring neuronal activity is associated with using of new technologies including optical imaging (two – photon microscopy), high density neuronal recordings in freely moving animals, patch-clamp recording and viral vectors [14,15]. The application of these techniques provide detailed insight of neuronal and synaptic function in both categories of neurons – principal (pyramidal, excitatory, glutamatergic) as well as inhibitory interneurons (GABAergic).
In addition to their prominent role in precise spatio-temporal inhibitory control of the activity of principal neurons INs has been associated with their synchronization and oscillation and generation of several types of neurodegenerative disorders [16,17].
Various approaches to sort GABAergic interneurons into distinct subgroups have been adopted. In an effort to overcome the problems associated with the lack of consensus on the classification and nomenclature of cortical interneurons, a meeting devoted to this topic was held in 2005 in S. Ramon y Cajal’s birthplace, Petilla de Aragon (Navarra, Spain). At this meeting a list of terms with characterization (the “Petilla convention”) describing the morphological, physiological and molecular features of neocortical interneurons was accepted. It is expected that this proposal could improve the methodology of studying the cortical interneurons.
Classification of GABAergic interneurons
Cortical interneurons are classified morphologically by their dendritic and axonal ramifications and neurochemical characteristics and functionally by firing properties and synaptic targets. On the basis of expression of selective markers three classes non-overlapping neocortical GABAergic INs are distinguished:
Parvalbumin immunoreactive neurons (PV+) represent the largest class of GABAergic interneurons accounting for about 40 % of cortical interneurons (Fig. 1, Fig. 2). PV+ interneurons are present in all neocortical layers except for layer 1 (L 1). PV+ neurons can be divided into two classes, basket and chandelier cells (also axo-axonic cells) exhibiting fast-spiking electrophysiological profiles [9,18]. A third type of PV+ neurons are multipolar bursting cells located mainly in upper L2. These neurons differ from fast-spiking basket cells but data describing their morphology and functional characteristics are sparse [19].
Somatostatin immunoreactive neurons represent 30 % of the neocortical INs and are divided into Martinotti cells and non-Martinotti cells.
The third group of neocortical INs expressing the ionotropic serotonin receptor 5HT3aR are present in all cortical layers except for layer 1, and represent 30 % of the cortical INs. These cells co-express vasoactive intestinal polypeptide (VIP).
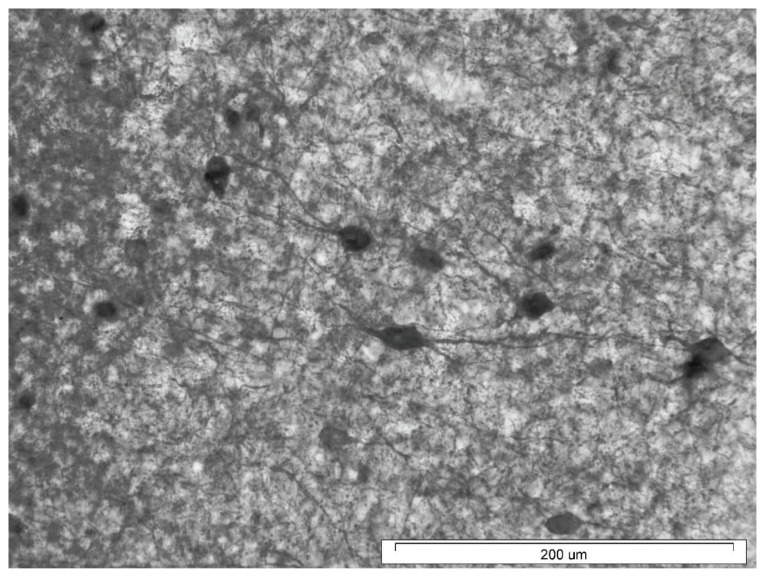
Fig. 1.
Representative low-power photomicrograph illustrating the distribution of PV- immunoreactive neurons in neocortex (somatosensory area) of the rat. Scale bar = 500 μm.
Fig. 2.
High-power photomicrograph of multipolar PV+ neuron in neocortex of the rat (somatosensory cortex, layer 3). Scale bar = 200 μm.
The main function of interneurons is to control the activity of principal (pyramidal) cells and another classification divides them according their axonal targets:
The perisomatic group - the largest population of interneurons containing basket and chandelier cells providing perisomatic inhibition. These cells control the spiking output of the principal cells.
The dendrite targeting group – target specific dendritic domains of principal cells.
The interneuron – specific group – their axons preferentially contact other interneurons but avoid principal cells.
Long – range group – this group of inhibitory cell projects over large distances. Some axons or axon collaterals innervate subcortical structures or contralateral hemisphere [20,21,22].
The goal of our review is to collect basic structural, developmental and functional data related to PV+ neurons in the neocortical formation.
Parvalbumin + interneurons
Tremendous number of new data and concepts were collected in recent years describing the phenotypic characteristics of PV+ GABAergic interneurons and their operations in cortical microcircuits and larger neocortical assemblies acting as substrate in neurodegenerative processes [23].
PV+ GABAergic neurons are the largest subpopulation of neocortical GABAergic interneurons, accounting for 40 % of the whole population, which modulate the activity of pyramidal projecting neurons [19]. The substrate for such modulation is feedback, feedforward and lateral inhibition. All PV+ neurons belong to the fast-spiking cells category which display narrow action potentials, little or no spike-frequency adaptation, rapid membrane kinetics and produce precisely timed spikes. On the basis of their axonal ramification, PV+ interneurons of the cortex are described as “basket cells” (BCs) or “chandelier cells” (ChCs, also axo-axonic cells) [24,25]. The distribution of PV+ neurons is not uniform with the interspecies and interareal differences [12]. PV+ cell bodies are distributed across cortical layers L 2–6. Basket cells are concentrated in L 2–3 and L5. Chandelier cells are more numerous in L2–3 than L 4–6 [18,24,26,27].
PV+ basket cells
PV+ basket cells (BCs) are the most abundant type of inhibitory interneurons found in all neocortical areas and layers, except in L1 and it represents the largest population of INs in the neocortex. BCs were first described by R. y Cajal [7] in cerebellar cortex and named according to their terminal axonal arborizations surrounding the somata of target neurons, later named Purkyně cells. BCs somata has round or oval shape and smooth dendrites. Dendritic ramification radiates from the cell body and most BCs have dense local and horizontal axon arborization. In the neocortex, they form synaptic contacts with many neighbouring pyramidal neurons and interneurons targeting their cell bodies and proximal dendritic segments and regulate the activity of local neuronal circuits with high temporal precision. BCs similarly as other neocortical interneurons are generated in medial ganglionic eminence (MGE).
Three subclasses of BCs were differentiated accordance with classical descriptions. Large (LBCs) and small BCs (SBCs). In the neocortex LBCs are large, aspiny multipolar neurons (cell body 20 – 25 μm) that have their synapses (20 – 40 %) on target cells somata. Three to five primary dendrites originate from the cell body radiating in all directions. Their axons ascend to give rise to many long horizontally and vertically projecting axon collaterals in addition to their side branches which extend up to a distance of 900 –1000 μm from the cell body to terminate in pericellular plexuses (baskets) around the somata and proximal dendrites of other neurons [28]. LBCs are the primary source of horizontal inhibition and are sole inhibitory interneurons under direct thalamic (excitatory) influence, which is prevailed in 3 – 5 layers while approx. 50 % of them are located in layer 4 [9,29]. Axonal ramifications of BCs in L4 are most frequently local while in L5 were described as local and translaminar axons as well. Some BCs in layers 2–3 and 5–6 have axonal ramifications that span several columns. These findings indicate that some basket cells provide not only local inhibition in layer and column where they are located but also more distant translaminar and transcolumnar inhibition [9,13,19,28].
SBCs are similarly aspiny multipolar but also bi-tufted or bipolar cells with cell bodies up to 20 μm. Twenty – 30 % of their synaptics target is on the cell somata. Their axons exhibited frequently short curvy axonal branches distributed within the same layer as cell bodies [30]. Axonal arborizations are dense and obviously limited to one layer. The horizontal extent is more limited than in LBCs up to 300 μm.
Third category of BC is named according to their axonal plexuses resembling a “messy bird’s nest” as nest basket cells (NBCs) ([31]). NBCs are small (up to 20 μm) oval or multipolar cell bodies, giving rise to radially projecting aspiny dendrites. Axonal arborization is compact and forms a nest-like complicated plexuses around the cell body. About 2/3 of the axonal arborization lies within 150 μm from the soma indicating probably a local intracolumnar inhibitory effect. To activate one NBC, a larger number of pyramidal neurons is rather necessary for activation of other interneurons [31,32].
The comparative analyses of BCs in rat and monkey indicate that the axon originates from the cell body or one of the primary dendrites. Axons spread predominantly horizontally and axonal tree was mainly located within the same cortical layer as the soma. Axons are highly branched and establish local, dense synaptic connections with postsynaptic neurons. Morphometric analysis of basket cells from monkeys and rats indicate similarity in parametric measures. The soma area was about 90 μm2 in both representatives, number of primary dendrites was 6 – 7, total dendritic length was 2000 – 2300 μm. The average axonal horizontal and vertical spans were 750 – 810 μm respectively 550 – 570 μm [33].
PV+ chandelier cells (ChCs)
The chandelier cells are named according to the structure of their axon terminals which resembles a candelabrum and were first described by [34]. Chandelier cells represent aspiny interneurons, with oval, multipolar or bi-tufted cell body, and smooth radially oriented dendrites. Most of the ChCs originate in the most ventral MGE in rat starting at E 13.5. Smaller contingent is generated in the preoptic area. The peak of ChCs genesis is evident between E16–17. The axon exhibits extensive branching of its preterminal segments, which form short vertical rows of boutons resembling rows of candles in a chandelier. [34–36]. These terminals, also called cartridges selectively innervate only the axon initial segment (AIS) of pyramidal cells on which they form symmetrical (inhibitory) synapses (Fig. 3). ChCs never innervate other interneurons [24, 27]. ChCs have been found in layers II – VI and their higher density was demonstrated in L2, L3 and L6 [19, 37]. ChCs similarly as basket cells fire high frequency minimally adapting and have a higher firing frequency compared to basket cells. ChCs are considered to be the most powerful cortical inhibitory neurons due to the fact of being inhibitory (GABAergic) and distributed in majority of the cortical layers and also due to the AIS of pyramidal (excitatory) neurons being in strategical region where the action potential is generated. They typically express CBPs PV and CB, but not CR. The expression of neuropeptide is blocked with the exception of corticotropin – releasing factor [38]. ChCs represent a minority of the PV+ neurons population and their role in gamma oscillations should be clarified. Recently it was demonstrated that ChCs target the distal region of the axon initial segment containing voltage-gated K v 1.2 channels associated with the adhesion molecule Caspr2. The proximal region of the AIS is innervated by somatostatin (GABAergic) interneuron [39–41].
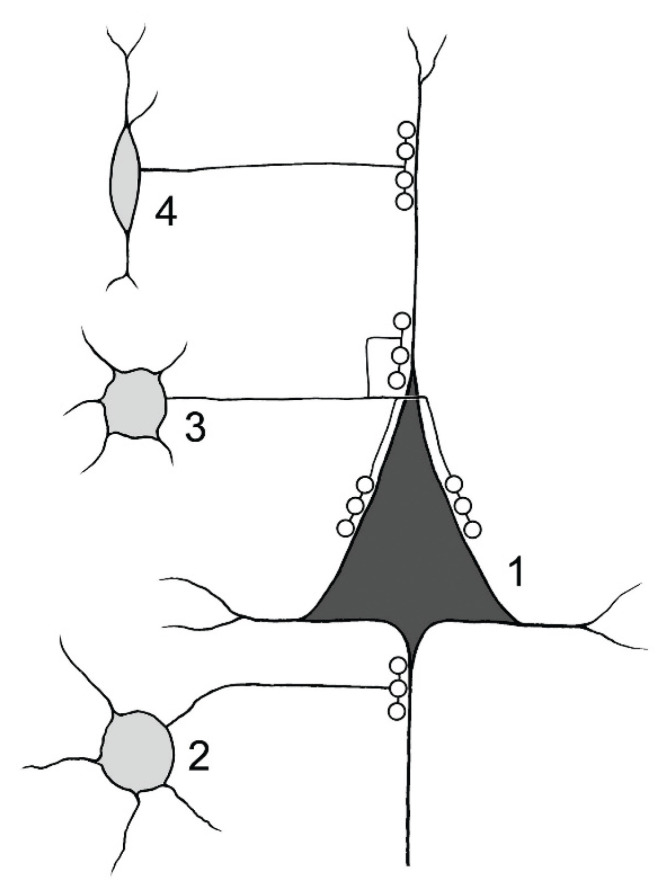
Fig. 3.
Synaptic contacts of inhibitory interneurons onto postsynaptic domains of pyramidal neuron 1). 2) chandelier cell (axo-axonic) innervating the axon initial segment of pyramidal cell. 3) basket cell innervating perisomatic domain of pyramidal cell. 4) somatostatin – ir neuron. Axon terminals innervate mainly peripheral dendrites of pyramidal neurons.
ChCs in some conditions can depolarize postsynaptic pyramidal cells due to elevated chloride potential in the axon initial segment [42].
More detailed analysis discovered two types of ChCs terminals in the mouse neocortex. Simple terminals were made up of 1 or two rows of boutons, each row consisting of 3 – 5 boutons. The complex terminals were cylinder-like structures containing multiple rows of boutons.
Simple terminals were detected throughout the cerebral cortex and claustroamygdaloid complex. The complex terminals were more frequent in associative temporal as well as in singular areas [41].
In the human neocortex complex, basket formations were most frequently found in area 4 followed by areas 3b, 13 and 18. They were mostly observed in layer 4 followed by layer 5. Majority of innervated neurons were pyramidal cells (70 %), while the remaining (30 %) were multipolar cells. Most multipolar cells expressed PV. Chandelier axon terminals exhibit the highest density in layer 2, followed by other layers. Concerning areal distribution of chandelier axon terminals their highest density was in prefrontal areas (9, 10, 11, 12, 13, 14) and temporal areas (20, 21, 22, 38). In other words, the sensory motor areas had high densities of complex basket formations and low densities of chandelier terminals, where association areas exhibited high densities of chandelier terminals and low densities of complex basket formations.
Long-range parvalbumin + efferent cortical neurons
Although expression of PV is evaluated as one of the characteristic features of GABAergic inhibitory cortical interneurons a recent studies indicate expression of PV also in long-range cortico-striatal, commissural and descending cortical neurons [43] demonstrated with the using of transgenic mouse lines retrogradely labelled PV+ neurons in the auditory cortex after injection of tracer into the auditory striatum. Corticostriatal PV+ neurons were observed in all subdivisions of the auditory cortex and the majority of them were located in the infragranular layers. Their axonal and dendritic morphology is similar to cortical PV+ basket cells.
In addition to PV+ corticostriatal projection, there were found long-range GABAergic somatostatin (SOM) expressing neurons in the auditory cortex regulating the activity of projecting neurons in the auditory sector of the striatum. [44] described PV expressing neurons entering the contralateral hemisphere via corpus callosum.
Part of the giant pyramidal cells in the monkey ‘s motor cortical area (area 4) contain PV [45] and Kv3. 1 potassium channels [46]. The PV labelling was lower than in the cortical interneurons.
Expression of PV was recently found also in human Betz cells - gigantopyramidal neurons of primary motor cortical area (BA 4). Szocsis et al. [47] demonstrated that around 70 % of Betz cells in the human cortex expressed PV, but in less intensity than the cortical interneurons.
Expression of PV and Kv3. 1 potassium channels in the Betz cells may be related to their fast-spiking characteristics and generation of frequent and thin spikes with short duration. Thus PV expression may be preconditioned for faster operation compared to other pyramidal neurons [47]. Another feature supporting functional characteristics of human PV+ Betz cell is direct thalamic input and high density of inhibitory perisomatic innervation. This inhibitory somatic input originates from fast- spiking PV+ basket cells.
PV as a member of calcium-binding proteins (CBPs) family
Parvalbumin (PV), calbindin (CB) and calretinin (CR) belong to the large family of EF-hand CBPs, which are characterized by the presence of a variable number of helix-loop-helix motifs binding Ca2++ ions with high affinity. Each PV molecule binds two calcium ions
Parvalbumin is a small, cytosolic low molecular Ca2++ binding protein freely mobile and completely fills axons, dendrites, neuronal somata and nuclei due its water solubility. Immunocytochemistry of PV in neocortex indicate strong positivity of cell body and nucleus in comparison with neuronal processes. In addition, PV was found in association with microtubules, postsynaptic densities and intracellular membranes [48,49]. Despite many years of intensive research which collected new data related to PV structure and physiochemical properties, the physiological functions are still not clear. PV consists of 106–113 amino acids residues and its molecular mass is 11–12 kDa. The alpha and beta isoforms were described which show differences in amino acid residues in 11 positions. Beta isoforms are also referred to as oncomodulins. PV exhibits high affinity to Ca2+ ions which compete with Mg2+ ions for the same binding sites. The affinity of PV with peptides is probably negative and no target proteins have been found for PV [50].
Physiological role of PV is oriented to manipulation with Ca2++ ions, namely to their transport, buffering and a protection of neurons against calcium overload. In neurons PV expression depends on their activity. Decreased activity causes a decrease of PV expression and decrease of PV messenger RNA.
PV is often described as a “calcium buffer”, but its activity is more complex than absorbing its excess levels. Initially PV accelerates the decay of calcium transient levels in axon boutons and dendrites and later slowly releases calcium and transmitter. This modulation facilitates coordinated rhythmic inhibition, which some authors regard as the main function of the PV interneuron system [51,52].
Development of PV+ neurons
In rodents, the embryonic subpallium (ganglionic eminence) generates all GABAergic INs. In non-human primates and in humans GABAergic neuronal progenitors were found in ganglionic eminence and also in the cortical subventricular zone.
During mouse brain development, the majority of PV+ interneurons migrating to the neocortex and hippocampus comes from the rostral part of the medial ganglionic eminence (MGE). MGE generates interneurons from E 13.5 [53]. Some PV+ interneurons are generated in the embryonic preoptic area [54]. Basket cells are born in embryonic days E13 to E17, while chandelier cells are generated later from E15 – to E18 [18].
The prenatal and postnatal development of PV+ neuron is complicated a process associated with extensive reorganization of axon and synaptic connectivity. During the first postnatal week in the mouse cortex, the majority of PV+ neurons migrate to their laminar destination and form synaptic contacts. The end of the first week is the period when many of PV+ neurons undergo apoptosis, where they do not integrate into the neuronal circuits [55, 56]. Parvalbumin expression appears between P 12 – P 14 and at that interval terminate the migration and integration of PV+ neurons or their disintegration and pruning and neurons obtain their electrophysiological properties. Myelination of proximal axonal branches occurs during first and second postnatal week while myelination of more distal axonal segments is evident during third and fourth postnatal week [55].
Molecular mechanism controlling interneuron development is very complicated and based on genetic cascades of transcription factors regulating all phases of this process. At the core of this development is the transcription factor Lhx6. LHX6 controls tangential and also radial migration of GABAergic interneurons from MGE to the cortex and their differentiation into PV expressing and SOM expressing cells. Lhx6 also supports the survival of MGE-derived interneurons [57,58].
Afferentation and Synaptic contacts on PV- ir neurons
For understanding the role of PV+ neurons in cortical circuits, it is important to reveal their excitatory and inhibitory inputs. PV+ interneurons cell bodies are targeted by several afferent systems. More than 90 % of afferents on PV+ interneurons are excitatory from pyramidal neurons. Small proportion of inhibitory inputs originate at other GABAergic interneurons such as SOM+ interneurons and other PV+ neurons. Dominant excitatory input and consecutive efferent innervation targeting pyramidal neurons by means of axo-somatic contacts is a substrate for strategic position of fast-spiking basket cells. PV+ neurons receive inhibitory inputs on proximal portions of dendrites while excitatory cortical inputs on distal dendrites [59].
Mapping afferent inputs of PV+ GABAergic neurons in L2 and L3 of barrel field with using monosynaptic rabies virus reveals two basic sources of afferent monosynaptic input. Local input is mainly from layer IV excitatory cells which drive the activity of PV+ cells to sharpen sensory responses. In addition, barrel field PV+ neurons are targeted from other distant sensory cortices (secondary somatosensory, visual, auditory) and from the thalamus. PV+ cells in L2 and L3 also receive projections from inhibitory L1 interneurons complementing inhibitory projections from other PV+ interneurons. Proportion of long-range cortical versus subcortical inputs as 64 % respectively 36 % of total inputs.
PV+ fast-spiking neurons are interconnected through chemical and electrical synapses. These connections were initially described on the cell bodies and proximal dendrites between neighbouring basket cells [32, 60]. However, more recent reports indicate that gap junctions are distributed also at the distal dendrites of PV+ neurons [61].
Quantitative analysis of axodendritic and axosomatic inhibitory inputs to PV+ neurons in the mice somatosensory cortex indicate that the density of GABAergic inputs was higher on the cell bodies than the dendrites, however GABAergic inputs per PV+ neuron was 6-fold more numerous on the dendrites than on the cell bodies. PV input terminates according to previous results on cell bodies of PV+ neurons [62,63] but recently published data indicate that the majority of PV inputs terminate on the dendrites and less frequently on cell bodies of PV+ neurons [64]. It was revealed in the same experiment that somatostatin (SOM) input also preferred dendritic termination than the somatic compartment of PV+ neurons. The density of SOM inputs to PV neurons was less than half of PV neurons and thus the dendrite of PV+ neurons are innervated most abundantly by PV neurons and less frequently by SOM neurons. Third category of GABAergic neurons expressing VIP peptide in contrast to PV and SOM inputs terminate on the cell bodies of PV+ neurons. VIP inputs represent approximately 60 % of GABAergic inputs to PV+ cell bodies and PV+ neurons are inhibited by the axosomatic input of VIP neurons. VIP neurons with bitufted or bipolar morphology and with vertically oriented axonal ramification may control the activity of pyramidal cells in cortical columns and simultaneously translaminary disinhibit PV neurons through the axosomatic inputs [64]. In addition to VIP neurons, the somatic compartment of PV+ neurons is targeted by CCK+ neurons which represent approximately 33 % of the axosomatic inputs [65].
Autaptic connections
Autapses are synapses made by a neuron onto itself. Inhibitory autapses are self-innervating synaptic connections in GABAergic interneurons. Several experimental studies reported GABAergic autapses in neocortical deep as well as superficial PV-expressing basket cells in rodents [66–68]. [68] analysed GABAergic autapses also in human neocortical tissue resected from frontal, temporal and occipital areas and demonstrated that GABA A receptors mediated inhibition is present in both species. Autaptic contacts were found in PV expressing basket cells in perisomatic position and on proximal dendrites. Comparison of human and mouse (somatosensory cortex) autaptic mechanism revealed similarity in structure and functional characteristics.
BCs exhibit autaptic (self-targeted) synaptic connections which are perisomatic and provide inhibitory feedback. It is estimated that 85–90 % of PV+ interneurons generate autaptic connections which represents about 40 % of inhibitory input to the perisomatic region. Autapses mediate fast modulation of PV+ interneurons and contribute to the regularity of spike timing and to synchronization of the PV+ interneurons. In addition, PV expressing basket cells self-inhibition may contribute to pyramidal cell disinhibition related to several cortical processes [52,66–68].
Efferentation of PV+ interneurons
Many PV+ neurons are basket cells and their axons target perisomatic regions of pyramidal neurons but only 30 % contact directly somas [28]. In addition to innervation of excitatory pyramidal neurons, PV+ interneurons innervate also other interneurons. PV+ cells together with VIP+ cells target Martinotti cells (MC). Such inhibitory connection was demonstrated in several sensory cortical areas in L2 and L3. Paired recordings revealed stronger synaptic input onto MC from PV+ cells than VIP cells. Inhibition of MC by other interneurons result in disinhibition of pyramidal neurons [69].
Electrical synapses between GABAergic interneurons
Simultaneous recording from paired cells revealed that inhibitory interneurons can be connected by two types of connections. In addition to chemical GABA-mediated synaptic contacts, inhibitory interneurons can also be connected via electrical synapses [70,71]. Electrical synapses are specialized zones of neuronal membrane where connexin-based (Cx36) gap junction channels bridge the plasmatic membrane of two neurons. Gap junctions act as low-resistance pathways which enable diffusion of small molecules and support synchronous activity of connected cells. Such arrangement increases the probability of synchronization of action potentials within networks of inhibitory neurons [72,73]. Such synchronization of firing activity of GABAergic neurons could coordinate the activity of other neuronal populations and can promote oscillatory rhythmic activity of neuronal nets [74]. Electrical synapses form a network of fast-spiking neocortical cells which have an important role in coordinating cortical activity [75,76]. Gap junctions are common between BCs and ChCs are typically dendro-dendritic or dendro-somatic and their effect is functionally excitatory [62]. According to [60] approximately 60 % of PV+ interneurons pairs were connected by gap junctions and 70 % by chemical synapses.
Although five classes of electrically coupled GABAergic neurons have been described so far, fast-spiking neurons including BCs and ChCs expressing PV are highly interconnected via electrical and GABAergic synapses in young and adult rodent neocortex. [60] demonstrated that electrical coupling among GABAergic PV+ interneuron is maintained in the adult neocortex. Other types of GABAergic interneurons exhibit different patterns of connectivity. For example, somatostatin-positive neurons are highly interconnected by electrical synapses but classical GABAergic synapses are rare [71]. Electrical synapses were not found among pyramidal neurons or between FS cells and other cortical neurons.
The spatial extent of the neuronal network formed by electrical synapses can extend over 100 μm and can contain about 100 neurons [77].
Receptors of PV-ir neurons
PV+ interneurons are the main source of inhibition in the cerebral cortex. Whereas their morphological and physiological characteristics have been studied extensively, the receptor equipment is a more recent field of investigation. Analysis of the three glutamate receptors (AMPA, NMDA, kainate) indicate their different expression in GABAergic interneurons. Different GABAergic interneurons exhibit lower GluR-B than pyramidal neurons. PV+ interneurons are characterized by AMPA receptors with high Ca++ permeability while VIP-positive interneurons are equipped with AMPA receptors with lower Ca++ permeability [78].
Studies on NMDA receptor expression indicate that cortical GABAergic interneurons express NR2A and NR2B and that PV+ and SOM+ neurons express preferentially NR2D subunit. Data about expression of metabotropic glutamate receptors in cortical GABAergic interneurons are sparse. Only part of SOM+, CB+ and CR+ neurons express mGluR1a while this subunit was not demonstrated in PV+ interneurons [79]. GABA A receptor subunits alpha1 and beta 2–3 are expressed in FS cells [79]
One of the neuromodulatory sources of activity onto the GABAergic interneuron is through nicotinic acetylcholine signalling. Nicotinic signalling plays important role in cognitive and memory processes. Neocortical interneurons are the major target of basal forebrain derived nicotinic signalling [80]. Recently, it was demonstrated that cortical interneurons express cholinergic receptors (nAChR) with distinct subunits compositions. Cholinergic transmission influences several types of interneurons including chandelier cells. Development of ChC axonal arborization requires some level of activity in subcortical cholinergic neurons located in the basal forebrain. Cholinergic system regulates the development of their axonal arborization as well as the signalling process in cortical networks [81].
Inhibitory neurons and circuits in superficial layers of the neocortex
The majority of neocortical neurons are excitatory (80 %) releasing glutamate onto their axonal targets. Excitatory neurons in layers L2/3 are pyramidal neurons with vertically oriented apical dendrites. Their short axonal projections innervate locally layer L2/3 and long axonal projections innervate distant cortical areas. L2 and L3 excitatory neurons are innervated by L4 and L5 excitatory neurons. In addition, L3 neurons are also innervated by the thalamus even though the main thalamic input terminates in L4.
Pyramidal neurons in layer L2/3 have lower firing rates than the excitatory neurons in deeper layers L4/5. Several reports demonstrated that a small population of excitatory neurons is more active than the vast majority which are much less active. These differences may be explained by strong inhibition influencing the majority of pyramidal neurons located in superficial cortical layers.
Approximately 20 % of neocortical neurons are GABAergic inhibitory interneurons. In L2/3, the largest group of interneurons (50 % of GABAergic population) expresses serotonin receptor 5-HT3A corresponding to the non-fast spiking GABAergic neurons. Subclass of this group expresses vasoactive intestinal peptide (VIP). The second largest group of L2/3 interneurons (30 %) are PV+ cells. These fast-spiking PV+ neurons densely innervate excitatory neurons, and are capable of firing at very high frequencies and producing their strong inhibition. PV+ neurons are divided into two classes:
Basket cells – synaptically target the soma and proximal dendrites of pyramidal neurons.
Chandelier (axo-axonic) cells which innervates the axon initial segment of pyramidal neurons.
PV+ basket and chandelier cells inhibit the perisomatic region of pyramidal neurons and control the frequency of their spiking.
The third group of L2/3 GABAergic interneurons (20 %) express the neuropeptide somatostatin also termed Martinotti cells [82] innervate distal dendrites of pyramidal neurons including their apical tuft in layer L1 (Fig. 4).
Fig. 4.
Simplified schematic presentation of cortical interneuron connectivity in cortical layers L 2/3. Different types of interneurons (white) provide inhibition to a pyramidal neuron (black). PVb – parvalbumin + basket cells target perisomatic region of pyramidal neurons. PVch – parvalbumin+ chandelier cells target axon initial segment of pyramidal neuron. M – Martinotti cells expressing somatostatin inhibit the distal dendritic segments of pyramidal neurons and VIP- positive interneuron. Vasoactive intestinal peptide (VIP) – positive interneurons innervate other interneurons (Martinotti and basket cells). Parvalbumin+ cells (basket and chandelier cells) are under excitatory influence of pyramidal neuron. Mutual and autaptic connections of basket cells are also depicted. 1 – 3 cortical layers. Modified according Laurenço et al. [97].
These three groups of interneurons differ in many physiological features. Among them is the average spontaneous firing rate which is highest in PV+ neurons, intermediate in SOM neurons and lowest in 5HT3A receptor expressing neurons. The spontaneous firing rate in all groups is significantly higher than in excitatory neurons in their vicinity. The sparse firing in excitatory neurons in comparison with high firing rates of inhibitory neurons indicate that the GABAergic neurons may be responsible for supressing the activity of excitatory neurons. The strongest output of excitatory pyramidal neuron is to PV+ neurons. The PV+ neurons strongly innervate other PV+ neurons and nearby excitatory neurons. These data indicate that excitatory neurons and PV+ neurons form highly connected networks and that strong excitatory inputs onto PV+ neurons contribute to their high firing rates and to the low firing rate of pyramids [14, 83, 84]. Since PV+ neurons innervate perisomatic compartments of many pyramidal neurons control their output and synchronize large networks of pyramidal cells. PV+ cells thus generate several brain rhythms associated with cognitive functions [85, 86].
VIP- expressing interneurons are mostly present in L2/3 and inhibit other interneurons (preferentially SOM+ neurons), thus providing disinhibition of cortical circuits [21].
Inhibitory neurons and circuits in layer 5
Layer 5 represents an infragranular layer with distinct morphological and physiological properties and with specific targeting of pyramidal neurons. Pyramidal neurons of layer 5 in contrast with pyramidal neurons located in superficial layers 2/3 fire at high rates and their firing rate exhibits reduction during sensory stimulation. A current research focused on cortical layer 5 inhibitory microcircuits revealed architecture of intralaminar as well as translaminar inhibition.
Intralaminar inhibition is based on activity of fast-spiking basket cells. PV+ cells are excited by pyramidal cells and in turn project onto the surrounding L5 pyramidal cells. It is estimated that each PV cell inhibits more than 1000 pyramidal cells [87]. This massive inhibition of pyramidal cells is derived from basket cells because projection from L5 chandelier cells onto L5 pyramidal cells was not reported [42]. Thus in L5 PV+ basket cells represent dominant inhibitory force [42].
In addition to intralaminar connections, activity of L5 neurons is regulated by a number of inhibitory influences from other cortical layers. Translaminar inhibition of L5 is based on input from GABAergic neurons located in other cortical layers that regulate activity of L5 pyramidal neurons. Other possibility is influence of translaminar excitatory afferents that activate local GABAergic cells. Other source of translaminar inhibition within L5 is input from interneurons in layers 2/3, 4 and 6.
Inhibition of other interneurons in L5 takes place at the final activity of the projecting neurons influenced by means of descending projections of many subcortical structures.
L5 PV+ interneurons form chemical and electrical synapses with other PV cells and thus form PV networks showing network synchrony and generating oscillations [71]. In contrast, another type of inhibitory interneurons expressing SOM and innervating dendrites of pyramidal neurons are not mutually interconnected, but do inhibit PV interneurons [88]. SOM interneurons can inhibit the dendritic compartment of pyramidal neurons and simultaneously disinhibit their somatic compartment by supressing PV+ cells. SOM interneurons also inhibit other interneurons, including VIP cells. In L5 PV and SOM cells are innervated by VIP cells. The dendritic compartment of L5 pyramidal neurons is innervated by SOM and VIP positive neurons. The somatic and perisomatic compartment is innervated by PV+ basket cells. AIS is a target of PV+ chandelier (axo-axonic) cells [42, 89].
Phylogenetic aspects of distribution of PV+ neurons
The phylogenetic variation in distribution of calcium-binding proteins (PV, CB, CR) suggests the existence of two major cortical formations. The first is characterized by a high degree of morphological differentiation of neocortical areas and balanced representation of the three calcium-binding proteins. This formation is evident in rodents, carnivores, lagomorphs, tree shrews and primates. In the second cortical formation there is less differentiated cortical plate, the lack or significant reduction of layer 4, the prevalence of magnocellular neurons and prominence of CR and CB positive interneurons in comparison to PV+ neurons. The second cortical formation is present in ungulates, cetaceans and artiodactyls (the superorder cetungulata). The prevalence of CB- and CR- positive neurons in cetaceans and artiodactyls is evaluated as an ancestral trait as these CBPs occur preferentially in phylogenetically older brain systems [90–92].
The distribution of neurons expressing CBPs has been described in the brains of laboratory animals such as rats, mauses, cats, macaque monkeys and in the human cerebral cortex. Several papers described main patterns of distribution of CBPs in other mammalian orders [12, 90, 91, 93].
PV + neurons in the neocortex of monotremata in platypus are present in layers III – VI and their morphology resembles basket cells. The primary sensory and motor areas contain more PV and CB immunoreactivity than other cortical areas. Large numbers of PV-ir neurons are observed in primary sensory representation of the bill [12]. In echidna PV+ large multipolar or pyramidal-like neurons were described in deep cortical layers (V and VI). There are more PV+ neurons in posterior cortical regions in both representatives.
In marsupials, low expression of PV+ neurons was demonstrated and it prevailed in the primary visual cortex. In insectivorous hedgehog, PV+ prevailed in layer V and their morphology resemble large stellate neurons.
Metabolism of PV+ neurons
Mitochondria are involved in many cellular functions including neuronal energy metabolism, redox homeostasis, macromolecule biosynthesis and others. The subpopulation of GABAergic interneurons expressing PV is characterized by high metabolic demands which are frequently associated with several brain disorders exhibiting mitochondrial dysfunction [94]. PV+ interneurons are subdivided into fast- spiking chandelier cells and basket cells. Several functional characteristics of BCs are associated with high level of their metabolism. BCs generate high-frequency action potentials (>50 Hz at 22 °C and >150 Hz at 34 °C) without accommodation.
Their morphology with multiple and extensively branching dendrites and dense axonal ramification allows them to receive prevailing excitatory inputs from pyramidal neurons and much smaller inhibitory inputs from other inhibitory interneurons (somatostatin interneurons, other PV+ neurons and autaptic terminals) and innervate pyramidal neurons. Their fast inhibitory control significantly influences activity of excitatory postsynaptic pyramidal neurons, namely their synchronized oscillatory activity specifically in gamma oscillations [95].
For their optimal function which covers fast-spiking generation, synaptic activities in extensive axonal arbor and for maintaining of axonal and dendritic transport and optimal mitochondrial function PV interneurons require continuous supply of oxygen and glucose. All these processes and namely fast-spiking signalling are more energy expensive. Such high metabolic demands correspond with greater number of mitochondria compared with other neurons and with generation of high levels of ATP in mitochondria [96]. In several studies was demonstrated bidirectional relationship between mitochondrial and PV dysfunction. PV knockout is associated with increase of mitochondrial volume and density while PV upregulation resulted in decrease of mitochondrial volume (Table 2 in [94]).
Generation of fast-spiking activity of PV-interneurons is related to K, 3.1 and K, 3.2 subunits of K+ channels which are specifically expressed in PV-interneurons and are implicated in their optimal rhythmic oscillatory activities [94].
Perineuronal nets in the PV+ neurons
Perineuronal nets (PNNs) are specialized extracellular matrix structures forming stable meshwork that surround cell bodies, axon initial segments and proximal dendrites of PV expressing GABAergic interneurons. In neocortex they are located around PV+ basket cells [97]. Basic structure of PNNs is hyaluronic acid (HA) which is secreted by membrane bound hyaluronic synthase attached to the cell surface. HA is formed by long polysaccharide composed of saccharide units and connected with chondroitin sulphate proteoglycans (aggrecan, versican, neurocan, brevican) by link proteins. HA has three-dimensional structure and is an important component of extracellular matrix. Aggrecan is most frequent protein in PNNs serving also as an immunohistochemical marker for their identification. Negatively charged long polysaccharide chain binds metal ions (Fe2+, Cu2+, Co2++, Zn2++) which have protective role against oxidative damage to HA. Anionic binding sites within the PNNs maintain non-toxic concentrations of metal ions in the local environment of neurons they enwrap [98]. Lectin staining for PNNs indicate the greatest density in layers 3 and 5 where are also PV+ neurons concentrated. PNNs distribution is nonhomogeneous and can differ between cortical regions and layers. PNN density is greater in primary sensory cortices than in associative cortical areas [52].
Several papers reported data regarding diurnal variations in the structure and physiology of PV+ cells and PNNs. Diurnal rhythmicity of PV expression was demonstrated in medial prefrontal cortex. The intensity of PNNs and PV levels was higher in the dark phase compared to the light phase [99].
In mouse visual cortex PNN degradation decreases inhibitory activity of PV+ interneurons [100]. PNNs play an important role in neural development where they limit central nervous system plasticity and stabilize synapses [98,101].
Parvalbumin and neuroplasticity
The brain is plastic and has capacity to remodel its neuronal activity in response to changing conditions throughout life. Many aspects of brain plasticity were identified by studies examining this phenomenon at cortical level. Cortical plasticity is associated with several processes including ageing, stress, ischemia, injury, spinal cord lesion and sensory learning [102–104]. During postnatal development structural and functional plasticity allows the cerebral cortex to adapt to the environment. These critical periods are needed to establish an optimal adaptation to the environment and several abilities can only be acquired during these periods [105]. An optimal excitatory/inhibitory balance is required for plasticity thus critical period onset is triggered by the maturation of the fast-spiking inhibitory PV interneurons which gradually become surrounded by the perineuronal nets [106].
A substantial body of evidence supports the view that inhibitory connections develop later than the excitatory ones and confirms crucial role of GABAergic inhibitory system in cortical plasticity both in development and adulthood [105–107]. The construction of the neocortex into a canonical circuits composed of excitatory and inhibitory cells enable hierarchical neuronal computation.
Such arrangement amplifies thalamic input and integrates that input with flow of information from other cortical regions to realize sensory processing, memory and learning [104].
Within the neocortex prevail excitatory neurons, whereas GABAergic inhibitory interneurons represent about 20 % of cortical neurons [9, 11]. Most abundant inhibitory neurons are PV expressing interneurons and here we review how these interneuron subtypes participate on some mechanisms of plasticity.
PV neurons generated at different times differ in connectivity, in PV and GAD 67 expression and in how they are regulated during experience- related plasticity [108].
Perineuronal nets preferentially surround PV- positive neurons, their proximal dendrites and their synapses. PNNs are an activity dependent and for formation of PNNs is necessary PV- positive neurons activity. Structure of PNNs fluctuates over the course of an animal’s life in response to sensory experience [109]. Regular development of PNN is necessary for termination of developmental critical period and genetic knockout pf PNNs components prevents critical period closure [110, 111].
There is evidence of mutual influence between the structure and maturity of PNNs and the activity of PV+ neurons [112]. Developed PNNs are necessary component for termination of critical periods. Genetic knockout of PNN components prevent critical period closure.
In mice, plasticity in the auditory cortex is associated with maternal behaviour. It was demonstrated that exposure to pups in Mecp2 mutant mouse results to an increase in expression of GAD67 and to overexpression of PV and PNNs associated with PV+ neurons in auditory cortex [113].
Pharmacogenetic suppression of PV+ cells re-introduced the ability of the visual V 1 network to show ocular dominance plasticity after critical period termination. Donato et al. [114] demonstrated that low level of PV expression was observed when plasticity was induced during learning. Activation of PV+ neurons prevented the switch to plasticity – associated low-PV level [20, 115].
The prefrontal cortex (PFC) regulates several cognitive functions, including attention, inhibiting emotional impulses, decision making and language comprehension [116]. PV neurons represent a major group of GABAergic interneurons in the PFC. In the mouse cortex, PV neurons appear at first in the sensory cortical areas (P 10 – P 13) and later (P 14) in PFC. The expression of PV could correlate with functional maturation of PV neurons. In Ueno et al. experiments was demonstrated that the expression of PV was reduced in the PFC and that PV neurons exhibit to show immature characteristics in the mature PFC including reduction of fully developed PNNs. Another important finding of this study is that PNNs in the mature PFC are not typical lattice-like structures and do not have the major components of PNNs and tenascin-R [107].
PNNs are part of the molecular brakes that decrease plasticity and close the critical period. An emerging view is that brain is genetically determined but also plastic. Adult plasticity is reduced by molecular brakes that limit its recovery after critical period closure. However, plasticity can be reopened after critical period closure either by reinstalling lower levels of inhibition or by amplifying the molecular brakes. In this process disrupting of PNNs cold play important role [117,107].
An important role of PV interneurons surrounding PNNs in the hippocampus and the anterior cingulate cortex in contextual fear memory was explained in Shi et al. study [118]. Increasing PNN expression in these cortical formations enhances the recall and reconsolidation of both recent and remote fear memory. Otherwise, removal of PNNs impairs consolidation and reconsolidation of both recent and remote fear memory. In the medial prefrontal cortex PNN removal disrupts long-term memories as well as fear conditioned memories [119].
Then the expression of PNNs surrounding PV interneurons is important in memory consolidation and storage and exert modulatory effect on behavioural plasticity.
PNNs also regulates other types of memory among them social memory, spatial memory and object recognition memory. The main neurodegenerative disease associated with memory loss is Alzheimer’s disease and several components of the extracellular matrix are implicated in progression of this condition [120].
Modification of PNNs and chondroitin sulphate proteoglycans has various effect on local synaptic properties and there is an overall increase in network activity caused to an overall reduction of inhibitory activity. PNNs degradation produce a variety of electrophysiological responses in PV interneurons but in most case is evident reduction of their function (a decrease in firing rate) and their returning to a less mature state. In contrast PNNs removal produced only few changes in electrophysiological properties of principal neurons [121].
Conclusions
Parvalbumin expressing GABAergic interneurons are the largest population of inhibitory neocortical cells. The majority of PV+ interneurons generate from the rostral part of the medial ganglionic eminence. The molecular mechanism controlling the interneuron development is complicated and is driven by genetic cascades of transcription factors. At the core of this process lays the transcription factor Lhx6.
The distribution of PV+ neurons is not uniformed within interspecies and interareal differences. The basis of axonal ramification is described as basket or chandelier (axo-axonic) cells. PV+ neurons are distributed across cortical layers 2 – 6 with prevalence to supragranular layers. All PV+ neurons belong to the category of fast-spiking neurons providing very fast, strong, and precise inhibition to their postsynaptic cells. In addition to strong inhibitory effects, they have been shown to generate gamma oscillations. Their extensive axonal arborization inhibits mainly the cell body, proximal dendrites and axon initial segment of their postsynaptic targets. Although PV+ cells are evaluated as an important component of cortical inhibitory interneuronal systems, it was demonstrated recently that some of them belong to the category of long-range GABAergic neurons projecting to distant cortical and subcortical areas. PV+ interneurons are targeted by several afferent systems.
Majority of afferents on PV+ interneurons are excitatory from pyramidal neurons and from the thalamus. Smaller proportion is inhibitory from other PV+ neurons and from SOM, VIP and CCK interneurons. BCs exhibit also autaptic synaptic (self-targeted) connections which represent about half of inhibitory input to the perisomatic region. Autapses contribute to the regularity of spike timing and to synchronization of PV+ interneurons and to pyramidal cells disinhibition.
Efferent connections of PV+ neurons innervate mainly excitatory pyramidal neurons but also other interneurons.
Inhibitory interneurons can be connected by chemical GABA-mediated synaptic contacts and also via electrical synapses (gap junctions). Gap junctions are common between BCs and ChCs. Electrical synapses form a network of fast-spiking neurons and increase the probability of their synchronization. PV+ cells form important components of neuronal circuits in the superficial as well as in the deep cortical layers.
PV+ interneurons have extraordinary energy requirements to support a high metabolic activity needed for optimal function of extensive axonal and dendritic ramification and for fast-spiking and gamma oscillation generation. The high metabolic demands correspond to a greater number of mitochondria compared to other neurons.
The functional importance of PV+ interneurons in operations of cortical microcircuits is stressed by the existence of intracellular as well as extracellular protective mechanisms. Intracellular parvalbumin protects neurons against calcium overload and its expression depends on activity of PV+ interneurons. Extracellular perineuronal nets form a stable meshwork of hyaluronic acid and associated proteoglycans that surround PV expressing GABAergic interneurons. Anionic binding sites within the nets maintain non-toxic concentration of metal ions in the local environment of neurons they enwrap.
The emerging literature suggests that preservation of these unique inhibitory neurons could support their role in several higher network functions.
Acknowledgements
This work was supported by Grant Agency of Charles University, Grant no. 35407.
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- 1.Filimonov IN. Archicortex and intermediate cortex. Moscow: Publ. Hause of the Acad. Med. Sci., Moscow; 1949. Comparative anatomy of the cerebral cortex of mammals. Paleocortex. [Google Scholar]
- 2.Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig: Leipzig: Barth; 1909. [Google Scholar]
- 3.Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120:701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- 4.Wiesel TN, Hubel DH. Ordered arrangement of orientation columns in monkeys lacking visual experience. J Comp Neurol, 1974;158:307–318. doi: 10.1002/cne.901580306. [DOI] [PubMed] [Google Scholar]
- 5.Brodal P. The Central nervous System. Oxford University Press; 1992. p. 464. [Google Scholar]
- 6.Szentagothai J. The ‘module-concept’ in cerebral cortex architecture. Brain Res, 1975;95:475–496. doi: 10.1016/0006-8993(75)90122-5. [DOI] [PubMed] [Google Scholar]
- 7.Ramón y Cajal S. Histology of the nervous system of man and vertebrates. II. Paris (in French): A Maloine; 1911. pp. 1–993. [Google Scholar]
- 8.Ramón y Cajal S. Recollections of my life. Vol. 1. Cambridge: MIT Press; 1937. p. 50. [Google Scholar]
- 9.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 10.Barinka F, Druga R. Calretinin expression in the mammalian neocortex: a review. Physiol Res. 2010;59:665–677. doi: 10.33549/physiolres.931930. [DOI] [PubMed] [Google Scholar]
- 11.DeFelipe J, Lopez-Cruz PL, Bielza C, Larranga P, Anderson S, Burkhalter A, Cauli B, et al. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci. 2013;14:202–216. doi: 10.1038/nrn3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hof PR, Glezer II, Conde F, Flagg RA, Rubin MB, Nimchinsky EA, Vogt Weisenhorn DM. Cellular distribution of the calcium-binding proteins parvalbumin, calbindin, and calretinin in the neocortex of mammals: phylogenetic and developmental patterns. J Chem Neuroanat. 1999;16:77–116. doi: 10.1016/S0891-0618(98)00065-9. [DOI] [PubMed] [Google Scholar]
- 13.Kawaguchi Y, Kondo S. Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J Neurocytol. 2002;31:277–287. doi: 10.1023/A:1024126110356. [DOI] [PubMed] [Google Scholar]
- 14.Petersen CC, Crochet S. Synaptic computation and sensory processing in neocortical layer 2/3. Neuron, 2013;78:28–48. doi: 10.1016/j.neuron.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Buzsaki G. Large-scale recording of neuronal ensembles. Nat Neurosci, 2004;7:446–451. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- 16.Selten MH, van Bokhoven H, Nadif Kasri N. Inhibitory control of the excitatory/inhibitory balance in psychiatric disorders. F1000Res, 2018;7:23. doi: 10.12688/f1000research.12155.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferguson BR, Gao WJ. PV Interneurons: Critical Regulators of E/I Balance for Prefrontal Cortex-Dependent Behavior and Psychiatric Disorders. Front Neural Circuits. 2018;12:37. doi: 10.3389/fncir.2018.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taniguch H, Lu J, Huang ZJ. The spatial and temporal origin of chandelier cells in mouse neocortex. Science, 2013;339:70–74. doi: 10.1126/science.1227622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tremblay R, Lee S, Rudy B. GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron, 2016;91:260–292. doi: 10.1016/j.neuron.2016.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roux L, Buzsaki G. Tasks for inhibitory interneurons in intact brain circuits. Neuropharmacology. 2015;88:10–23. doi: 10.1016/j.neuropharm.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gulyas AI, Hajos N, Freund TN. Interneurons containing calretinin are specialized to control other interneurons in the rat hippocampus. J Neurosci, 1996;16:3397–3411. doi: 10.1523/JNEUROSCI.16-10-03397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gulyas AI, Hajos N, katona I, Freund TF. Interneurons are the local targets of hippocampal inhibitory cells which project to the medial septum. Eur J Neurosci. 2003;17:1861–1872. doi: 10.1046/j.1460-9568.2003.02630.x. [DOI] [PubMed] [Google Scholar]
- 23.Ruden JB, Dugan LL, Konradi C. Parvalbumin interneuron vulnerability and brain disorders. Neuropsychopharmacology. 2021;46:279–287. doi: 10.1038/s41386-020-0778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gabbott PL, Bacon SJ. Local circuit neurons in the medial prefrontal cortex (areas 24a, b, c, 25 and 32) in the monkey: II. Quantitative areal and laminar distributions. J Comp Neurol. 1996;364:609–36. doi: 10.1002/(SICI)1096-9861(19960122)364:4<609::AID-CNE2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 25.Gabbott PL, Dickie BG, Vaid RR, Headlam AJ, Bacon SJ. Local-circuit neurones in the medial prefrontal cortex (areas 25, 32 and 24b) in the rat: morphology and quantitative distribution. J Comp Neurol. 1997;377:465–499. doi: 10.1002/(SICI)1096-9861(19970127)377:4<465::AID-CNE1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 26.Hendry SH, Jones EG, Emson PC, Lawson DE, Haizmann CW, Streit P. Two classes of cortical GABA neurons defined by differential calcium binding protein immunoreactivities. Exp Brain Res. 1989;76:467–472. doi: 10.1007/BF00247904. [DOI] [PubMed] [Google Scholar]
- 27.Gabbott PL, Bacon SJ. Local circuit neurons in the medial prefrontal cortex (areas 24a,b,c, 25 and 32) in the monkey: I. Cell morphology and morphometrics. J Comp Neurol. 1996;364:567–608. doi: 10.1002/(SICI)1096-9861(19960122)364:4<567::AID-CNE1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 28.Kisvarday ZF. GABAergic networks of basket cells in the visual cortex. Prog Brain Res, 1992;90:385–405. doi: 10.1016/S0079-6123(08)63623-7. [DOI] [PubMed] [Google Scholar]
- 29.Krimer LS, Zaitsev AV, Czanner G, Kroner S, Gonzales-Burgos G, Povysheva NV, Iyengar S, et al. Cluster analysis-based physiological classification and morphological properties of inhibitory neurons in layers 2–3 of monkey dorsolateral prefrontal cortex. J Neurophysiol. 2005;94:3009–3022. doi: 10.1152/jn.00156.2005. [DOI] [PubMed] [Google Scholar]
- 30.Fairen A, DeFelipe J, Regidor J. Non-pyramidal neurons: General account. In: Peters A, Jones EG, editors. Cerebral cortex, vol.1, Cellular components of the cerebral cortex. New York: Plenum Press; pp. 201–253. [Google Scholar]
- 31.Wang Y, Gupta A, Toledo-Rodriguez M, Wu CZ, Markram H. Anatomical, physiological, molecular and circuit properties of nest basket cells in the developing somatosensory cortex. Cereb Cortex. 2002;12:395–410. doi: 10.1093/cercor/12.4.395. [DOI] [PubMed] [Google Scholar]
- 32.Gupta A, Wang Y, Markram H. Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science. 2000;287:273–278. doi: 10.1126/science.287.5451.273. [DOI] [PubMed] [Google Scholar]
- 33.Povysheva NV, Zaitsev AV, Rotaru Dc, Gonzalez-Burgos G, Lewis DA, Krimer LS. Parvalbumin-positive basket interneurons in monkey and rat prefrontal cortex. J Neurophysiol. 2008;100:2348–60. doi: 10.1152/jn.90396.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szentagothai J, Arbib MA. Conceptual models of neural organization. Neurosci Res Program Bull. 1974;12:305–510. [PubMed] [Google Scholar]
- 35.Jones EG. Varieties and distribution of non-pyramidal cells in the somatic sensory cortex of the squirrel monkey. J Comp Neurol, 1975;160:205–267. doi: 10.1002/cne.901600204. [DOI] [PubMed] [Google Scholar]
- 36.Defelipe J, Gonzalez-Albo MC, Del Rio MR, Elston GN. Distribution and patterns of connectivity of interneurons containing calbindin, calretinin, and parvalbumin in visual areas of the occipital and temporal lobes of the macaque monkey. J Comp Neurol. 1999;412:515–526. doi: 10.1002/(SICI)1096-9861(19990927)412:3<515::AID-CNE10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 37.Inan M, Anderson SA. The chandelier cell, form and function. Curr Opin Neurobiol. 2014;26:142–148. doi: 10.1016/j.conb.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeFelipe J, Farinas I. The pyramidal neuron of the cerebral cortex: morphological and chemical characteristics of the synaptic inputs. Prog Neurobiol. 1992;39:563–607. doi: 10.1016/0301-0082(92)90015-7. [DOI] [PubMed] [Google Scholar]
- 39.Gonchar Y, Turney S, Price JL, Burkhalter A. Axo-axonic synapses formed by somatostatin-expressing GABAergic neurons in rat and monkey visual cortex. J Comp Neurol. 2002;443:1–14. doi: 10.1002/cne.1425. [DOI] [PubMed] [Google Scholar]
- 40.Howard A, Tamas G, Soltesz I. Lighting the chandelier: new vistas for axo-axonic cells. Trends Neurosci. 2005;28:310–316. doi: 10.1016/j.tins.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Inda MC, DeFelipe J, Munoz A. Morphology and distribution of chandelier cell axon terminals in the mouse cerebral cortex and claustroamygdaloid complex. Cereb Cortex. 2009;19:41–54. doi: 10.1093/cercor/bhn057. [DOI] [PubMed] [Google Scholar]
- 42.Naka A, Adesnik H. Inhibitory Circuits in Cortical Layer 5. Front Neural Circuits. 2016;10:35. doi: 10.3389/fncir.2016.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertero A, Zurita H, Normandin M, Apicella AJ. Auditory Long-Range Parvalbumin Cortico-Striatal Neurons. Front Neural Circuits. 2020;14:45. doi: 10.3389/fncir.2020.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zurita H, Feyen PLC, Apicella AJ. Layer 5 Callosal parvalbumin-expressing neurons: a distinct functional group of GABAergic neurons. Front Cell Neurosci. 2018;12:53. doi: 10.3389/fncel.2018.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Preuss TM, Kaas JH. Parvalbumin-like immunoreactivity of layer V pyramidal cells in the motor and somatosensory cortex of adult primates. Brain Res. 1996;712:353–357. doi: 10.1016/0006-8993(95)01531-0. [DOI] [PubMed] [Google Scholar]
- 46.Ichinohe N, Watakabe A, Miyashita T, Yamamori T, Hashikawa T, Rockland KS. A voltage-gated potassium channel, Kv3.1b, is expressed by a subpopulation of large pyramidal neurons in layer 5 of the macaque monkey cortex. Neuroscience. 2004;129:179–85. doi: 10.1016/j.neuroscience.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 47.Szocsics P, Papp P, Havas L, Watanabe M, Maglocky Z. Perisomatic innervation and neurochemical features of giant pyramidal neurons in both hemispheres of the human primary motor cortex. Brain Struct Funct. 2021;226:281–296. doi: 10.1007/s00429-020-02182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stichel CC, Singer W, Heizmann CW, Norman AW. Immunohistochemical localization of calcium-binding proteins, parvalbumin and calbindin-D 28k, in the adult and developing visual cortex of cats: a light and electron microscopic study. J Comp Neurol. 1987;262:563–577. doi: 10.1002/cne.902620409. [DOI] [PubMed] [Google Scholar]
- 49.Schwaller B. Cytosolic Ca2+ buffers. Cold Spring Harb Perspect Biol. 2010;2:a004051. doi: 10.1101/cshperspect.a004051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Permyakov EA, Uversky VN. What is parvalbumin for? Biomolecules. 2022:12. doi: 10.3390/biom12050656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aponte Y, Bischofberger J, Jonas P. Efficient Ca2+ buffering in fast-spiking basket cells of rat hippocampus. J Physiol. 2008;586:2061–2075. doi: 10.1113/jphysiol.2007.147298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bucher EA, Collins JM, King AE, Vickers JC, Kirkcaldie MTK. Coherence and cognition in the cortex: the fundamental role of parvalbumin, myelin, and the perineuronal net. Brain Struct Funct. 2021;226:2041–2055. doi: 10.1007/s00429-021-02327-3. [DOI] [PubMed] [Google Scholar]
- 53.Bartholome O, de la Brassinne Bonardeaux O, Neirinckx V, Rogister B. A Composite Sketch of Fast-Spiking Parvalbumin-Positive Neurons. Cereb Cortex Commun. 2020;1:tgaa026. doi: 10.1093/texcom/tgaa026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gelman D, Griveau A, Dehorter N, Teissier A, Varela C, Pla R, Pierani A, et al. A wide diversity of cortical GABAergic interneurons derives from the embryonic preoptic area. J Neurosci. 2011;31:16570–16580. doi: 10.1523/JNEUROSCI.4068-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Micheva KD, Kiraly M, Perez MM, Madison DV. Extensive structural remodeling of the axonal arbors of parvalbumin basket cells during development in mouse neocortex. J Neurosci. 2021;41:9326–9339. doi: 10.1523/JNEUROSCI.0871-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong FK, Marin O. Developmental Cell Death in the Cerebral Cortex. Annu Rev Cell Dev Biol. 2019;35:523–542. doi: 10.1146/annurev-cellbio-100818-125204. [DOI] [PubMed] [Google Scholar]
- 57.Christodoulou O, Maragkos I, Antonakou V, Denaxa M. The development of MGE-derived cortical interneurons: An Lhx6 tale. Int J Dev Biol. 2022;66:43–49. doi: 10.1387/ijdb.210185md. [DOI] [PubMed] [Google Scholar]
- 58.Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- 59.Kameda H, Hioki H, Tanaka YH, Tanaka T, Sohn J, Sonomura T, Furuta T, et al. Parvalbumin-producing cortical interneurons receive inhibitory inputs on proximal portions and cortical excitatory inputs on distal dendrites. Eur J Neurosci. 2012;35:838–854. doi: 10.1111/j.1460-9568.2012.08027.x. [DOI] [PubMed] [Google Scholar]
- 60.Galarreta M, Hestrin S. Electrical and chemical synapses among parvalbumin fast-spiking GABAergic interneurons in adult mouse neocortex. Proc Natl Acad Sci U S A, 2002;99(19):12438–43. doi: 10.1073/pnas.192159599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fukuda T. Structural organization of the gap junction network in the cerebral cortex. Neuroscientist. 2007;13:199–207. doi: 10.1177/1073858406296760. [DOI] [PubMed] [Google Scholar]
- 62.Tamas G, Buhl EH, Lorincz A, Somogyi P. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci. 2000;3:366–371. doi: 10.1038/73936. [DOI] [PubMed] [Google Scholar]
- 63.Tamas G, Somogyi P, Buhl EH. Differentially interconnected networks of GABAergic interneurons in the visual cortex of the cat. J Neurosci. 1998;18:4255–4270. doi: 10.1523/JNEUROSCI.18-11-04255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hioki H, Okamoto S, Konno M, Kameda H, Sohn J, Kuramoto E, Fujiyama F, et al. Cell type-specific inhibitory inputs to dendritic and somatic compartments of parvalbumin-expressing neocortical interneuron. J Neurosci. 2013;33:544–555. doi: 10.1523/JNEUROSCI.2255-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hioki H, Sohn J, Nakamura H, Okamoto S, Hwang J, Ishida Y, Takahashi M, et al. Preferential inputs from cholecystokinin-positive neurons to the somatic compartment of parvalbumin-expressing neurons in the mouse primary somatosensory cortex. Brain Res. 2018;1695:18–30. doi: 10.1016/j.brainres.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 66.Bacci A, Huguenard JR, Prince DA. Functional autaptic neurotransmission in fast-spiking interneurons: a novel form of feedback inhibition in the neocortex. J Neurosci. 2003;23:859–866. doi: 10.1523/JNEUROSCI.23-03-00859.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bacci A, Rudolph U, Huguenard JR, Prince DA. Major differences in inhibitory synaptic transmission onto two neocortical interneuron subclasses. J Neurosci. 2003;23:9664–9674. doi: 10.1523/JNEUROSCI.23-29-09664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Szegedi V, Paizs M, Baka J, Barzo P, Molnar G, Tamas G, lamsa K. Robust perisomatic GABAergic self-innervation inhibits basket cells in the human and mouse supragranular neocortex. Elife. 2020:9. doi: 10.7554/eLife.51691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walker F, Mock M, Feyerabend M, Guy J, Wagener RJ, Schubert D, Staiger JF, et al. Parvalbumin- and vasoactive intestinal polypeptide-expressing neocortical interneurons impose differential inhibition on Martinotti cells. Nat Commun. 2016;7:13664. doi: 10.1038/ncomms13664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–75. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- 71.Hestrin S, Galarreta M. Electrical synapses define networks of neocortical GABAergic neurons. Trends Neurosci. 2005;28:304–309. doi: 10.1016/j.tins.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 72.Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 73.Gibson JR, Beierlein M, Connors BW. Functional properties of electrical synapses between inhibitory interneurons of neocortical layer 4. J Neurophysiol. 2005;93:467–480. doi: 10.1152/jn.00520.2004. [DOI] [PubMed] [Google Scholar]
- 74.Buzsaki G. Rhytms of the brain. Oxford: Oxford University Press; 2006. [Google Scholar]
- 75.Fukuda T, Kosaka T. The dual network of GABAergic interneurons linked by both chemical and electrical synapses: a possible infrastructure of the cerebral cortex. Neurosci Res. 2000;38:123–130. doi: 10.1016/S0168-0102(00)00163-2. [DOI] [PubMed] [Google Scholar]
- 76.Fukuda T, Kosaka T. Gap junctions linking the dendritic network of GABAergic interneurons in the hippocampus. J Neurosci. 2000;20:1519–1528. doi: 10.1523/JNEUROSCI.20-04-01519.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Amitai Y, Gibson JR, Beierlein M, Patrick SL, Ho AM, Connors BW, Golomb D. The spatial dimensions of electrically coupled networks of interneurons in the neocortex. J Neurosci. 2002;22:4142–4152. doi: 10.1523/JNEUROSCI.22-10-04142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Blatow M, Caputi A, Burnashev N, Monyer H, Rozov A. Ca2+ buffer saturation underlies paired pulse facilitation in calbindin-D28k-containing terminals. Neuron. 2003;38:79–88. doi: 10.1016/S0896-6273(03)00196-X. [DOI] [PubMed] [Google Scholar]
- 79.Blatow M, Caputi A, Monyer H. Molecular diversity of neocortical GABAergic interneurones. J Physiol. 2005;562(Pt 1):99–105. doi: 10.1113/jphysiol.2004.078584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Demars MP, Morishita H. Cortical parvalbumin and somatostatin GABA neurons express distinct endogenous modulators of nicotinic acetylcholine receptors. Mol Brain. 2014;7:75. doi: 10.1186/s13041-014-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Steinecke A, Bolton MM, Taniguchi H. Neuromodulatory control of inhibitory network arborization in the developing postnatal neocortex. Sci Adv. 2022;8(10):eabe7192. doi: 10.1126/sciadv.abe7192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fanselow EE, Richardson KA, Connors BW. Selective, state-dependent activation of somatostatin-expressing inhibitory interneurons in mouse neocortex. J Neurophysiol. 2008;100:2640–2652. doi: 10.1152/jn.90691.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Avermann M, Tomm C, Mateo C, Gerstner W, Petersen CC. Microcircuits of excitatory and inhibitory neurons in layer 2/3 of mouse barrel cortex. J Neurophysiol. 2012;107:3116–3134. doi: 10.1152/jn.00917.2011. [DOI] [PubMed] [Google Scholar]
- 84.Adesnik H, Bruns W, Taniguchi H, Huang ZJ, Scanziani M. A neural circuit for spatial summation in visual cortex. Nature. 2012;490:226–231. doi: 10.1038/nature11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Buzsaki G. Neural syntax: cell assemblies, synapsembles, and readers. Neuron, 2010;68(3):362–385. doi: 10.1016/j.neuron.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Buzsaki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci, 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Packer AM, Yuste R. Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J Neurosci, 2011;31:13260–13271. doi: 10.1523/JNEUROSCI.3131-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tuncdemir SN, Wamsley B, Stam FJ, Osakada F, Goulding M, Callaway EM, Rudy B, et al. Early somatostatin interneuron connectivity mediates the maturation of deep layer cortical circuits. Neuron. 2016;89:521–535. doi: 10.1016/j.neuron.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kawaguchi Y, Otsuka T, Morishima M, Ushimaru M, Kubota Y. Control of excitatory hierarchical circuits by parvalbumin-FS basket cells in layer 5 of the frontal cortex: insights for cortical oscillations. J Neurophysiol. 2019;121:2222–2236. doi: 10.1152/jn.00778.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Glezer II, Hof PR, Leranth C, Morgane PJ. Calcium-binding protein-containing neuronal populations in mammalian visual cortex: a comparative study in whales, insectivores, bats, rodents, and primates. Cereb Cortex. 1993;3:249–272. doi: 10.1093/cercor/3.3.249. [DOI] [PubMed] [Google Scholar]
- 91.Glezer II, Hof PR, Morgane PJ. Comparative analysis of calcium-binding protein-immunoreactive neuronal populations in the auditory and visual systems of the bottlenose dolphin (Tursiops truncatus) and the macaque monkey (Macaca fascicularis) J Chem Neuroanat. 1998;15:203–237. doi: 10.1016/S0891-0618(98)00022-2. [DOI] [PubMed] [Google Scholar]
- 92.Hof PR, Schmitz C. Current trends in neurostereology - introduction to the special issue “Recent advances in neurostereology”. J Chem Neuroanat. 2000;20:3–5. doi: 10.1016/S0891-0618(00)00072-7. [DOI] [PubMed] [Google Scholar]
- 93.Hof PR, Bogaert YE, Rosenthal RE, Fiskum G. Distribution of neuronal populations containing neurofilament protein and calcium-binding proteins in the canine neocortex: regional analysis and cell typology. J Chem Neuroanat. 1996;11:81–98. doi: 10.1016/0891-0618(96)00126-3. https://doi.org/10.1016/0891-0618(96)00117-2, https://doi.org/10.1016/0891-0618(96)00126-3. [DOI] [PubMed] [Google Scholar]
- 94.Pinna A, Colasanti A. The neurometabolic basis of mood instability: the parvalbumin interneuron link-a systematic review and meta-analysis. Front Pharmacol. 2021;12:689473. doi: 10.3389/fphar.2021.689473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Buzsaki G. The structure of consciousness. Nature. 2007;446(7133):267. doi: 10.1038/446267a. [DOI] [PubMed] [Google Scholar]
- 96.Gulyas AI, Buzsaki G, Freund TF, Hirase H. Populations of hippocampal inhibitory neurons express different levels of cytochrome c. Eur J Neurosci. 2006;23:2581–2594. doi: 10.1111/j.1460-9568.2006.04814.x. [DOI] [PubMed] [Google Scholar]
- 97.Favuzzi E, Marques-Smith A, Deogracias R, Winterflood CM, Sanchez-Aguilera A, Mantoan L, Maeso P, et al. Activity-dependent gating of parvalbumin interneuron function by the perineuronal net protein brevican. Neuron. 2017;95:639–655 e10. doi: 10.1016/j.neuron.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 98.Burket JA, Webb JD, Deutsch SI. Perineuronal Nets and Metal Cation Concentrations in the Microenvironments of Fast-Spiking, Parvalbumin-Expressing GABAergic Interneurons: Relevance to Neurodevelopment and Neurodevelopmental Disorders. Biomolecules. 2021:11. doi: 10.3390/biom11081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Harkness JH, Gonzalez AE, Bushana PN, Jorgensen ET, Hegarty DM, Di Nardo AA, Prochiantz A, et al. Diurnal changes in perineuronal nets and parvalbumin neurons in the rat medial prefrontal cortex. Brain Struct Funct. 2021;226:1135–1153. doi: 10.1007/s00429-021-02229-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lensjo KK, Lepperod ME, Dick G, Hafting T, Fyhn M. Removal of perineuronal nets unlocks juvenile plasticity through network mechanisms of decreased inhibition and increased gamma activity. J Neurosci. 2017;37(5):1269–1283. doi: 10.1523/JNEUROSCI.2504-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McRae PA, Porter BE. The perineuronal net component of the extracellular matrix in plasticity and epilepsy. Neurochem Int. 2012;61:963–972. doi: 10.1016/j.neuint.2012.08.007-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Higo N, Nishimura Y, Murata Y, Oishi T, Yoshino-Saito K, Takahashi M, Tsuboi F, et al. Increased expression of the growth-associated protein 43 gene in the sensorimotor cortex of the macaque monkey after lesioning the lateral corticospinal tract. J Comp Neurol. 2009;516:493–506. doi: 10.1002/cne.22121. [DOI] [PubMed] [Google Scholar]
- 103.Cheatwood JL, Emerick AJ, Kartje GL. Neuronal plasticity and functional recovery after ischemic stroke. Top Stroke Rehabil. 2008;15:42–50. doi: 10.1310/tsr1501-42. [DOI] [PubMed] [Google Scholar]
- 104.Rupert DD, Shea SD. Parvalbumin-positive interneurons regulate cortical sensory plasticity in adulthood and development through shared mechanisms. Front Neural Circuits. 2022;16:886629. doi: 10.3389/fncir.2022.886629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327-. [DOI] [PubMed] [Google Scholar]
- 106.Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- 107.Ueno H, Suemitsu S, Okamoto M, Matsumoto Y, Ishihara T. Parvalbumin neurons and perineuronal nets in the mouse prefrontal cortex. Neuroscience. 2017;343:115–127. doi: 10.1016/j.neuroscience.2016.11.035. [DOI] [PubMed] [Google Scholar]
- 108.Caroni P. Regulation of Parvalbumin Basket cell plasticity in rule learning. Biochem Biophys Res Commun. 2015;460:100–103. doi: 10.1016/j.bbrc.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 109.Magnowska M, Gorkiewicz T, Suska A, Wawrzyniak M, Rutkowska-Wlodarczyk I, Kaczmarek L, Wlodarczyk J. Transient ECM protease activity promotes synaptic plasticity. Sci Rep. 2016;6:27757. doi: 10.1038/srep27757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hou X, Yoshioka N, Tsukano H, Sakai A, Miyata S, Watanabe Y, Yanagawa Y, et al. Chondroitin Sulfate Is Required for Onset and Offset of Critical Period Plasticity in Visual Cortex. Sci Rep. 2017;7:12646. doi: 10.1038/s41598-017-04007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ribic A. Stability in the face of change: lifelong experience-dependent plasticity in the sensory cortex. Front Cell Neurosci. 2020;14:76. doi: 10.3389/fncel.2020.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Devienne G, Picaud S, Cohen I, Piquet J, Tricoire L, Testa D, Di Nardo AA, et al. Regulation of perineuronal nets in the adult cortex by the activity of the cortical network. J Neurosci. 2021;41:5779–5790. doi: 10.1523/JNEUROSCI.0434-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Krishnan K, Lau BY, Ewall G, Huang ZJ, Shea SD. MECP2 regulates cortical plasticity underlying a learned behaviour in adult female mice. Nat Commun. 2017;8:14077. doi: 10.1038/ncomms14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Donato F, Rompani SB, Caroni P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature. 2013;504:272–276. doi: 10.1038/nature12866. [DOI] [PubMed] [Google Scholar]
- 115.Hensch TK. Bistable parvalbumin circuits pivotal for brain plasticity. Cell. 2014;156:17–19. doi: 10.1016/j.cell.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Medina J. Brain rules. Seattle: Pear press; 2008. pp. 1–301. [Google Scholar]
- 117.Bernard C, Prochiantz A. Otx2-PNN Interaction to Regulate Cortical Plasticity. Neural Plast. 2016;2016:7931693. doi: 10.1155/2016/7931693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shi W, Wei X, Wang X, Du S, Liu W, Song J, Wang Y. Perineuronal nets protect long-term memory by limiting activity-dependent inhibition from parvalbumin interneurons. Proc Natl Acad Sci U S A. 2019;116:27063–27073. doi: 10.1073/pnas.1902680116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hylin MJ, Orsi SA, Moore AN, Dash PK. Disruption of the perineuronal net in the hippocampus or medial prefrontal cortex impairs fear conditioning. Learn Mem. 2013;20:267–273. doi: 10.1101/lm.030197.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fawcett JW, Fyhn M, Jendelova P, Kwok JCF, Ruzicka J, Sorg BA. The extracellular matrix and perineuronal nets in memory. Mol Psychiatry. 2022;27:3192–3203. doi: 10.1038/s41380-022-01634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wingert JC, Sorg BA. Impact of perineuronal nets on electrophysiology of parvalbumin interneurons, principal neurons, and brain oscillations: a review. Front Synaptic Neurosci. 2021;13:673210. doi: 10.3389/fnsyn.2021.673210. [DOI] [PMC free article] [PubMed] [Google Scholar]