Abstract
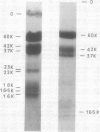
The major cytokinin binding protein of wheat germ (CBP) was extensively purified employing chromatography on Cibacron F3GA-Sepharose CL6B and concanavalin A-agarose as key purification steps. The major polypeptides present in the purified CBP preparations have molecular weights of 60,000 ± 4,000, 42,000 ± 3,000, and 37,000 ± 3,000, respectively. A protein kinase that catalyzes the phosphorylation of CBP (CBP kinase) was extensively purified from wheat germ by affinity chromatography on casein-Sepharose 4B and CBP-Sepharose 4B. The purification procedure resolves CBP kinase from an abundant casein kinase that does not phosphorylate CBP. CBP kinase catalyzes the phosphorylation of casein, phosvitin, CBP, and the wheat germ cyclic AMP-binding protein cABPII. CBP kinase phosphorylates the major 60,000 dalton subunit of CBP as well as 16,000 to 18,000 dalton polypeptides present in CBP preparations. CBP fractions with differing activities as substrates for CBP kinase were partly resolved by gel filtration and by chromatography on DEAE-Sephacel.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auricchio F., Migliaccio A. In vitro inactivation of oestrogen receptor by nuclei: prevention by phosphatase inhibitors. FEBS Lett. 1980 Aug 11;117(1):224–226. doi: 10.1016/0014-5793(80)80950-1. [DOI] [PubMed] [Google Scholar]
- Davies J. R., Polya G. M. Purification and properties of a high specific activity protein kinase from wheat germ. Plant Physiol. 1983 Mar;71(3):489–495. doi: 10.1104/pp.71.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaille J. G., Peters K. A., Fischer E. H. Isolation and properties of the rabbit skeletal muscle protein inhibitor of adenosine 3',5'-monophosphate dependent protein kinases. Biochemistry. 1977 Jul 12;16(14):3080–3086. doi: 10.1021/bi00633a006. [DOI] [PubMed] [Google Scholar]
- Erion J. L., Fox J. E. Purification and Properties of a Protein Which Binds Cytokinin-active 6-Substituted Purines. Plant Physiol. 1981 Jan;67(1):156–162. doi: 10.1104/pp.67.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. E., Erion J. L. A cytokinin binding protein from higher plant ribosomes. Biochem Biophys Res Commun. 1975 May 19;64(2):694–700. doi: 10.1016/0006-291x(75)90376-9. [DOI] [PubMed] [Google Scholar]
- Heyns W., De Moor P. A 3(17)beta-hydroxysteroid dehydrogenase in raterythrocytes. Conversion of 5alpha-dihydrotestosterone into 5alpha-androstane-3beta,17beta-diol and purification of the enzyme by affinity chromatography. Biochim Biophys Acta. 1974 Jul 17;358(1):1–13. doi: 10.1016/0005-2744(74)90251-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin P. P., Key J. L. Histone Kinase from Soybean Hypocotyls: PURIFICATION, PROPERTIES, AND SUBSTRATE SPECIFICITIES. Plant Physiol. 1980 Sep;66(3):360–367. doi: 10.1104/pp.66.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Moore F. H. A Cytokinin-binding Protein from Wheat Germ: Isolation by Affinity Chromatography and Properties. Plant Physiol. 1979 Oct;64(4):594–599. doi: 10.1104/pp.64.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G., Guilfoyle T. J., Key J. L. Isolation and Characterization of a Chromatin-associated Protein Kinase from Soybean. Plant Physiol. 1978 Jun;61(6):1023–1030. doi: 10.1104/pp.61.6.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G., Guilfoyle T. J., Key J. L. Isolation and preliminary characterization of a casein kinase from cauliflower nuclei. Plant Physiol. 1978 Sep;62(3):434–437. doi: 10.1104/pp.62.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polya G. M., Bowman J. A. Ligand Specificity of a High Affinity Cytokinin-binding Protein. Plant Physiol. 1979 Sep;64(3):387–392. doi: 10.1104/pp.64.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polya G. M., Bowman J. A. Resolution and Properties of Two High Affinity Cyclic Adenosine 3':5'-monophosphate-Binding Proteins from Wheat Germ. Plant Physiol. 1981 Sep;68(3):577–584. doi: 10.1104/pp.68.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph R. K., McCombs P. J., Tener G., Wojcik S. J. Evidence for modification of protein phosphorylation by cytokinins. Biochem J. 1972 Dec;130(4):901–911. doi: 10.1042/bj1300901a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranu R. S. Isolation of a translational inhibitor from wheat germ with protein kinase activity that phosphorylates initiation factor eIF-2. Biochem Biophys Res Commun. 1980 Dec 16;97(3):1124–1132. doi: 10.1016/0006-291x(80)91492-8. [DOI] [PubMed] [Google Scholar]
- Rosen O. M., Rangel-Aldao R., Erlichman J. Soluble cyclic AMP-dependent protein kinases: review of the enzyme isolated from bovine cardiac muscle. Curr Top Cell Regul. 1977;12:39–74. [PubMed] [Google Scholar]
- Rychlik W., Zagórski W. Purification and characterisation of adenosine-3',5'-phosphate-independent protein kinase from wheat germ. Eur J Biochem. 1980 May;106(2):653–659. doi: 10.1111/j.1432-1033.1980.tb04613.x. [DOI] [PubMed] [Google Scholar]
- Smilowitz H., Hadjian R. A., Dwyer J., Feinstein M. B. Regulation of acetylcholine receptor phosphorylation by calcium and calmodulin. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4708–4712. doi: 10.1073/pnas.78.8.4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel N. L., Tash J. S., Means A. R., Schrader W. T., O'Malley B. W. Phosphorylation of hen progesterone receptor by cAMP dependent protein kinase. Biochem Biophys Res Commun. 1981 Sep 16;102(1):513–519. doi: 10.1016/0006-291x(81)91549-7. [DOI] [PubMed] [Google Scholar]