Abstract
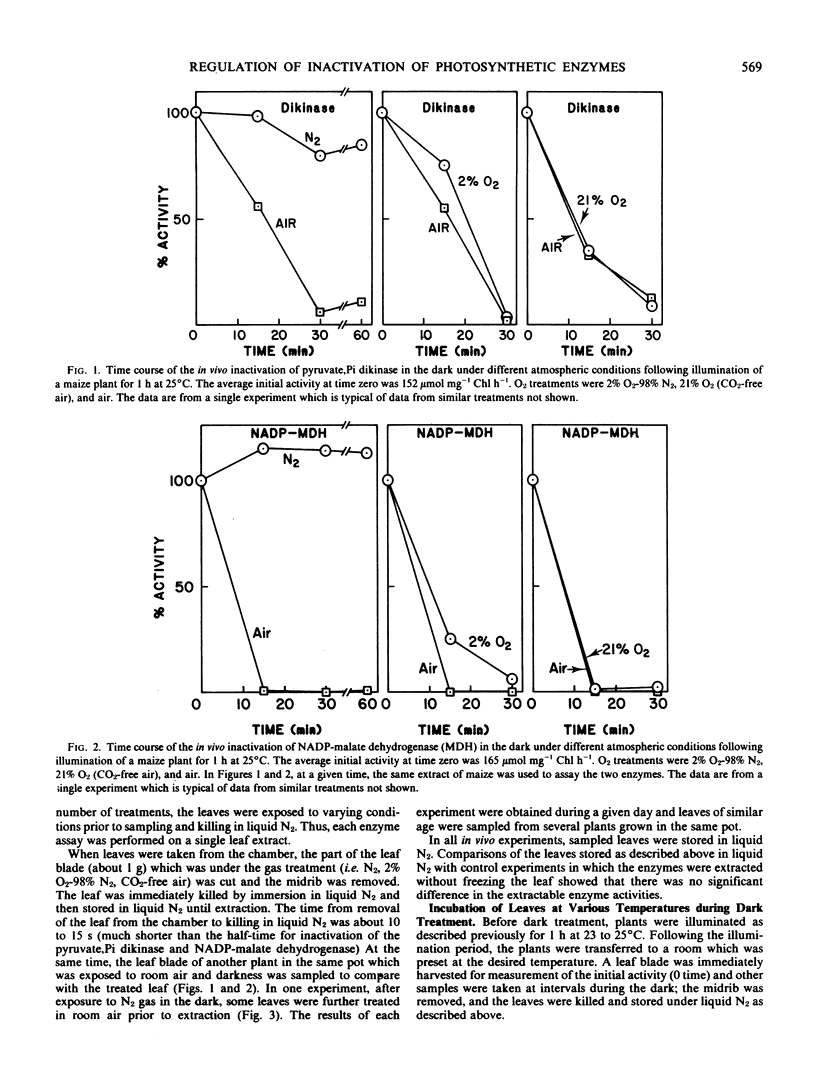
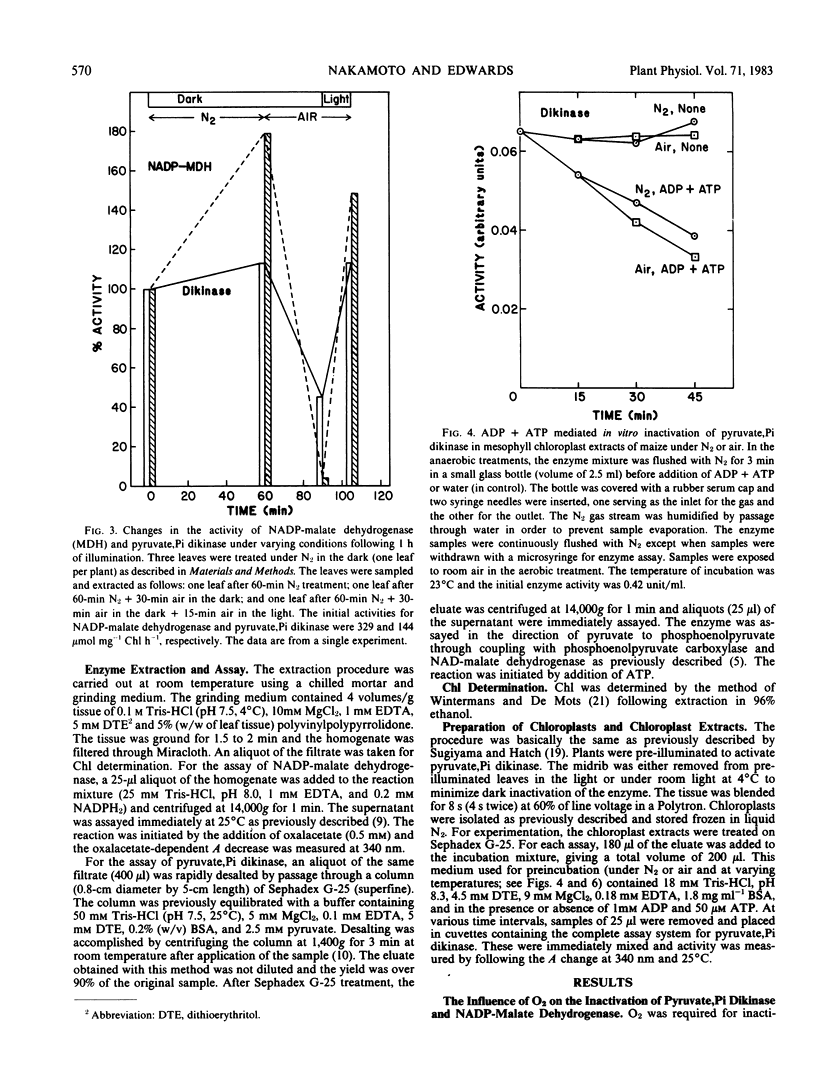
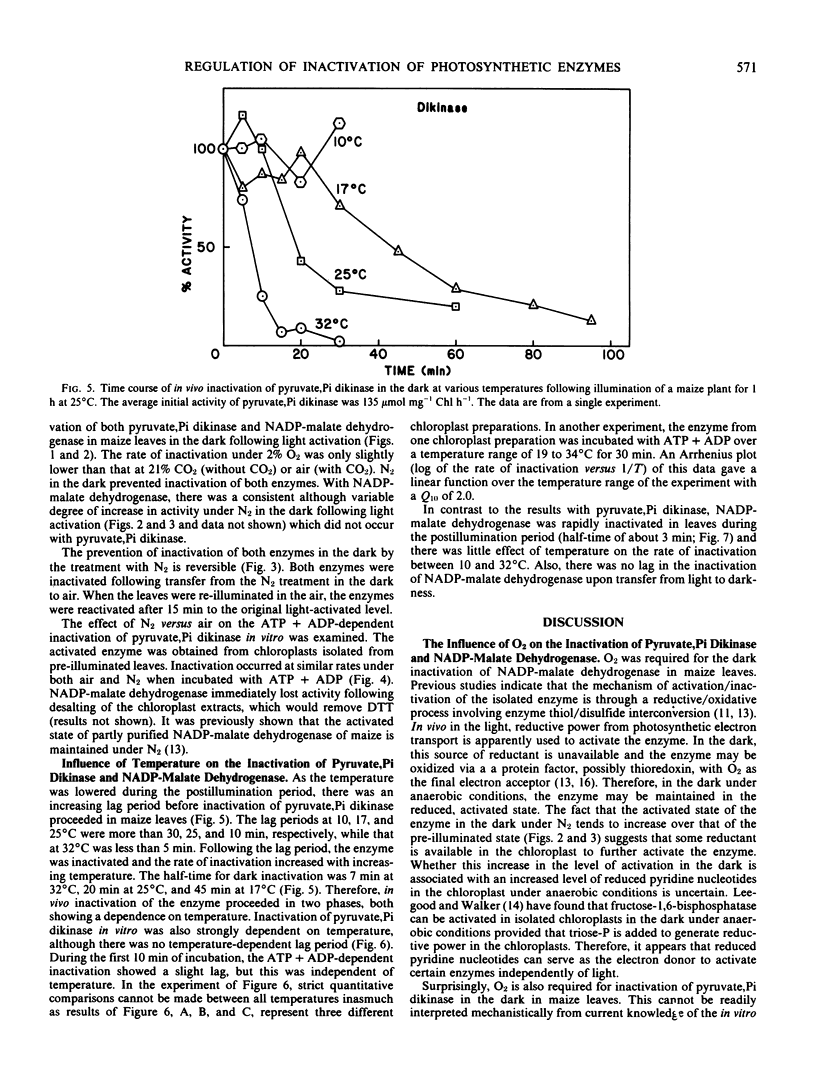
The influence of oxygen and temperature on the inactivation of pyruvate, Pi dikinase and NADP-malate dehydrogenase was studied in Zea mays. O2 was required for inactivation of both pyruvate, Pi dikinase and NADP-malate dehydrogenase in the dark in vivo. The rate of inactivation under 2% O2 was only slightly lower than that at 21% O2. The in vitro inactivation of pyruvate, Pi dikinase, while dependent on adenine nucleotides (ADP + ATP), did not require O2.
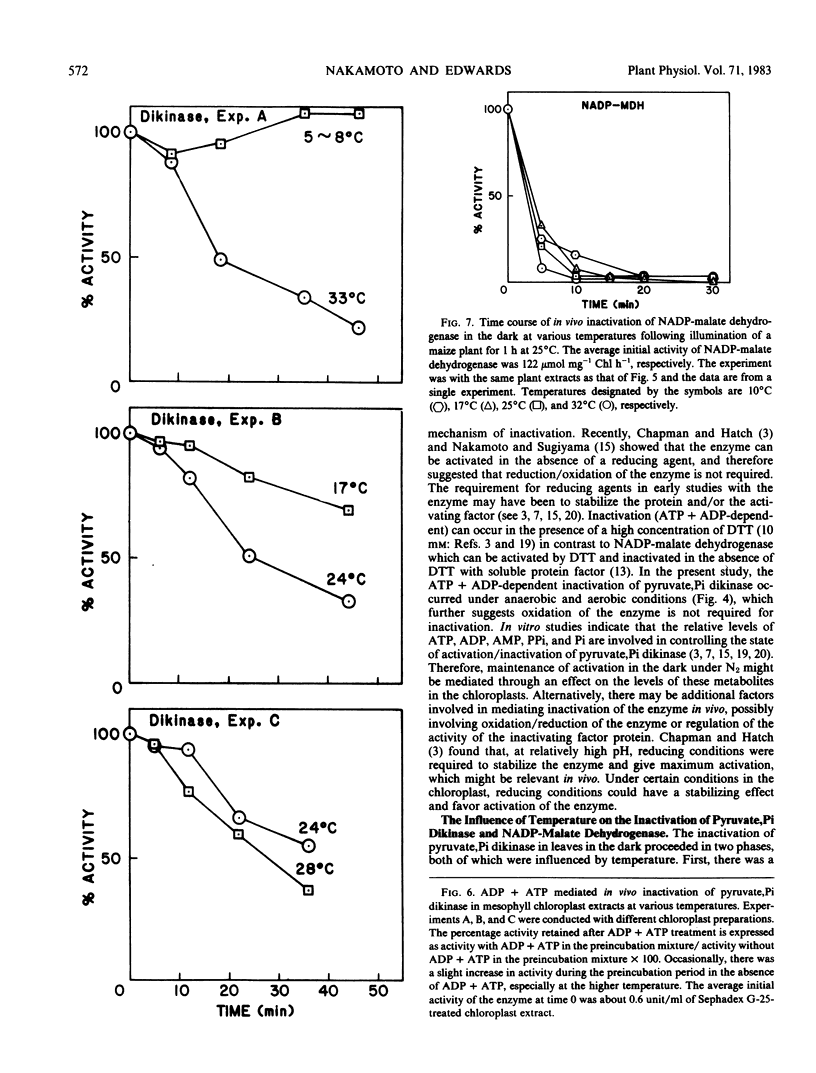
The postillumination inactivation of pyruvate, Pi dikinase in leaves was strongly dependent on temperature. As temperature was decreased in the dark, there was a lag period of increasing length (e.g. at 17°C there was a lag of about 25 minutes) before inactivation proceeded. Following the lag period, the rate of inactivation decreased with decreasing temperature. The half-time for dark inactivation was about 7 minutes at 32°C and 45 minutes at 17°C. The inactivation of pyruvate, Pi dikinase in vitro following extraction from illuminated leaves was also strongly dependent on temperature, but occurred without a lag period. In contrast, NADP-malate dehydrogenase was rapidly inactivated in leaves (half-time of approximately 3 minutes) during the postillumination period without a lag, and there was little effect of temperature between 10 and 32°C. The results are discussed in relation to known differences in the mechanism of activation/inactivation of the two enzymes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chapman K. S., Hatch M. D. Regulation of C4 photosynthesis: mechanism of activation and inactivation of extracted pyruvate, inorganic phosphate dikinase in relation to dark/light regulation. Arch Biochem Biophys. 1981 Aug;210(1):82–89. doi: 10.1016/0003-9861(81)90166-1. [DOI] [PubMed] [Google Scholar]
- Hatch M. D. Regulation of enzymes in C4 photosynthesis. Curr Top Cell Regul. 1978;14:1–27. [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. NADP-specific malate dehydrogenase and glycerate kinase in leaves and evidence for their location in chloroplasts. Biochem Biophys Res Commun. 1969 Mar 10;34(5):589–593. doi: 10.1016/0006-291x(69)90778-5. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. Studies on the mechanism of activation and inactivation of pyruvate, phosphate dikinase. A possible regulatory role for the enzyme in the C4 dicarboxylic acid pathway of photosynthesis. Biochem J. 1969 May;112(5):549–558. doi: 10.1042/bj1120549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmerhorst E., Stokes G. B. Microcentrifuge desalting: a rapid, quantitative method for desalting small amounts of protein. Anal Biochem. 1980 May 1;104(1):130–135. doi: 10.1016/0003-2697(80)90287-0. [DOI] [PubMed] [Google Scholar]
- Jacquot J. P., Buchanan B. B. Enzyme Regulation in C(4) Photosynthesis : PURIFICATION AND PROPERTIES OF THIOREDOXIN-LINKED NADP-MALATE DEHYDROGENASE FROM CORN LEAVES. Plant Physiol. 1981 Aug;68(2):300–304. doi: 10.1104/pp.68.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H. S., Hatch M. D. Properties and regulation of leaf nicotinamide-adenine dinucleotide phosphate-malate dehydrogenase and 'malic' enzyme in plants with the C4-dicarboxylic acid pathway of photosynthesis. Biochem J. 1970 Sep;119(2):273–280. doi: 10.1042/bj1190273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., Hatch M. D. Regulation of C4 photosynthesis: characterization of a protein factor mediating the activation and inactivation of NADP-malate dehydrogenase. Arch Biochem Biophys. 1977 Nov;184(1):290–297. doi: 10.1016/0003-9861(77)90353-8. [DOI] [PubMed] [Google Scholar]
- Leegood R. C., Walker D. A. Activation of fructose 1,6-bisphosphatase in darkened intact chloroplasts by NADPH. Arch Biochem Biophys. 1981 Dec;212(2):644–650. doi: 10.1016/0003-9861(81)90408-2. [DOI] [PubMed] [Google Scholar]
- Nakamoto H., Sugiyama T. Partial characterization of the in vitro activation of inactive pyruvate, pi dikinase from darkened maize leaves. Plant Physiol. 1982 Apr;69(4):749–753. doi: 10.1104/pp.69.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirahashi K., Hayakawa S., Sugiyama T. Cold lability of pyruvate, orthophosphate dikinase in the maize leaf. Plant Physiol. 1978 Nov;62(5):826–830. doi: 10.1104/pp.62.5.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]


