Abstract
Background
Vesicoureteral reflux (VUR) is one of the most common risk factors of urinary tract infection (UTI) among children. Various treatment modalities including antibiotic prophylaxis, surgical or endoscopic corrections and conservative treatment were used depending on the severity of VUR. The aim of this study is to compare the effectiveness of these treatment modalities in children with VUR grades II–IV by conducting a systematic review and network meta-analysis.
Methods
A systematic search from different databases was performed from their earliest records to December 2022 without any language restriction. Only randomised controlled trials were included in this study. Effectiveness of treatment modalities was mainly compared by UTI. Other outcomes for renal scarring and resolution by renal units were also measured between treatments.
Results
A total of 11 studies with 1447 children were included in this study. While comparing with antibiotic prophylaxis in network meta-analysis for UTI recurrence, surgical treatment probably lowers the rate of UTI recurrence (Log OR −0.26, 95% CI −0.54 to 0.02, high quality). However, endoscopic treatment (Log OR 0.2, 95% CI −1.41 to 1.81, high quality) and conservative treatment (Log OR 0.15, 95% CI −0.45 to 0.75, high quality) revealed probably inferior to antibiotic treatment.
Conclusion
Both pairwise and network meta-analytic results probably showed no difference between the treatments in terms of their impact on UTI recurrence, progression of previous renal scars, or formation of new renal scars in children with VUR grades II–IV. These findings may offer a better understanding of each treatment and evidence-based suggestions for the choice of treatment, which should be individualised and based on the patient’s risk factors.
Keywords: Statistics, Nephrology
WHAT IS ALREADY KNOWN ON THIS TOPIC
Reimplantation surgery provides a significantly better reflux resolution in children with vesicoureteral reflux (VUR).
WHAT THIS STUDY ADDS
There is no significant difference in urinary tract infection (UTI) recurrence rate, renal scar progressions and new renal scar formation in VUR grades II–IV between antibiotic prophylaxis, endoscopic surgery and reimplantation surgery.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The choice of treatment should be individualised and risk-based approach. Physicians’ and parents’ preference should also be considered because of no significant differences between antibiotic prophylaxis, endoscopic surgery and reimplantation surgery in preventing UTI recurrence and renal scarring.
Introduction
Primary vesicoureteral reflux (VUR), the reflux of urine into the ureter or the kidney due to anti-reflux failure in vesicoureteral junction,1 is a common risk factor of urinary tract infection (UTI) among children. The incidence of VUR among normal children is 0.5%–3%.2 However, in those with UTI combined with VUR, the incidence rises to 30%–40%.3 4 It is also a potential risk factor for various renal problems like pyelonephritis, renal scarring and chronic kidney disease.5
The grading of VUR is mostly defined by the use of radiographic classification based on the degree of filling and dilatation of the ureter, renal pelvis and calyces by the International Reflux Study group.6 Voiding cystourethrogram is the gold standard for diagnosing VUR and defining its severity. The severity of VUR can also be easily assessed with distal ureter diameter ratio and VUR index score which can also predict for resolution.7–9
Spontaneous resolution of VUR can be observed in about more than 80% of grades I and II, around 45% of grade III, and less than 10% of grades IV and V.10 Various treatment modalities including antibiotic prophylaxis (AbxP), surgical (Sx Rx) or endoscopic corrections (Endo Rx), and conservative treatment without antibiotic prophylaxis (no AbxP) are used depending on the severity of VUR and physicians’ preference.11 Each treatment’s effectiveness varies in preventing UTI and renal damage. Success rate also differs in each surgical correction method.12 13 With good resolution rates, non-operative management, such as AbxP and no AbxP, are preferred treatments for low-grade VUR. However, Sx Rx is reserved for high-grade VUR due to a potential risk of renal damage.14
Previous meta-analytic studies15–17 examined treatments mostly for low grades (I, II) and high grades (III, IV, V). However, in practice, children with grade V VUR is associated with a very high risk of recurrent UTI and renal scarring, and therefore, AbxP alone may not be sufficient for these patients and rarely enrolled in randomised controlled study. On the contrary, surgery is rarely used to treat grade I VUR patients. Having the high probability of rapid spontaneous resolution in VUR grade I, and concerning the high incidence of associated renal dysplasia or potential risk of renal damage in VUR grade V, the choice of treatment for these two grades is clear and more standardised. Therefore, most randomised controlled trials (RCTs) comparing AbxP, Endo Rx, or reimplantation include VUR grades II–IV patients. Herein, the aim of this study is to compare the effectiveness of these treatment modalities in managing children with VUR grades II–IV by conducting a systematic review and network meta-analysis.
Methods
Search strategy
A systematic search was conducted in different databases including PubMed, Embase and Google scholar using both free text and MESH terms (VUR; vesicoureteral reimplantation; endoscopic treatment or AbxP). All databases were searched from their inceptions to December 2022 without any language restriction. The search was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Involving a Network Meta-Analysis statement. The number of included and excluded studies was reported at each stage.
Selection criteria
Abstracts of the identified articles were manually reviewed, and full texts were assessed for those without clear eligibility. Only were RCT studies comparing any two of four treatments (vesicoureteral reimplantation, endoscopic treatment, AbxP, or surveillance with no AbxP) for managing primary VUR grades II–IV included in this study. Studies which examined treatments for VUR grades I–V and provided separate results for each grade were also eligible for inclusion.
Articles were excluded if treatment outcomes were not directly compared or if duplicate data on the same cohort were reported. Studies with primary VUR grade I or V and those with secondary VUR, such as posterior urethral valves, neurological abnormalities, other urological abnormalities, and kidney transplants, were also excluded.
Treatment modalities
Different treatment modalities for VUR grades II–IV reported in the included studies were AbxP, no AbxP, Sx Rx and Endo Rx.
Data extraction
Two investigators (C-LC and C-HC) extracted the data from each eligible study, including UTI, renal scarring for both old lesion progression and new scars formation, as well as resolution of VUR by cases and renal units. Another four investigators (C-KH, S-SDY and S-JC) checked the accuracy of extracted data, and a custom piloted spreadsheet was used for comparing those data for each variable of interest.
Outcomes
Primary outcome was to compare the rate of UTI according to the criteria defined by each study between treatment modalities.
Secondary outcomes were the rate of worsening of previous renal scars (ie, progression of old lesions) and formation of new renal scars usually followed by technetium-99 m-labelled dimercaptosuccinic acid (99mTc-DMSA) scintigraphy and also the resolution rate of VUR.
Risk-of-bias assessment
The Cochrane Collaboration risk of bias tool (RoB2) was used, and risks of bias, such as selection, performance, detection, attrition and reporting bias, were evaluated for each included study. Each item was rated as either low risk of bias, some concern (either lack of information or uncertainty over the potential for bias) or high risk of bias.
Statistical analysis
Pairwise comparisons between studies were performed by RevmanV. 5.4 software (www.cochrane.org), and R program software was used for conducting network meta-analysis. Frequentist model was adopted using netmeta package for estimating each treatment’s effect. The statistical heterogeneity between the studies was measured by I2 and Qtotal showing the overall inconsistency in the network. Network consistency was checked with netsplit method. We conducted a pooled analysis of dichotomous outcomes using ORs for pairwise comparisons and ORs in logarithmic scale (log ORs) for comparisons in network meta-analysis. Random-effect method was used to overcome the high heterogeneity between studies.
Certainty of the evidence
The certainty of the results from both pairwise comparisons and network meta-analysis was accessed with the methods provided in GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) handbook. Overall certainty of evidence was based on risk of bias, inconsistency, indirectness, imprecision and publication bias. Each result was graded into high, moderate, low or very low certainty.
Patient and public involvement statement
Patients or the public were not involved in the conduct of this systematic review and network meta-analysis study. The analyses were restricted to studies on children with VUR grade II–IV. The main target audience includes paediatricians, urologists, nephrologists and clinicians who have special interest in children with VUR.
Results
Search strategy and study characteristics
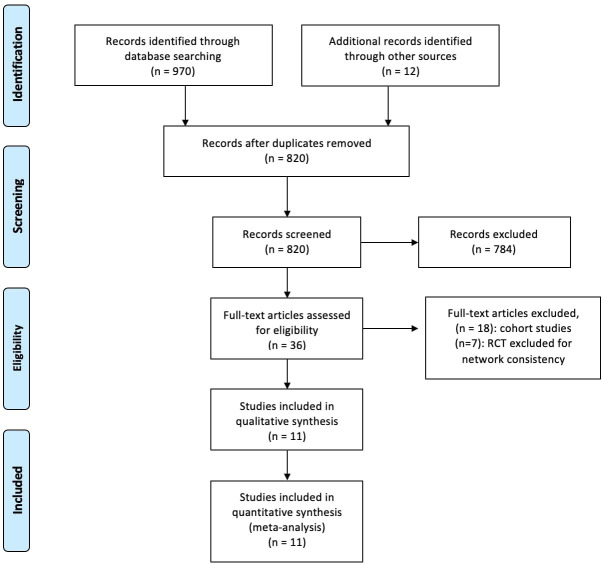
The selection of articles was conducted according to the PRISMA guidelines, and a total of 820 studies were initially selected. A final sample of 11 studies including 1447 children with VUR grades II–IV were included, and the detailed process of selection is demonstrated in figure 1. All 11 studies were RCTs and all of which were published in English language. The oldest age of enrolled children was 18 years. Follow-up periods varied from 1 to 5 years. Characteristics of the included studies are summarised in table 1.
Figure 1.
Research flow chart. RCT, randomised controlled trial.
Table 1.
Study characteristics of included studies
| Author/year | Country | VUR grade | Age | Follow-up | UTI definition | Comparisons |
| Hari 201520 | India | VUR grade III, IV | <12 years | 1 year | (+) UC | AbxP versus no AbxP |
| Craig 200921 | Australia | VUR grade III, IV | <18 years | 1 year | (+) UC | AbxP versus no AbxP |
| Pennesi 200822 | Italy | VUR grade II, III, IV | <2.5 years | 4 years | Febrile UTI | AbxP versus no AbxP |
| Olbing 199218 | Germany | VUR grade III, IV | <11 years | 5 years | No information | AbxP versus Sx Rx |
| Jodal 200619 | US | VUR grade III, IV | <11 years | 5 years | (+) UC | AbxP versus Sx Rx |
| Weiss 199223 | US | VUR grade III, IV | < 10 years | 4.5 years | No information | AbxP versus Sx Rx |
| BRSG 198324 | UK | VUR grade III or grade II with scarring |
>1 year | 2 years | (+) UC | Sx Rx versus AbxP |
| Garcia-Aparicio 201325 | Spain | VUR grade II, III, IV | >1 year | 5 years | No information | Endo Rx versus Sx Rx |
| Capozza 200226 | Italy | VUR grade II, III, IV | >1 year | 1 year | (+) UC | Endo Rx versus AbxP |
| Brandström 201127 | Sweden | VUR grade III, IV | 1–2 years | 2 years | Febrile UTI | Endo Rx versus AbxP versus no AbxP |
| Salih 202128 | Egypt | VUR grade III, IV | 10 years | 2 years | No information | Endo Rx versus Sx Rx |
AbxP, antibiotic prophylaxis; Sx Rx, surgical treatment; Endo Rx, endoscopic treatment; UC, urine culture; UTI, urinary tract infection; VUR, vesicoureteral reflux.
Risk of bias
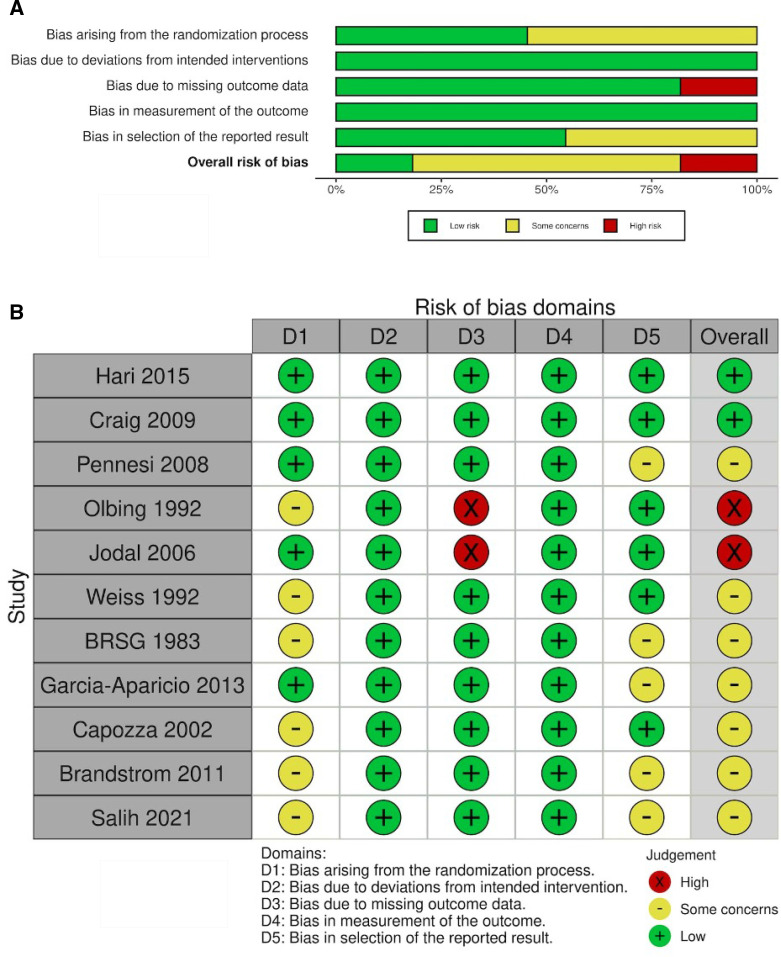
Nearly half of the included studies reported unclear information about randomisation, allocation and blinding of outcome assessment. Two studies18 19 had severe missing outcome data, and they were rated as high risk of bias in missing outcome data. Half of the included studies were considered for some concern as having bias in selection of reported results. For the overall bias, approximately 20% of the included studies were considered having a high risk of bias, and the results were summarised in figure 2A and B.
Figure 2.
(A) Risk of bias graph: each risk of bias component displayed as percentage across papers. (B) Risk of bias summary: each risk of bias component for each paper.
Evaluation of inconsistency and fitness of the model of the network meta-analysis
The network evidence of UTI for four treatment modalities was demonstrated with network graph (figure 3) including a total of nine studies. Our model showed two strong arms (AbxP vs no AbxP and AbxP vs Sx Rx) each including three studies, and it consisted of a closed loop between AbxP, Sx Rx and Endo Rx. Both results of direct and indirect methods calculated by the netsplit method did not show a significant difference between them. Therefore, no inconsistency was found in our model. For the fitness of model, only the studies which reported the outcomes of VUR grades II–IV were included, and fixed effect model was used due to overall low heterogeneity among studies (Q value=0.91).
Figure 3.
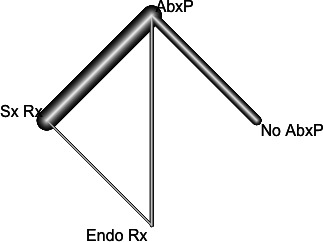
Network graph of each treatment for urinary tract infection.
Synthesis of results
In this study, the effectiveness of treatment modalities was pooled analysed with primary outcomes (UTI) simultaneously measured by network meta-analysis. Other outcomes such as renal scarring and resolution by renal units (RRU) were only analysed by pairwise meta-analysis due to limited studies between treatments.
Urinary tract infection
A total of 9 studies19–27 including 1013 participants reported the incidence of post-treatment UTI. The definitions of UTI were positive urine culture and symptomatic or febrile UTI. Some studies did not report information about UTI definition.
Pairwise comparisons of UTI between different treatment modalities
There was no significant difference in UTI recurrence among the treatment modalities. Sx Rx was associated with less UTI than AbxP, but the difference was not significant (OR=0.75, 95% CI 0.43 to 1.29, p=0.3). Endo Rx showed a higher risk of UTI than AbxP, but the difference was not significant (OR=2.03, 95% CI 0.89 to 4.64, p=0.09). Finally, there was no significant difference in UTI recurrence between AbxP or no AbxP (OR=1.07, 95% CI 0.51 to 2.24, p=0.86). All results for each treatment comparison are reported in table 2.
Table 2.
Results for pairwise comparisons of different treatment modalities
| Outcomes | Treatment comparisons Treatment (1) versus (2)—references of included studies |
Treatment (1) Total E/C (n/n) |
Treatment (2) Total E/C (n/n) |
OR (95% CI) |
| UTI | Sx Rx versus AbxP20–22 | 50/238 | 63/235 | 0.75 (0.43 to 1.29) |
| Endo Rx versus AbxP26 27 | 20/105 | 10/90 | 2.03 (0.89 to 4.64) | |
| AbxP versus No AbxP19 23 24 | 26/152 | 24/145 | 1.07 (0.51 to 2.24) | |
| Endo Rx versus Sx Rx25 | 2/22 | 0/19 | Not estimable | |
| Progression of old lesions | AbxP versus Sx Rx18 23 24 | 52/270 | 43/264 | 1.23 (0.79 to 1.93) |
| AbxP versus No AbxP22 | 1/50 | 9/50 | 0.09 (0.01 to 0.76) | |
| Formation of new renal scars | AbxP versus Sx Rx18 23 | 33/223 | 36/215 | 0.86 (0.51 to 1.44) |
| AbxP versus No AbxP27 | 0/69 | 9/68 | Not estimable | |
| AbxP versus Endo Rx27 | 0/69 | 6/66 | Not estimable | |
| Endo Rx versus No Abxb27 | 6/66 | 9/68 | 0.66 (0.22 to 1.96) | |
| RRU | Sx Rx versus Endo Rx25 28 | 77/80 | 66/80 | 5.02 (1.47 to 17.13) |
| Sx Rx versus AbxP24 | 67/69 | 17/65 | 94.59 (20.87 to 428.74) | |
| Endo Rx versus AbxP26 | 40/52 | 10/30 | 8.33 (3.14 to 22.13) |
AbxP, antibiotic prophylaxis; E/C, events/cases; Endo Rx, endoscopic treatment; RRU, resolution by renal units; Sx Rx, surgical treatment; UTI, urinary tract infection.
Results from network meta-analysis
Sx Rx showed the lowest risk of UTI compared with other treatments reporting in figure 4. However, the mixed comparison results were not significant with low heterogeneity.
Figure 4.
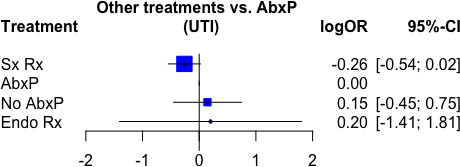
Comparison of urinary tract infection (UTI) recurrence after each treatment of vesicoureteral reflux (VUR).
Progression of old lesions
A total of four studies18 22–24 were pooled for the analysis. Three studies compared AbxP and Sx Rx, and one compared AbxP and no AbxP. The pooled result showed that AbxP had potential for more progression of old lesions than Sx Rx (OR=1.23, 95% CI 0.79 to 1.93, p=0.36), but the result was not significant.
Formation of new renal scar
A total of 3 studies18 23 27 with 641 participants were included. Two studies comparing AbxP and Sx Rx were pooled for pairwise comparison, and no significant result was found between them (OR=0.86, 95% CI 0.51 to 1.44, p=0.56). Another study compared AbxP, no AbxP and Endo Rx, and the results for these comparisons are reported in table 2.
Resolution by renal units
Of 4 studies24–26 28 which reported corrected VUR by renal units, 2 studies consisting of 160 participants compared Sx Rx and Endo Rx. The other two studies compared Sx Rx and AbxP as well as Endo Rx and AbxP. Sx Rx showed a significantly better resolution rate of VUR than Endo Rx (OR=5.02, 95% CI 1.47 to 17.13, p=0.01). Both Sx Rx and Endo Rx showed better resolutions than AbxP, and the results are reported in table 2.
Complications
Most of the included studies did not report about complications except two studies.19 25 Ureteral stricture is one of possible complications of Sx Rx. Long-term report of IRS study showed postoperative unilateral obstruction (6.6%, 10 in 151 patients) in which 7 patients (4.7%) needed further surgery.19 Garcia-Aparicio et al also reported mild postoperative complications with haematuria (5.2%) and bladder spasm (5.2%).25
Certainty of the evidence
About two-third of the results from pairwise comparison were rated as moderate certainty as there were high risk of bias in randomisation process and outcome data. Overall certainty of the evidence and summary of findings table for pairwise comparison were presented in table 3. For network meta-analysis, only surgical treatment was found having moderate certainty and the rest having high certainty. Certainty of evidence for each treatment was integrated with the results and the overall summary of findings were reported in table 4.
Table 3.
Summary of findings of GRADE analysis for pairwise comparisons
| Outcomes | No of participants (studies) Follow-up | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects | |
| Risk with control | Risk difference with intervention* | ||||
|
UTI recurrence (AbxP vs No AbxP) follow-up: range 1–4 years |
297 (3 RCTs) | High | OR 1.07 (0.51 to 2.24) | 166 per 1000 | 10 more per 1000 (74 fewer to 142 more) |
|
UTI recurrence (Sx Rx vs AbxP) follow-up: range 2–5 years |
473 (3 RCTs) | Moderate | OR 0.75 (0.43 to 1.29) | 268 per 1000 | 53 fewer per 1000 (132 fewer to 53 more) |
|
UTI recurrence (Endo Rx vs AbxP) follow-up: range 1–2 years |
195 (2 RCTs) | High | OR 2.03 (0.89 to 4.64) | 111 per 1000 | 91 more per 1000 (11 fewer to 256 more) |
|
UTI recurrence (Endo Rx vs Sx Rx) follow-up: median 1 year |
41 (1 RCT) | High | Not estimable | 0 per 1000 | 0 fewer per 1000 (0 fewer to 0 fewer) |
|
Progression of old lesion (AbxP vs Sx Rx) follow-up: range 2–5 years |
534 (3 RCTs) | Moderate | OR 1.23 (0.79 to 1.93) | 163 per 1000 | 30 more per 1000 (30 fewer to 110 more) |
|
Progression of old lesion (AbxP vs No AbxP) follow-up: median 4 years |
100 (1 RCT) | High | OR 0.09 (0.01 to 0.76) | 180 per 1000 | 161 fewer per 1000 (178 fewer to 37 fewer) |
|
Formation of new renal scars (AbxP vs Sx Rx) follow-up: range 4–5 years |
438 (2 RCTs) | Moderate | OR 0.86 (0.51 to 1.44) | 167 per 1000 | 20 fewer per 1000 (74 fewer to 57 more) |
|
Formation of new renal scars (Endo Rx vs No AbxP) follow-up: median 2 years |
134 (1 RCT) | Moderate‡ | OR 0.66 (0.22 to 1.96) | 132 per 1000 | 41 fewer per 1000 (100 fewer to 98 more) |
|
RRU (Sx Rx vs Endo Rx) follow-up: range 2–5 years |
160 (2 RCTs) | Moderate‡ | OR 5.02 (1.47 to 17.13) | 825 per 1000 | 134 more per 1000 (49 more to 163 more) |
|
RRU (Sx Rx vs AbxP) follow-up: median 2 years |
134 (1 RCT) | Moderate‡ | OR 94.59 (20.87 to 428.74) | 262 per 1000 | 709 more per 1000 (619 more to 732 more) |
|
RRU (Endo Rx vs AbxP) follow-up: median 1 years |
82 (1 RCT) | Moderate‡ | OR 8.33 (3.14 to 22.13) | 333 per 1000 | 473 more per 1000 (278 more to 584 more) |
GRADE Working Group grades of evidence.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Bold values were Odd ratios(OR) and risk differences for each treatment comparison.
*The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
†Unclear explanation of randomisation process in two studies and some missing data in one study.
‡Unclear explanation of randomisation process.
RCTs, randomised controlled trials; RRU, resolution by renal units; UTI, urinary tract infection.
Table 4.
Summary of findings of GRADE analysis for network meta-analysis
| Patient or population: VUR grades II–IV Setting: various treatment modalities in children with VUR grade II–IV Interventions: surgical, endoscopic and conservative treatment Comparison: antibiotic prophylaxis Outcome: UTI recurrence |
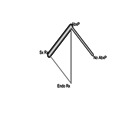 Network geometry* |
|||
| Total studies: 9 RCTs Total participants: 1013 |
NMA estimate effect† (95% CI) | NMA Certainty in the evidence | Ranking‡ (P-score) |
Interpretation |
|
Surgical treatment (Sx Rx) |
−0.26 (−0.54 to 0.02) | Moderate§ | 0.85 | Probably superior |
|
Antibiotic prophylaxis (AbxP) |
Reference comparator | Reference comparator | 0.43 | Reference comparator |
|
Endoscopic treatment (Endo Rx) |
0.2 (−1.41 to 1.81) | High | 0.38 | Probably inferior |
|
Conservative treatment (No AbxP) |
0.15 (−0.45 to 0.75) | High | 0.31 | Probably inferior |
GRADE Working Group grades of evidence.
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
*Lines represent direct comparisons.
†Network estimate effects are reported as Log OR and the results are expressed in 95% CI since the frequentist model has been conducted.
‡Ranking is calculated by P-score by netrank function.
§Unclear explanation of randomisation process in two studies and some missing data in one study.
RCTs, randomised controlled trials; UTI, urinary tract infection; VUR, vesicoureteral reflux.
Discussion
To our knowledge, this is the first network meta-analysis that compared different treatment modalities for patients with VUR grades II–IV. The effectiveness of each treatment in preventing the occurrence of post-treatment UTI was simultaneously compared by conducting network meta-analysis. Sx Rx showed the best outcome in reducing post-treatment UTI among patients with VUR grades II–IV followed by AbxP, no AbxP and Endo Rx consecutively. However, mixed comparison results showed no significant differences. Pairwise comparisons for post-treatment UTI, progression of old lesions and formation of new renal scar showed no significant differences between the treatment modalities. However, Sx Rx provided a better resolution rate of VUR grades II–IV than Endo Rx and AbxP.
Children with VUR have a high spontaneous resolution rate within the first 4–5 years of life.29 30 Male sex, young age, unilateral VUR have good resolution rate. Besides, it is also believed that VUR alone is not likely to cause renal damage without the presence of UTI.31 Risk factors for UTI include young age, high-grade VUR, female sex and circumcision status in boys. Presence of bladder bowel dysfunction is also one of the important factors that influence VUR resolution rate and increase UTI risk.32
ABxP is commonly used for children with VUR to prevent UTI recurrence. However, several studies have examined age, gender and VUR severity to determine the efficacy of AbxP, and the results remain controversial. Swedish reflux study27 and randomised intervention for children with vesicoureteral reflux trial33 supported using AbxP because of its significant reduction in UTI recurrence, but PRIVENT (Prevention of Recurrent Urinary Tract Infection in Children with Vesicoureteric Reflux and Normal Renal Tracts) study21 found a limited effect of AbxP. A recent meta-analysis17 comparing all grades of VUR showed that recurrent UTI was less in AbxP than no AbxP group. In our study, there was no significant difference between AbxP and other treatments for UTI and renal damage. This may be due to differences in age, gender and VUR severity of included studies.
Antibiotic resistance is an emerging problem for AbxP,34 and this may affect the treatment outcomes. Adverse effects of long-term antibiotic use such as allergic reaction, weaken immune system and Clostridium difficile infection should also be considered. Becoming less effectiveness of AbxP, active surveillance without AbxP can be an alternative option. Being alert for febrile UTI and early treatment to prevent renal damage are necessary. Therefore, understanding and compliance of the parents play an important role for active surveillance.
Ureteral reimplantation has been used for decades with the most successful outcome for the correction of VUR. The principle of surgical correction is to mimic or strengthen the antireflux mechanism by creating the longer ureteral segment passing the tunnel between bladder mucosa and muscularis propria. Lich-Gregoir extravesical antireflux technique, Cohen intravesical reimplantation and Politano-Leadbetter combined intravesical and extravesical reimplantation technique are most commonly used methods.35 Sx Rx included in our study are open ureteral reimplantation methods, mostly Cohen and Politano-Leadbetter technique. Despite a significant better RRU in Sx Rx, no significant difference was found in recurrent UTI and renal damage in our study. These results coincide with other meta-analyses.16 17
Another treatment option for VUR is Endo Rx which has been introduced over the last two decades.36 Different bulking agents can be injected at ureteric orifice with the Traditional Subureteric Teflon Injection technique or hydrodistension implantation technique (HIT) including the double HIT.37 However, the choice of bulking agents may impact the safety and efficacy of Endo Rx as granuloma formation due to foreign body reaction, migration from injection site and periureteric fibrosis. Dextranomer/hyaluronic acid showed low complication rates with short-term hematuria (0.2%–0.8%), ureteral obstruction (0.5%–1.3%), calcification (0.5%) and late ureteral implantation (2.7%).38 Although Endo Rx showed significantly lower resolution rate than Sx Rx, it is less invasive and uses easier technique than Sx Rx. However, clinicians must balance risks and benefits of each procedure as well as their own surgical experiences.
Limitations of this study should be addressed. For low risks of bias, only randomised control studies were included in this study. As many studies did not report separate data for VUR grades II–IV, they were excluded from current study for network consistency and transitivity. Mixed treatment comparison could be performed by network meta-analysis only for UTI recurrence, and the rest parameters could only be compared with pairwise comparisons. Moreover, robotic-assisted surgery has been used to correct VUR in children with body weight>10 kg39 40 while our study did not include it in this study. Therefore, future research should consider including robotic assisted surgery as one of the treatment modalities. Last, but not least, our study could not consider patients’ age, febrile or symptomatic UTI, follow-up times, and publication years because of limited included studies.
Conclusion
The results from both pairwise and network meta-analyses suggest that there is probably no difference between the treatments concerning their impact on UTI recurrence, progression of previous renal scars, or the formation of new renal scars in children with VUR grades II–IV. These findings could offer valuable evidence-based insights for guiding treatment selection, emphasising the importance of individualised approaches based on each patient’s specific risk factors.
Supplementary Material
Acknowledgments
The authors would like to thank the team of Taipei Tzu Chi Hospital Library and the research team of Taipei Tzu Chi Hospital for their support in the database search and the development of this paper.
Footnotes
Contributors: C-LC and C-HC conceptualised and designed the study, performed data collection, data analysis and drafted the original manuscript. S-SDY, C-KH and S-JC coordinated and supervised data collection, and critically reviewed the manuscript. All authors reviewed and approved the final manuscript as submitted and agree to be accountable for all aspects of the work. S-JC willingly accepted the role of guarantor for this study.
Funding: This study was supported by a grant from the Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation (Grant No. TCRD-TPE-110-12).
Competing interests: No, there are no competing interests.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Chandra M. Reflux nephropathy, urinary tract infection, and voiding disorders. Curr Opin Pediatr 1995;7:164–70. 10.1097/00008480-199504000-00009 [DOI] [PubMed] [Google Scholar]
- 2.Sargent MA. What is the normal prevalence of vesicoureteral reflux Pediatr Radiol 2000;30:587–93. 10.1007/s002470000263 [DOI] [PubMed] [Google Scholar]
- 3.Hunziker M, Colhoun E, Puri P. Prevalence and predictors of renal functional abnormalities of high grade vesicoureteral reflux. J Urol 2013;190:1490–4. 10.1016/j.juro.2013.01.068 [DOI] [PubMed] [Google Scholar]
- 4.Hoberman A, Charron M, Hickey RW, et al. Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med 2003;348:195–202. 10.1056/NEJMoa021698 [DOI] [PubMed] [Google Scholar]
- 5.Keren R, Shaikh N, Pohl H, et al. Risk factors for recurrent urinary tract infection and renal Scarring. Pediatrics 2015;136:e13–21. 10.1542/peds.2015-0409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lebowitz RL, Olbing H, Parkkulainen KV, et al. International system of radiographic grading of vesicoureteric reflux. Pediatr Radiol 1985;15:105–9. 10.1007/BF02388714 [DOI] [PubMed] [Google Scholar]
- 7.Arlen AM, Kirsch AJ, Leong T, et al. Validation of the ureteral diameter ratio for predicting early spontaneous resolution of primary vesicoureteral reflux. J Pediatr Urol 2017;13:383. 10.1016/j.jpurol.2017.01.012 [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Roig M, Ridley DE, McCracken C, et al. Vesicoureteral reflux index: predicting primary vesicoureteral reflux resolution in children diagnosed after age 24 months. J Urol 2017;197:1150–7. 10.1016/j.juro.2016.12.008 [DOI] [PubMed] [Google Scholar]
- 9.Ntoulia A, Back SJ, Shellikeri S. Contrast-enhanced voiding urosonography (cevus) with the intravesical administration of the ultrasound contrast agent optison™ for vesicoureteral reflux detection in children: a prospective clinical trial. Pediatr Radiol 2018;48:216–26. 10.1007/s00247-017-4026-3 [DOI] [PubMed] [Google Scholar]
- 10.Shimada K, Taguchi K, Koike H, et al. Spontaneous resolution of reflux in children with primary VUR. Nihon Hinyokika Gakkai Zasshi 1990;81:982–7. 10.5980/jpnjurol1989.81.982 [DOI] [PubMed] [Google Scholar]
- 11.Springer A, Subramaniam R. Relevance of current guidelines in the management of VUR. Eur J Pediatr 2014;173:835–43. 10.1007/s00431-013-2253-7 [DOI] [PubMed] [Google Scholar]
- 12.Teixeira CBB, Cançado M de P, Carvalhaes J de A. Cançado, and J.T.D.A. carvalhaes, primary vesicoureteral reflux: conservative therapy or surgical intervention. J Bras Nefrol 2014;36:10–7. 10.5935/0101-2800.20140004 [DOI] [PubMed] [Google Scholar]
- 13.Williams G, Hodson EM, Craig JC. Interventions for primary vesicoureteric reflux. Cochrane Database Syst Rev 2019;2. 10.1002/14651858.CD001532.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teixeira CBB, Cançado M de P, Carvalhaes J de A. Primary vesicoureteral reflux: conservative therapy or surgical intervention. J Bras Nefrol 2014;36:10–7. 10.5935/0101-2800.20140004 [DOI] [PubMed] [Google Scholar]
- 15.Wheeler D, Vimalachandra D, Hodson EM, et al. Antibiotics and surgery for vesicoureteric reflux: a meta-analysis of randomised controlled trials. Arch Dis Child 2003;88:688–94. 10.1136/adc.88.8.688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mina-Riascos SH, Fernández N, García-Perdomo HA. Effectiveness and risks of endoscopic management compared to vesicoureteral reimplantation in patients with high-grade vesicoureteral reflux: systematic review and network meta-analysis. Eur J Pediatr 2021;180:1383–91. 10.1007/s00431-021-03948-w [DOI] [PubMed] [Google Scholar]
- 17.Xie M, Xu X, Cao Z, et al. Do various treatment modalities of vesicoureteral reflux have any adverse effects in pediatric patients? A meta-analysis. Urol Int 2021;105:1002–10. 10.1159/000518603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olbing H, Claësson I, Ebel KD, et al. Renal scars and parenchymal thinning in children with vesicoureteral reflux: a 5-year report of the International reflux study in children (European branch). J Urol 1992;148:1653–6. 10.1016/s0022-5347(17)36995-1 [DOI] [PubMed] [Google Scholar]
- 19.Jodal U, Smellie JM, Lax H, et al. Ten-year results of randomized treatment of children with severe vesicoureteral reflux. final report of the International reflux study in children. Pediatr Nephrol 2006;21:785–92. 10.1007/s00467-006-0063-0 [DOI] [PubMed] [Google Scholar]
- 20.Hari P, Hari S, Sinha A, et al. Antibiotic prophylaxis in the management of vesicoureteric reflux: a randomized double-blind placebo-controlled trial. Pediatr Nephrol 2015;30:479–86. 10.1007/s00467-014-2943-z [DOI] [PubMed] [Google Scholar]
- 21.Craig JC, Simpson JM, Williams GJ, et al. Antibiotic prophylaxis and recurrent urinary tract infection in children. N Engl J Med 2009;361:1748–59. 10.1056/NEJMoa0902295 [DOI] [PubMed] [Google Scholar]
- 22.Pennesi M, Travan L, Peratoner L, et al. Is antibiotic prophylaxis in children with vesicoureteral reflux effective in preventing pyelonephritis and renal scars? A randomized, controlled trial. Pediatrics 2008;121:e1489–94. 10.1542/peds.2007-2652 [DOI] [PubMed] [Google Scholar]
- 23.Weiss R, Duckett J, Spitzer A. Results of a randomized clinical trial of medical versus surgical management of infants and children with grades III and IV primary vesicoureteral reflux (united states). the international reflux study in children. J Urol 1992;148:1667–73. 10.1016/s0022-5347(17)36998-7 [DOI] [PubMed] [Google Scholar]
- 24.Prospective trial of operative versus non-operative treatment of severe vesicoureteric reflux: two years' observation in 96 children. Br Med J (Clin Res Ed) 1983;287:171–4. 10.1136/bmj.287.6386.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Aparicio L, Rovira J, Blazquez-Gomez E, et al. Randomized clinical trial comparing endoscopic treatment with dextranomer hyaluronic acid copolymer and cohen's ureteral reimplantation for vesicoureteral reflux: long-term results. J Pediatr Urol 2013;9:483–7. 10.1016/j.jpurol.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 26.Capozza N, Caione P. Dextranomer/hyaluronic acid copolymer implantation for vesico-ureteral reflux: a randomized comparison with antibiotic prophylaxis. J Pediatr 2002;140:230–4. 10.1067/mpd.2002.121380 [DOI] [PubMed] [Google Scholar]
- 27.Brandström P, Jodal U, Sillén U, et al. The Swedish reflux trial: review of a randomized, controlled trial in children with dilating vesicoureteral reflux. J Pediatr Urol 2011;7:594–600. 10.1016/j.jpurol.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 28.Salih EM, Eldamanhory H, Selmy GI, et al. Comparison of subureteral endoscopic injection of dextranomer/hyaluronic acid and lich-gregoir ureteral reimplantation in the treatment of pediatric primary vesicoureteral reflux: a prospective randomized study. J Laparoendosc Adv Surg Tech A 2021;31:719–23. 10.1089/lap.2020.0973 [DOI] [PubMed] [Google Scholar]
- 29.Estrada CR, Passerotti CC, Graham DA, et al. Nomograms for predicting annual resolution rate of primary vesicoureteral reflux: results from 2,462 children. J Urol 2009;182:1535–41. 10.1016/j.juro.2009.06.053 [DOI] [PubMed] [Google Scholar]
- 30.Hajiyev P, Burgu B. Contemporary management of vesicoureteral reflux. Eur Urol Focus 2017;3:181–8. 10.1016/j.euf.2017.08.012 [DOI] [PubMed] [Google Scholar]
- 31.Swerkersson S, Jodal U, Sixt R, et al. Urinary tract infection in small children: the evolution of renal damage over time. Pediatr Nephrol 2017;32:1907–13. 10.1007/s00467-017-3705-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elder JS, Diaz M. Vesicoureteral reflux--the role of bladder and bowel dysfunction. Nat Rev Urol 2013;10:640–8. 10.1038/nrurol.2013.221 [DOI] [PubMed] [Google Scholar]
- 33.Mattoo TK, Chesney RW, Greenfield SP, et al. Renal scarring in the randomized intervention for children with vesicoureteral reflux (RIVUR) trial. Clin J Am Soc Nephrol 2016;11:54–61. 10.2215/CJN.05210515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng C-H, Tsai M-H, Huang Y-C, et al. Antibiotic resistance patterns of community-acquired urinary tract infections in children with vesicoureteral reflux receiving prophylactic antibiotic therapy. Pediatrics 2008;122:1212–7. 10.1542/peds.2007-2926 [DOI] [PubMed] [Google Scholar]
- 35.Austin JC, Cooper CS. Vesicoureteral reflux: surgical approaches. Urol Clin North Am 2004;31:543–57, 10.1016/j.ucl.2004.04.018 [DOI] [PubMed] [Google Scholar]
- 36.Calisti A, Oriolo L, Perrotta ML, et al. Endoscopic subureteral injection for vesicoureteral reflux and the risk of overtreatment. Minerva Pediatr 2009;61:1–7. [PubMed] [Google Scholar]
- 37.Yap T-L, Chen Y, Nah SA, et al. STING versus HIT technique of endoscopic treatment for vesicoureteral reflux: a systematic review and meta-analysis. J Pediatr Surg 2016;51:2015–20. 10.1016/j.jpedsurg.2016.09.028 [DOI] [PubMed] [Google Scholar]
- 38.Kirsch AJ, Cooper CS, Läckgren G. Non-animal stabilized hyaluronic acid/dextranomer gel (nasha/dx, deflux) for endoscopic treatment of vesicoureteral reflux: what have we learned over the last 20 years? Urology 2021;157:15–28. 10.1016/j.urology.2021.07.032 [DOI] [PubMed] [Google Scholar]
- 39.Finkelstein JB, Levy AC, Silva MV, et al. How to decide which infant can have robotic surgery? Just do the math. J Pediatr Urol 2015;11:170. 10.1016/j.jpurol.2014.11.020 [DOI] [PubMed] [Google Scholar]
- 40.Herz D, Fuchs M, Todd A, et al. Robot-assisted laparoscopic extravesical ureteral reimplant: a critical look at surgical outcomes. J Pediatr Urol 2016;12:402. 10.1016/j.jpurol.2016.05.042 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.